Published online Sep 14, 2013. doi: 10.3748/wjg.v19.i34.5700
Revised: June 8, 2013
Accepted: July 23, 2013
Published online: September 14, 2013
AIM: To investigate time-dependent changes caused by temporal portal vein obstruction and subsequent reperfusion in the lobe with or without an occluded portal vein.
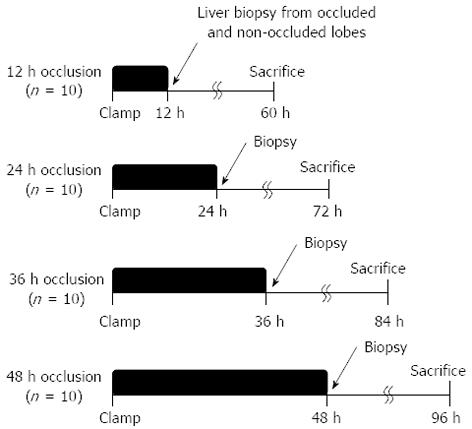
METHODS: The portal vein (PV) of the anterior lobe of the liver of a male Wistar rat (8 wk-old) was obstructed (70%) for 12, 24, 36 and 48 h, respectively, and models were sacrificed at 48 h after reperfusion (each group: n = 10). The histological changes and the status of liver regeneration were compared between a liver biopsy performed on each lobe after temporary obstruction of the portal vein in the same rat liver, and the liver extracted at the time of sacrifice (48 h after reperfusion).
RESULTS: With regard to the obstructed lobe, the liver weight/body weight ratio significantly decreased according to obstruction time. On the other hand, in the non-obstructed lobe, there were no significant differences within each group. The duration of PV occlusion did not seem to be strong enough to introduce liver weight increase. Stimulation of liver regeneration was brought about in the non-occluded lobe by 12-h occlusion, and was sustained even at 48 h after reperfusion. The obstructed lobe atrophied with the passage of time in the obstructed state. However, the proliferating-cell nuclear antigen labeling index also increased at 48 h after reperfusion, and a repair mechanism was observed.
CONCLUSION: Temporary blood flow obstruction of the portal vein may become a significant trigger for liver regeneration, even with an obstruction of 12 h.
Core tip: This paper describes the chronological effects of temporary portal venous branch ligation on liver regeneration in rats. These results imply that, in the future, it might be possible to control liver regeneration. In the clinical setting, we have just completely occluded the portal venous branch irreversibly.
- Citation: Hamasaki K, Eguchi S, Soyama A, Hidaka M, Takatsuki M, Fujita F, Kanetaka K, Minami S, Kuroki T. Chronological changes in the liver after temporary partial portal venous occlusion. World J Gastroenterol 2013; 19(34): 5700-5705
- URL: https://www.wjgnet.com/1007-9327/full/v19/i34/5700.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i34.5700
Permanent obstruction of the portal vein, as clinically applied in portal branch ligation (PBL) or percutaneous transhepatic portal venous embolization, evokes liver regeneration[1-4]. This technique enables relatively major hepatic resection for malignancy in an occluded liver lobe[5-9]. In addition, PBL has been used to induce a regenerative stimulus for transplanted hepatocytes or pancreatic islet cells for cell therapy[10,11].
Although short term temporary occlusion can induce some degree of liver regeneration, an investigation of liver regeneration caused by temporary portal vein obstruction, as well as time-dependent changes resulting from reperfusion, has not yet been performed[12]. Therefore, the current study aimed to examine time-dependent changes in a lobe with a portal vein occlusion of an unobstructed portal vein caused by temporary portal vein obstruction and reperfusion as the central focus.
Male Wistar rats (200-240 g, Japan SLC Inc., Shizuoka, Japan) were used for the experiments. All animals were maintained at 24 °C with a 12-h light-dark cycle and given free access to tap water and standard laboratory chow. The animals were treated in accordance with the guidelines stated in the University of Nagasaki Research Animal Resources during all experimental procedures.
