Published online Sep 14, 2013. doi: 10.3748/wjg.v19.i34.5645
Revised: June 29, 2013
Accepted: August 4, 2013
Published online: September 14, 2013
AIM: To investigate the quality of topical 2% diltiazem formulations extemporaneously compounded by retail pharmacies openly offering drug-compounding services.
METHODS: A participating healthcare professional wrote 12 prescriptions for compounded 2% diltiazem cream, with 2 refills allowed per prescription. The 12 sets of prescriptions were filled, at intervals of 1-2 wk between refills, at 12 different independent retail pharmacies that openly offer drug-compounding services in a major metropolitan region. The 36 resultant preparations, provided as jars or tubes, were shipped, as soon as each was filled, at ambient temperature to the study core laboratory for high-performance liquid chromatography (HPLC) analysis, within 10 d of receipt. For the HPLC analysis, 8 different samples of the topical diltiazem, each approximately 1 g in weight, were taken from prespecified locations within each container. To initiate the HPLC analysis, each sample was transferred to a 100 mL volumetric flask, to which methanol was added. The HPLC analysis was conducted in accordance with the laboratory-validated method for diltiazem in cream, ointment, and gel formulations. The main outcome measures were potency (percentage of label claim) and content uniformity of the compounded topical 2% diltiazem formulations.
RESULTS: Of the 36 prescriptions filled, 30 were packaged in jars and 6 were packaged as tubes. The prescriptions were specifically for cream formulations, but 6 of the 12 pharmacies compounded 2% diltiazem as an ointment; for another pharmacy, which had inadequate labeling, the dosage form was unknown. The United States Pharmacopoeia (USP) standard for potency is 90%-115% of label claim. Of the 36 preparations, 5 (13.89%) were suprapotent and 13 (36.11%) were subpotent. The suprapotent prescriptions ranged in potency from 117.2% to 128.5% of label claim, and the subpotent prescriptions ranged in potency from 34.8% to 89.8% of label claim. Fourteen (38.9%) preparations lacked content uniformity according to the USP standard of 90%-110% potency and < 6% relative standard deviation. Of the 30 formulations packaged in jars, 12 (40%) lacked content uniformity, while of the 6 formulations packaged in tubes, 2 (33.3%) lacked content uniformity. Nine of the 12 pharmacies (75%) failed USP potency or content-uniformity specifications for at least 1 of the 3 prescription fills. For 5 of the 12 pharmacies (41.7%), the mean potency across all three prescription fills was < 90% of label claim.
CONCLUSION: Patients prescribed topical 2% diltiazem for treatment of anal fissure frequently receive compounded formulations that are misbranded with respect to potency and that lack content uniformity.
Core tip: The use of topical 2% diltiazem hydrochloride for treating anal fissures is supported by multiple clinical trials and is recommended in published practice parameters. As no commercially manufactured formulation of topical 2% diltiazem has been approved yet by the Food and Drug Administration for the treatment of anal fissure, prescriptions for the medication need to be extemporaneously compounded by retail pharmacies. Employing high-performance liquid chromatography analysis of topical 2% diltiazem formulations compounded by a sampling of pharmacies, we found a notable trend toward lack of content uniformity and misbranding of potency, suggesting that many patients might not receive the anticipated relief of anal-fissure pain.
- Citation: Shah M, Sandler L, Rai V, Sharma C, Raghavan L. Quality of compounded topical 2% diltiazem hydrochloride formulations for anal fissure. World J Gastroenterol 2013; 19(34): 5645-5650
- URL: https://www.wjgnet.com/1007-9327/full/v19/i34/5645.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i34.5645
The use of topical 2% diltiazem hydrochloride for treating anal fissures by lowering anal sphincter pressure has been explored in multiple clinical trials since 2000[1-5]. Diltiazem hydrochloride, a calcium channel blocker, is well known as an oral treatment for hypertension and angina[6]. In 2010, the Standard Practice Task Force of the American Society of Colon and Rectal Surgeons (ASCRS) published revised practice parameters for managing anal fissure, assigning to each practice parameter a grade of recommendation and a class of evidence[7]. Noting that conservative (nonsurgical) therapy is safe and should be the first step for managing anal fissure[8-10], the ASCRS task force stated that topical formulations of calcium channel blockers may be appropriately used to treat anal fissure and that these drugs seemed to have a lower incidence of adverse effects than topical nitrates, such as nitroglycerin. This practice parameter was accorded the highest grade of recommendation and the second highest class of evidence by the task force[7].