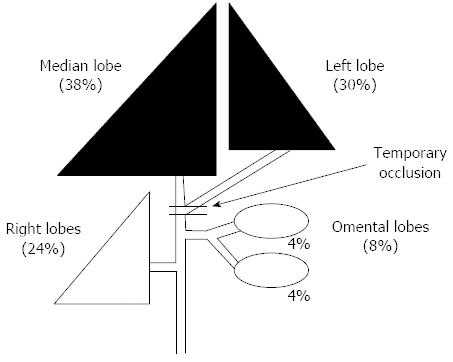
The portal vein of the anterior lobe (medial and left lobes) of the liver of a male Wistar rat (8 wk old) was occluded (70%) for 12, 24, 36 and 48 h, respectively (Figure 1). Rats to be sacrificed were prepared at 48 h after each reperfusion (models for each group: n = 10, Figure 2). The histological changes over time and the status of liver regeneration were compared between liver biopsies performed from each lobe after temporary obstruction of the portal vein in the same rat liver, and the liver extracted at the time of sacrifice (48 h after reperfusion).
The body weights and liver weights were recorded following the sacrifice of the rats to compare the rate of liver regeneration. The liver weight was expressed as a percentage of the body weight (%) and used as an index.
Formalin-fixed paraffin embedded (4 μm) sections were used for hematoxylin-eosin (HE) staining. Proliferating-cell nuclear antigen (PCNA) immunostaining was performed to examine hepatocyte proliferation using a mouse monoclonal antibody against PCNA (clone-PC 10; Dako, Kyoto, Japan)[13]. Briefly, liver tissue specimens were fixed in 10% buffered formalin, embedded in paraffin and then cut into 5 μm sections. The deparaffinized sections were heated in a microwave three times in phosphate-buffered saline (PBS) for 5 min each and were then washed three times with PBS for 5 min each. After blocking endogenous peroxidase activity, the specimens were washed three times with PBS for 5 min each. The sections were incubated with an antibody against PCNA overnight at 4 °C. After washing several times with PBS, biotin-labeled secondary antibody was added for 1 h at room temperature. After washing several times with PBS, the tissue peroxidase activity was visualized using diaminobenzidine.
The PCNA labeling index (PCNA LI) was then determined as the number of PCNA-positive cells among 1000 counted cells.
All of the data were expressed as the mean ± SD. The Mann-Whitney U-test was used for data analysis. A level of P < 0.05 was considered statistically significant.
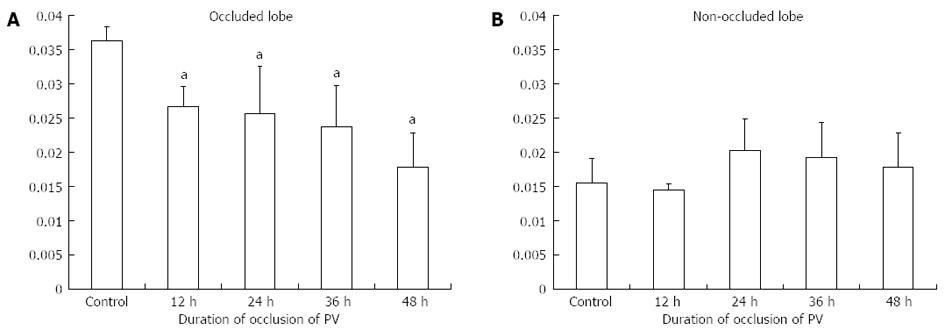
With regard to the obstructed lobe, the liver weight/body weight ratio significantly decreased with increasing obstruction time (Figure 3). On the other hand, in the non-obstructed lobe, there were no significant differences within each group. The duration of PV occlusion did not seem to be strong enough to induce an increased in liver weight.