No commercially manufactured formulation of topical 2% diltiazem has been approved by the United States Food and Drug Administration (FDA) for the treatment of anal fissure. Consequently, colon and rectal surgeons, gastroenterologists, and other physicians who want to follow ASCRS practice parameters and prescribe a topical calcium channel blocker for treatment of anal fissure have to write prescriptions for a product that will be extemporaneously compounded by retail pharmacies. Directions for compounding 2% diltiazem as a topical formulation are readily available in published literature. For example, propylene glycol, hydroxyethyl cellulose, and heated purified water are mixed with diltiazem, and the resulting formulation is packaged in a tight, light-resistant container, usually a tube or jar[11]. A few pharmacies that specialize in compounding services advertise the availability of compounded topical 2% diltiazem on the internet. However, many nonspecialized retail pharmacies also fulfill prescriptions by compounding the product.
In 2006, the FDA investigated the quality of compounded products, collecting active pharmaceutical ingredients (API) and finished compounded drug samples during unannounced visits to compounding pharmacies throughout the country. All API samples passed analysis, but a third of the 36 compounded samples that were collected failed analysis by being either subpotent or suprapotent or by lacking content uniformity. The United States Pharmacopoeia (USP) standard for potency is 90%-115% of label claim[12]. Because the API samples passed analysis, the FDA observed that the failures of the samples in the analysis were directly related to faulty compounding processes at the pharmacies, including the lack of proper in-process controls and end-product testing[13].
To examine the quality of compounded formulations of topical 2% diltiazem, we undertook a high-performance liquid chromatography (HPLC) analysis of preparations gathered from retail pharmacies in a major metropolitan region.
A healthcare professional was asked to write prescriptions for extemporaneously compounded 2% diltiazem cream for fulfillment by retail pharmacies in the greater New York metropolitan region. The selection criteria, intended to locate retail pharmacies that might have experienced pharmacists on staff with competency at compounding, included stipulations that the pharmacies be independent, not parts of retail pharmacy chains, and that they openly offer drug-compounding services by means of online or other advertising. A total of 12 qualifying retail pharmacies were selected from different parts of the metropolitan region.
The participating healthcare professional wrote 12 prescriptions, with 2 refills allowed per prescription, so that 3 prescriptions could be filled at each of the 12 pharmacies (36 total fills) for compounded 2% diltiazem cream. The prescriptions were filled at each of the 12 pharmacies during May 2012 and June 2012, at intervals of 1-2 wk in between refills. As soon as any prescription was filled by a retail pharmacy, it was collected and shipped at ambient temperature in a prepared bubble-wrap mailer to DermPathe Pharmaceuticals (Branchberg, NJ, United States), the laboratory engaged for the HPLC analysis. The compounded formulations of topical 2% diltiazem were provided by the retail pharmacies as either jars or tubes.
Upon receipt of each package, DermPathe logged the time and date and stored the compounded formulation at ambient temperature. HPLC analysis was conducted in accordance with the DermPathe-validated method for diltiazem in cream, ointment, and gel formulations. The analysis included an assessment of potency (percentage of label claim) and content uniformity for each of the compounded formulations. For the analysis of each formulation, 8 different samples of the topical diltiazem, each approximately 1 g in weight, were taken from pre-specified locations within the compounded formulation container. If the compounded formulation was provided in a jar, 4 samples were taken from the top of the jar: 1 at the top front, 1 at the top center, 1 at the top right back corner, and 1 at the top left back corner. In the middle layer of the jar, 1 sample was taken from the center and 1 from the middle right corner. At the bottom of the jar, 1 sample was taken from the center and 1 from the bottom left corner. If the compounded formulation was provided in a tube, the tube was sectioned horizontally and opened up. Then 2 samples were drawn from the top left and top right of the tube, 2 more from the middle left and middle right of the tube, 2 from the bottom left and bottom right of the tube, and the final 2 randomly on the left and on the right between the middle and the bottom of the tube.
Each sample was transferred to a 100 mL volumetric flask, to which methanol was added to fill approximately 80% of the flask volume. The solution was sonicated for 1 h, and the flask was then filled to volume with methanol and mixed thoroughly by shaking. Filtered through a 0.45-micron filter, the solution was then transferred into HPLC vials for analysis using a Waters 2695 HPLC system with a 2487 dual wavelength detector (Waters Corporation, Milford, MA, United States). A Luna C8 150 mm × 4.6 mm, 5 mm column with a C8 Security Guard column (Phenomenex, Torrance, CA, United States) was used as the stationary phase. The mobile phase consisted of an acetate buffer, acetonitrile, and methanol, in a 50:25:25 ratio. The acetate buffer contained 8.2 g of anhydrous sodium acetate and 1.16 g of D-10-camphorsulphonic acid in 1000 mL of water, with pH adjusted to 6.2 with 1 mol/L sodium hydroxide. The flow rate was set at 2 mL per minute, the detector wavelength was set at 240 nm, and the column temperature was set to 30 °C.
Descriptive statistics were used in this study. For categorical variables, frequencies and percentages are reported. For continuous variables, the number of observations, mean ± SD, and relative standard deviation are reported. Statistical analyses were performed using Excel 2010 (Microsoft, Redmond, WA, United States).
Thirty-six prescriptions for compounded topical 2% diltiazem were written, filled, and shipped to DermPathe for analysis. Of these preparations, 30 were packaged in jars and 6 were packaged as tubes (only 2 of the 12 retail pharmacies used tubes for packing). One of the 12 retail pharmacies failed to label each of the 3 filled prescriptions of topical 2% diltiazem with the drug name; the label on each of the 3 formulations from this pharmacy simply read “compound.”
The prescriptions were specifically for cream formulations. Five of the 12 pharmacies compounded 2% diltiazem as a cream, using lipoderm, but 6 of the 12 pharmacies compounded 2% diltiazem as an ointment, using petrolatum. For 1 pharmacy, the same that had the inadequate labeling, the dosage form was unknown.
The preparations were analyzed by DermPathe within 10 d of receipt. Prior to being analyzed, the products were stored at room temperature. In the published directions for preparing topical 2% diltiazem, the shelf life of the preparation is given as 30 d when stored at room temperature[11]. At the time of HPLC analysis, there were no visible signs of product degradation in any of the jars or tubes.
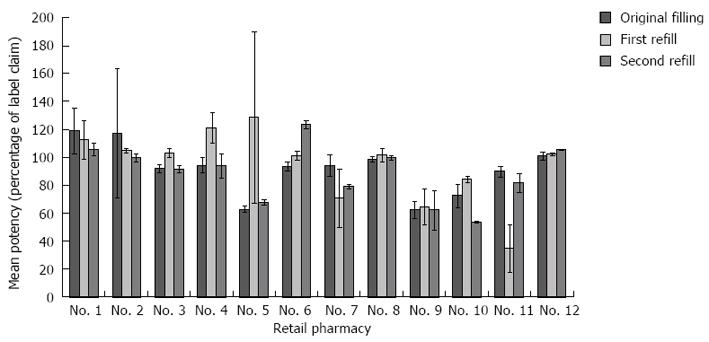
Of the 36 prescriptions, 18 (50.0%) were misbranded for potency according to the USP standard. Five (13.9%) of the prescriptions were suprapotent (that is, the measured drug activity was > 115% of label claim) (Figure 1). The suprapotent prescriptions ranged in potency from 117.2% to 128.5% of label claim. No retail pharmacy produced more than 1 suprapotent formulation. Thirteen (36.1%) of the prescriptions were subpotent (that is, the measured drug activity was < 90% of label claim) (Figure 1). The subpotent prescriptions ranged in potency from 34.8% to 89.8% of label claim. Only 3 of the 12 pharmacies compounded each of the 3 prescriptions they filled without misbranding potency. For 3 of the 12 pharmacies, all 3 of the filled prescriptions were subpotent.