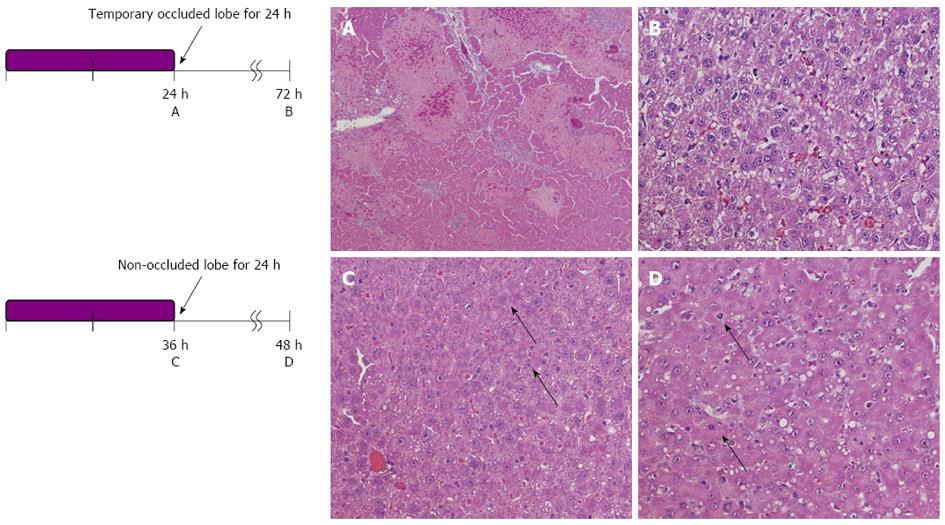
Liver histology was investigated under HE staining (Figure 4). In the occluded liver lobe, before reperfusion, coagulative necrosis was observed around the central vein in proportion to the occlusion time. However, after 48 h of reperfusion, the above-mentioned necrotic area decreased. On the other hand, in the non-occluded liver lobe, hepatocytes became hypertrophic, and some mitoses were observed.
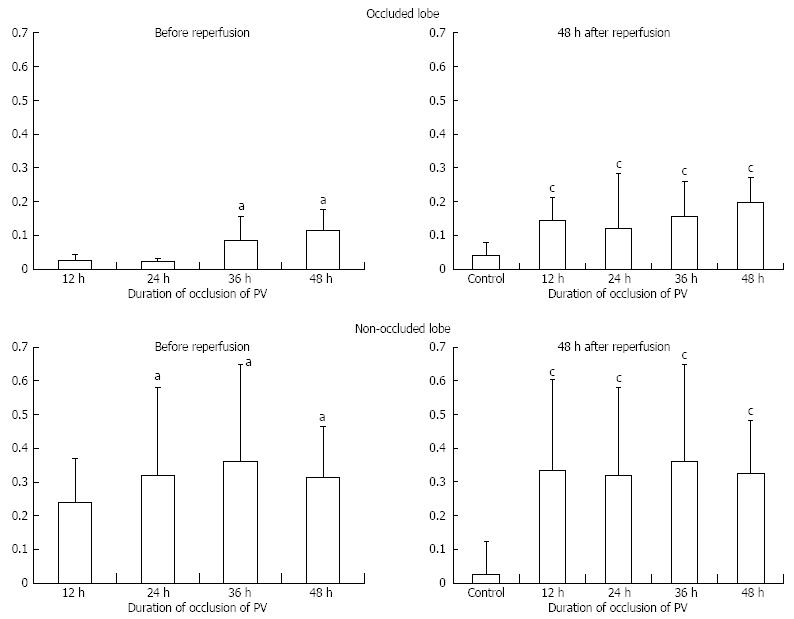
In the non-obstructed lobe, there were no significant differences in the PCNA LI within each group (Figure 5). LI seemed to peak at 36 h of biopsy (non-obstructed models at 12, 24, 36 and 48 h = 24%, 32%, 36% and 31%, Figure 5C). In the non-occluded lobe at 48 h after reperfusion (models for each group = 33%, 32%, 36% and 32%), the PCNA LI was still significantly increased at all points compared with the control (Figure 5D).