Of the 36 preparations, 14 (38.9%) lacked content uniformity according to the USP requirement of 90% to 110% potency and < 6% relative standard deviation[12]. Table 1 shows the potency variations at the different sample locations of these 14 preparations. Of the 30 formulations packaged in jars, 12 (40%) lacked content uniformity; of the 6 formulations packaged in tubes, 2 (33.3%) lacked content uniformity. In some of the jars the potency varied by more than 100%. In batch 4, provided as a jar, the potency at the top center of the jar was 99.8% while the potency at the bottom center of the jar was 231.7%. In batch 14, also provided as a jar, the potency at the top center of the jar was 72.4% while the potency at the bottom of the jar was 220.8%. Batch 32, provided as a tube, was overall subpotent and also lacked content uniformity: at the middle left of the tube, potency was 16.8%; at the bottom left of the tube, potency was 42.0%.
| Percentage of label claim (prescription number, type of packaging) | ||||||||||||||
| Sample location1 | 1, jar | 2, jar | 4, jar | 10, jar | 11, jar | 14, jar | 19, jar | 20, jar | 25, jar | 26, jar | 27, jar | 28, jar | 32, tube | 33, tube |
| Location 1 | 111.9% | 110.8% | 102.9% | 97.5% | 126.0% | 83.3% | 100.2% | 71.4% | 73.0% | 39.9% | 94.8% | 83.8% | 44.3% | 67.4% |
| Location 2 | 108.7% | 114.2% | 95.7% | 98.0% | 117.8% | 73.1% | 99.3% | 20.9% | 58.7% | 74.2% | 68.9% | 66.5% | 15.1% | 83.4% |
| Location 3 | 142.1% | 112.0% | 100.6% | 101.1% | 144.9% | 93.4% | 91.2% | 82.3% | 67.3% | 52.1% | 63.4% | 81.1% | 16.8% | 80.7% |
| Location 4 | 149.1% | 145.5% | 99.8% | 100.9% | 117.1% | 72.4% | 99.9% | 81.8% | 64.9% | 76.1% | 57.7% | 82.9% | 33.8% | 87.9% |
| Location 5 | 110.6% | 106.5% | 109.0% | 92.9% | 107.4% | 112.4% | 95.9% | 69.2% | 63.2% | 67.9% | 54.3% | 71.5% | 67.7% | 81.5% |
| Location 6 | 106.7% | 99.9% | 97.1% | 90.9% | 116.1% | 161.1% | 97.3% | 81.3% | 59.5% | 65.8% | 51.5% | 65.4% | 29.4% | 79.3% |
| Location 7 | 110.8% | 103.8% | 100.8% | 85.9% | 121.2% | 211.2% | 77.8% | 79.9% | 55.2% | 72.0% | 56.1% | 65.6% | 42.0% | 83.0% |
| Location 8 | 111.7% | 107.8% | 231.7% | 89.2% | 120.6% | 220.8% | 91.0% | 82.1% | 57.6% | 70.6% | 52.8% | 65.6% | 29.4% | 90.7% |
| Mean | 119.0% | 112.6% | 117.2% | 94.6% | 121.4% | 128.5% | 94.1% | 71.1% | 62.4% | 64.8% | 62.4% | 72.8% | 34.8% | 81.7% |
| Relative SD | 14.0% | 12.5% | 39.6% | 6.0% | 9.0% | 46.9% | 8.0% | 29.4% | 9.4% | 19.2% | 22.9% | 11.5% | 48.5% | 8.5% |
Nine of the 12 pharmacies failed USP potency or content-uniformity specifications for at least 1 of their 3 prescriptions. Three of the 12 pharmacies failed USP potency or content-uniformity specifications for all 3 of their prescriptions. When the potencies of the 3 time-separated prescriptions were averaged together for each of the 12 pharmacies, the mean potency was < 90% of label claim for 5 of the 12 pharmacies (Table 2).