On the other hand, hardly any positive cells were observed in a biopsy of the obstructed lobe (obstructed models at 12, 24, 36 and 48 h = 2%, 2%, 8% and 10%). However, at 48 h after reperfusion, an increase in LI was observed (models for each group = 14%, 12%, 15% and 19%).
Portal venous branch ligation or embolization (PBL or PBE) can induce atrophy of the ligated lobe, while inducing hypertrophy of a non-ligated lobe, which enables extended hepatectomy for a malignant tumor in a ligated lobe[14-16]. In addition, PBL has been used as a regenerative stimulator to induce transplanted cell proliferation in animal models for hepatocyte-based cell therapy[17-23]. The length of time of occlusion needed to induce remnant liver regeneration, i.e., temporary portal venous occlusion, remains unknown. In the present study. the stimulation of liver regeneration brought about in the non-occluded liver lobe was sustained, even after 48 h from reperfusion. Thus, temporary blood flow obstruction of the portal vein may be a significant trigger of liver regeneration, with an obstruction of at least 12 h. However, the duration of PV occlusion in this study did not seem to be long enough to induce an increase in liver weight.
Interestingly, there was no significant difference in PCNA LI in the non-occluded lobe according to the duration of portal vein occlusion up to 48 h. Therefore, in the clinical setting, the same extent of liver hypertrophy may be induced with temporary balloon occlusion in as short as 12 h, to minimize an invasive procedure, although there might be difference among species.
As a cell therapy, many investigator have used liver for the engraftment of many cell types[10-12,14-16]. In fact, transplanted cells (hepatocytes, pancreatic islet cells or genetically engineered cells) could be induced to proliferate using temporary portal venous occlusion. Although there must be some differences between humans and rodents in terms of liver regenerative activity, our results provide a new insight into temporary stimulation of liver regeneration for subsequent treatment procedures[24-26].
On the other hand, the PCNA labeling index of the obstructed lobe was also increased 48 h after portal venous reperfusion. This could be a repair mechanism for portal vein ischemia in the occluded lobe, although PCNA LI was lower compared to that in the non-occluded lobe that undergoes liver regeneration[14]. Although it was not observed up to 48 h, this result of the temporary occlusion of the lobe provided an interesting phenomena; however, the lack of temporal portal venous flow could become atrophic if portal venous ischemia had lasted longer. The duration for the “point of no return” should be investigated in further research.
In conclusion, a temporary blood flow obstruction of the portal vein may be a significant trigger for liver regeneration, even with an obstruction of 12 h. The histological changes in the unobstructed lobe and obstructed lobe in cases of temporary blood flow obstruction of the portal vein and at 48 h after reperfusion were described.
Permanent obstruction of the portal vein, as clinically applied in portal branch ligation (PBL) or percutaneous transhepatic portal venous embolization, evokes liver regeneration. This technique enables relatively major hepatic resection for malignancy in an occluded liver lobe. In addition, PBL has been used to induce a regenerative stimulus for transplanted hepatocytes or pancreatic islet cells for cell therapy.
Portal venous branch ligation or embolization can induce atrophy of the ligated lobe, while inducing hypertrophy of a non-ligated lobe, which enables extended hepatectomy for a malignant tumor in a ligated lobe. In addition, PBL has been used as a regenerative stimulator to induce transplanted cell proliferation in animal models of hepatocyte based cell therapy. The length of time of occlusion required to induce remnant liver regeneration, i.e., temporary portal venous occlusion, remains unknown.
The histological changes and the status of liver regeneration were compared between a liver biopsy performed on each lobe after temporary obstruction of the portal vein in the same rat liver, and the liver extracted at the time of sacrifice (48 h after reperfusion).