| Pharmacy No. | Label claim (mean ± SD) | Relative SD |
| 1 | 95.9% ± 9.8% | 8.7 |
| 2 | 107.3% ± 16.5% | 15.4 |
| 3 | 95.9% ± 2.2% | 2.2 |
| 4 | 103.3% ± 5.1% | 4.9 |
| 5 | 86.5% ± 20.1% | 23.2 |
| 6 | 106.2% ± 1.8% | 1.7 |
| 7 | 81.6% ± 6.5% | 8.0 |
| 8 | 100.1% ± 2.0% | 1.9 |
| 9 | 63.2% ± 3.8% | 6.0 |
| 10 | 70.4% ± 3.2% | 4.5 |
| 11 | 68.8% ± 6.5% | 9.4 |
| 12 | 102.9% ± 0.8% | 0.8 |
In this HPLC analysis of 36 preparations of compounded topical 2% diltiazem from 12 retail pharmacies, half of the preparations did not meet USP specifications for potency and almost 40% of the preparations did not meet USP specifications for content uniformity. Of the 12 retail pharmacies, only three were able to fill all three of the time-separated prescriptions consistently within USP specifications.
When compounded preparations of topical 2% diltiazem fall outside USP specifications for potency, they are more likely to be subpotent (36.1% of the prescriptions) than suprapotent (13.9% of prescriptions). With more than a third of the prescriptions of compounded 2% diltiazem being subpotent, such prescriptions might not routinely relieve anal fissure pain to the extent or with the speed established in clinical trials of topical 2% diltiazem[1,4,14-16]. In one of those trials, Carapeti et al[1] compared different diltiazem gel concentrations (0.1%, 0.5%, 1%, 2%, 5%, and 10% weight per volume) and found a dose-dependent effect on maximum resting anal sphincter pressure (MRP), with the maximal effect (28% reduction compared with pretreatment, P < 0.0001) achieved with the 2% formulation. The MRP was not lowered as effectively with the 1% concentration, while concentrations higher than the 2% produced no additional effect.
The potency of the 5 suprapotent preparations of topical 2% diltiazem did not exceed 128.5% of label claim. However, owing to a lack of content uniformity in some preparations, especially when packaged in jars, compounded diltiazem could be more than twice as potent as the label claim in some sections of a container. The level of suprapotency in these sections, as high as 231.7% of label claim in a section of one jar, could put patients at potential risk for drug-related side effects. Because diltiazem is also used as a hypertensive agent, the largest risk associated with suprapotent topical 2% diltiazem might be dizziness or postural hypotension. In studies that have evaluated topical 2% diltiazem for treatment of anal fissure, the side effect profile has been mild, and the most frequent side effects have been headache or anal pruritus[5,17-20]. However, there have been reports of postural hypotension associated with the use of topical diltiazem[21].
Azarnoff et al[12] conducted a similar HPLC analysis of compounded formulations of topical 0.3% nitroglycerin ointment for anal fissure. The investigators acquired 24 filled compounded prescriptions from 24 retail pharmacies across different geographic regions. They found that 7 (29.2%) of the 24 compounded formulations were subpotent and that 1 (4.8%) was suprapotent. Moreover, 5 (20.8%) of the 24 samples lacked content uniformity. In comparison, in the current study, in which 36 compounded preparations were acquired from 12 different pharmacies, 13 (36.11%) of the preparations were subpotent, 5 (13.9%) were suprapotent, and 14 (38.9%) lacked content uniformity. The relatively worse analytic performance of compounded topical 2% diltiazem formulations in the current study might be an artifact of study design. Another explanation might be that it is more difficult to prepare compounded diltiazem formulations rather than compounded nitroglycerin formulations in accordance with USP standards, although it is unclear why this might be the case.
In October 2012, an FDA report of an outbreak of fungal meningitis related to contaminated products produced by the New England Compounding Center (Framingham, MA, United States) brought to public awareness the practice and business of pharmacy compounding[22,23]. There are legitimate reasons for physicians to prescribe extemporaneously compounded drugs: for example, to provide patients with products like topical 2% diltiazem, currently recommended in the ASCR practice parameters for anal fissure but not approved by the FDA, or to create unique medications for specific patients, such as those who have documented allergies to certain drug ingredients or who require dosage forms different from those of FDA-approved drugs[24-26]. However, not only have both branded and generic drugs undergone FDA approval for safety and efficacy, they are also required by law to be produced under federal Good Manufacturing Practice (GMP) regulations in order to ensure their identity, quantity, potency, and purity[24]. In contrast, although extemporaneously compounded drugs might be formulated under professional pharmacy standards, these standards are inherently less rigorous than federal GMP quality standards. Because there is no federal surveillance of compounded drugs, the extent of quality and safety problems with compound drugs is unknown[24]. Clearly, a topical 2% diltiazem cream produced under GMP regulations is needed to avoid the large percentage of substandard compounded formulations of a drug specifically recommended by the practice parameters of a medical society.
There were limitations to this study. The sample size was small, and the collection of samples was restricted to a single major metropolitan area. None of the compounded formulations were analyzed for microbiologic content when received by the laboratory. Upon routine inspection of the formulations after 8 mo of refrigerated storage at 5 °C, the laboratory discovered mold on some of them, and all samples were discarded. A future study of compounded formulations of topical 2% diltiazem will need to include analyses of microbiologic content, of possible drug degradation, and of the release of diltiazem from the formulations.
In conclusion, this study shows that when patients are prescribed topical 2% diltiazem cream for treatment of anal fissure in a major metropolitan region, they receive compounded formulations from retail pharmacies that are misbranded in respect to potency approximately 50% of the time and that lack content uniformity approximately 40% of the time. Because approximately one third of the compounded preparations were subpotent, patients treated with compounded formulations of topical 2% diltiazem might not receive the anticipated relief of pain associated with anal fissure.
The use of topical 2% diltiazem hydrochloride for treating anal fissures is supported by multiple clinical trials and is recommended in published medical society practice parameters. In countries where no commercially manufactured formulation of topical 2% diltiazem is available, prescriptions for the medication need to be extemporaneously compounded by retail pharmacies. Up to now, the quality of these compounded diltiazem formulations has not been evaluated.
High-performance liquid chromatography analysis was undertaken with 36 preparations of compounded 2% topical diltiazem that were gathered from 12 different independent retail pharmacies in a major metropolitan area.
This is the first study to show that when patients are prescribed topical 2% diltiazem cream for treatment of anal fissure, they receive compounded formulations from retail pharmacies that are misbranded in respect to potency approximately 50% of the time and that lack content uniformity approximately 40% of the time. Because over one third of the compounded preparations were subpotent, patients treated with compounded formulations of topical 2% diltiazem might not receive the anticipated relief of anal-fissure pain.
By demonstrating that a sample of retail pharmacies compound a large percentage of substandard formulations of topical 2% diltiazem, the study underscores that this drug, recommended for use by medical society practice parameters, should be produced under Good Manufacturing Practice regulations to ensure its identity, quantity, potency, and purity.
Local absorption of diltiazem depends on skin thickness and local inflammation. It is also proportional to the medication amount. This study is similar to a 2007 study concerning compounded formulations of nitroglycerin ointment for anal fissure. The authors noticed a problem that is presumably unknown to gastroenterologists and surgeons: that compounded formulations of topical 2% diltiazem, recommended by the American Society of Colon and Rectal Surgeons for anal fissure therapy but not approved by the United States Food and Drug Administration, may be subpotent or suprapotent. Of 36 compounded preparations examined in the study, 38.9% lacked content uniformity, and 50% did not meet United States Pharmacopoeia specifications for potency.