This research is very important because it shows that a temporary obstruction of the portal vein as a trigger of liver regeneration. Recently, it has been used as a regenerative stimulator to induce transplanted cell proliferation. Thus, the manuscript approached an interesting subject from the surgical and scientific points of view; however, several aspects should be better evaluated.
P- Reviewers Helling TS, Ikeda K, Mizuno S, Silva R S- Editor Gou SX L- Editor Stewart GJ E- Editor Zhang DN
| 1. | Yu JH, Zhang WG, Jiang GX, Zhao JY, Li H, Wang ZD, Cui YF. Ischemia/reperfusion in clamped lobes facilitates liver regeneration of non-clamped lobes after selective portal vein ligation. Dig Dis Sci. 2012;57:3178-3183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | van Lienden KP, van den Esschert JW, de Graaf W, Bipat S, Lameris JS, van Gulik TM, van Delden OM. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol. 2013;36:25-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 293] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 3. | Rauchfuss F, Lambeck S, Claus RA, Ullmann J, Schulz T, Weber M, Katenkamp K, Guthke R, Bauer M, Settmacher U. Sustained liver regeneration after portal vein embolization --a human molecular pilot study. Dig Liver Dis. 2012;44:681-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Yang K, Du C, Cheng Y, Li Y, Gong J, Liu Z. Augmenter of liver regeneration promotes hepatic regeneration depending on the integrity of Kupffer cell in rat small-for-size liver transplantation. J Surg Res. 2013;183:922-928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, Nagino M. Portal vein embolization before extended hepatectomy for biliary cancer: current technique and review of 494 consecutive embolizations. Dig Surg. 2012;29:23-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Imamura H, Shimada R, Kubota M, Matsuyama Y, Nakayama A, Miyagawa S, Makuuchi M, Kawasaki S. Preoperative portal vein embolization: an audit of 84 patients. Hepatology. 1999;29:1099-1105. [PubMed] [Cited in This Article: ] |
| 7. | Okabe H, Beppu T, Nakagawa S, Yoshida M, Hayashi H, Masuda T, Imai K, Mima K, Kuroki H, Nitta H. Percentage of future liver remnant volume before portal vein embolization influences the degree of liver regeneration after hepatectomy. J Gastrointest Surg. 2013;17:1447-1451. [PubMed] [Cited in This Article: ] |
| 8. | Shindoh J, Vauthey JN, Zimmitti G, Curley SA, Huang SY, Mahvash A, Gupta S, Wallace MJ, Aloia TA. Analysis of the efficacy of portal vein embolization for patients with extensive liver malignancy and very low future liver remnant volume, including a comparison with the associating liver partition with portal vein ligation for staged hepatectomy approach. J Am Coll Surg. 2013;217:126-133; discussion 133-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 190] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 9. | Shindoh J, Truty MJ, Aloia TA, Curley SA, Zimmitti G, Huang SY, Mahvash A, Gupta S, Wallace MJ, Vauthey JN. Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J Am Coll Surg. 2013;216:201-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 194] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 10. | Eguchi S, Rozga J, Lebow LT, Chen SC, Wang CC, Rosenthal R, Fogli L, Hewitt WR, Middleton Y, Demetriou AA. Treatment of hypercholesterolemia in the Watanabe rabbit using allogeneic hepatocellular transplantation under a regeneration stimulus. Transplantation. 1996;62:588-593. [PubMed] [Cited in This Article: ] |
| 11. | Fogli L, Morsiani E, Bertanti T, Eguchi S, Azzena G, Demetriou AA. Pancreatic beta-cell replication in streptozotocin-diabetic rats: the effect of liver compensatory growth on intraportally engrafted islets. Pancreas. 1999;19:304-309. [PubMed] [Cited in This Article: ] |