P- Reviewers Kim YJ, Madalinski M S- Editor Zhai HH L- Editor A E- Editor Zhang DN
| 1. | Carapeti EA, Kamm MA, Phillips RK. Topical diltiazem and bethanechol decrease anal sphincter pressure and heal anal fissures without side effects. Dis Colon Rectum. 2000;43:1359-1362. [PubMed] [Cited in This Article: ] |
| 2. | DasGupta R, Franklin I, Pitt J, Dawson PM. Successful treatment of chronic anal fissure with diltiazem gel. Colorectal Dis. 2002;4:20-22. [PubMed] [Cited in This Article: ] |
| 3. | Knight JS, Birks M, Farouk R. Topical diltiazem ointment in the treatment of chronic anal fissure. Br J Surg. 2001;88:553-556. [PubMed] [Cited in This Article: ] |
| 4. | Kocher HM, Steward M, Leather AJ, Cullen PT. Randomized clinical trial assessing the side-effects of glyceryl trinitrate and diltiazem hydrochloride in the treatment of chronic anal fissure. Br J Surg. 2002;89:413-417. [PubMed] [Cited in This Article: ] |
| 5. | Bielecki K, Kolodziejczak M. A prospective randomized trial of diltiazem and glyceryltrinitrate ointment in the treatment of chronic anal fissure. Colorectal Dis. 2003;5:256-257. [PubMed] [Cited in This Article: ] |
| 6. | Elliott WJ, Ram CV. Calcium channel blockers. J Clin Hypertens (Greenwich). 2011;13:687-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 7. | Perry WB, Dykes SL, Buie WD, Rafferty JF. Practice parameters for the management of anal fissures (3rd revision). Dis Colon Rectum. 2010;53:1110-1115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Gough MJ, Lewis A. The conservative treatment of fissure-in-ano. Br J Surg. 1983;70:175-176. [PubMed] [Cited in This Article: ] |
| 9. | Shub HA, Salvati EP, Rubin RJ. Conservative treatment of anal fissure: an unselected, retrospective and continuous study. Dis Colon Rectum. 1978;21:582-583. [PubMed] [Cited in This Article: ] |
| 10. | Hananel N, Gordon PH. Re-examination of clinical manifestations and response to therapy of fissure-in-ano. Dis Colon Rectum. 1997;40:229-233. [PubMed] [Cited in This Article: ] |
| 11. | Allen LV Jr. Diltiazem hydrochloride 2% topical gel. Int J Pharm Compd. 2002;6:43. [Cited in This Article: ] |
| 12. | Azarnoff DL, Lee JC, Lee C, Chandler J, Karlin D. Quality of extemporaneously compounded nitroglycerin ointment. Dis Colon Rectum. 2007;50:509-516. [PubMed] [Cited in This Article: ] |
| 13. | Food and Drug Administration. 2006 Limited FDA survey of compounded drug products. Updated March 22, 2010. Available from: http: //www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/PharmacyCompounding/ucm204237.htm. [Cited in This Article: ] |
| 14. | Shrivastava UK, Jain BK, Kumar P, Saifee Y. A comparison of the effects of diltiazem and glyceryl trinitrate ointment in the treatment of chronic anal fissure: a randomized clinical trial. Surg Today. 2007;37:482-485. [PubMed] [Cited in This Article: ] |
| 15. | Sanei B, Mahmoodieh M, Masoudpour H. Comparison of topical glyceryl trinitrate with diltiazem ointment for the treatment of chronic anal fissure: a randomized clinical trial. Acta Chir Belg. 2009;109:727-730. [PubMed] [Cited in This Article: ] |
| 16. | Ala S, Saeedi M, Hadianamrei R, Ghorbanian A. Topical diltiazem vs. topical glyceril trinitrate in the treatment of chronic anal fissure: a prospective, randomized, double-blind trial. Acta Gastroenterol Belg. 2012;75:438-442. [PubMed] [Cited in This Article: ] |
| 17. | Nash GF, Kapoor K, Saeb-Parsy K, Kunanadam T, Dawson PM. The long-term results of diltiazem treatment for anal fissure. Int J Clin Pract. 2006;60:1411-1413. [PubMed] [Cited in This Article: ] |
| 18. | Jawaid M, Masood Z, Salim M. Topical diltiazem hydrochloride and glyceryl trinitrate in the treatment of chronic anal fissure. J Coll Physicians Surg Pak. 2009;19:614-617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 8] [Reference Citation Analysis (0)] |
| 19. | Tsunoda A, Kashiwagura Y, Hirose K, Sasaki T, Kano N. Quality of life in patients with chronic anal fissure after topical treatment with diltiazem. World J Gastrointest Surg. 2012;4:251-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 23] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Sajid MS, Whitehouse PA, Sains P, Baig MK. Systematic review of the use of topical diltiazem compared with glyceryltrinitrate for the nonoperative management of chronic anal fissure. Colorectal Dis. 2013;15:19-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