| 12. | Lainas P, Boudechiche L, Osorio A, Coulomb A, Weber A, Pariente D, Franco D, Dagher I. Liver regeneration and recanalization time course following reversible portal vein embolization. J Hepatol. 2008;49:354-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Ichikawa T, Taura N, Miyaaki H, Matsuzaki T, Ohtani M, Eguchi S, Takatsuki M, Soyama A, Hidaka M, Okudaira S. Human T-cell leukemia virus type 1 infection worsens prognosis of hepatitis C virus-related living donor liver transplantation. Transpl Int. 2012;25:433-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M, Ohta K, Yamaguchi T, Matsubara T, Takahashi T. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology. 2001;34:267-272. [PubMed] [Cited in This Article: ] |
| 15. | Aussilhou B, Lesurtel M, Sauvanet A, Farges O, Dokmak S, Goasguen N, Sibert A, Vilgrain V, Belghiti J. Right portal vein ligation is as efficient as portal vein embolization to induce hypertrophy of the left liver remnant. J Gastrointest Surg. 2008;12:297-303. [PubMed] [Cited in This Article: ] |
| 16. | Ribero D, Abdalla EK, Madoff DC, Donadon M, Loyer EM, Vauthey JN. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386-1394. [PubMed] [Cited in This Article: ] |
| 17. | Lesurtel M, Belghiti J. Temporary portal vein embolization as a starter of liver regeneration. J Hepatol. 2008;49:313-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Khuu DN, Nyabi O, Maerckx C, Sokal E, Najimi M. Adult human liver mesenchymal stem/progenitor cells participate in mouse liver regeneration after hepatectomy. Cell Transplant. 2013;22:1369-1380. [PubMed] [Cited in This Article: ] |
| 19. | Ghodsizad A, Fahy BN, Waclawczyk S, Liedtke S, Gonzalez Berjon JM, Barrios R, Mehrabi A, Karck M, Ruhparwar A, Kögler G. Portal application of human unrestricted somatic stem cells to support hepatic regeneration after portal embolization and tumor surgery. ASAIO J. 2012;58:255-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | am Esch JS, Schmelzle M, Fürst G, Robson SC, Krieg A, Duhme C, Tustas RY, Alexander A, Klein HM, Topp SA. Infusion of CD133+ bone marrow-derived stem cells after selective portal vein embolization enhances functional hepatic reserves after extended right hepatectomy: a retrospective single-center study. Ann Surg. 2012;255:79-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Gock M, Eipel C, Linnebacher M, Klar E, Vollmar B. Impact of portal branch ligation on tissue regeneration, microcirculatory response and microarchitecture in portal blood-deprived and undeprived liver tissue. Microvasc Res. 2011;81:274-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | van den Esschert JW, van Lienden KP, Alles LK, van Wijk AC, Heger M, Roelofs JJ, van Gulik TM. Liver regeneration after portal vein embolization using absorbable and permanent embolization materials in a rabbit model. Ann Surg. 2012;255:311-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Liska V, Slowik P, Eggenhofer E, Treska V, Renner P, Popp FC, Mirka H, Kobr J, Sykora R, Schlitt HJ. Intraportal injection of porcine multipotent mesenchymal stromal cells augments liver regeneration after portal vein embolization. In Vivo. 2009;23:229-235. [PubMed] [Cited in This Article: ] |
| 24. | Ozkan F, Peynircioglu B, Cil BE. Portal vein embolization and stem cell administration. Radiology. 2008;246:646; author reply 646-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 25. | Fürst G, Schulte am Esch J, Poll LW, Hosch SB, Fritz LB, Klein M, Godehardt E, Krieg A, Wecker B, Stoldt V. Portal vein embolization and autologous CD133+ bone marrow stem cells for liver regeneration: initial experience. Radiology. 2007;243:171-179. [PubMed] [Cited in This Article: ] |
| 26. | Dagher I, Nguyen TH, Groyer-Picard MT, Lainas P, Mainot S, Guettier C, Pariente D, Franco D, Weber A. Efficient hepatocyte engraftment and long-term transgene expression after reversible portal embolization in nonhuman primates. Hepatology. 2009;49:950-959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |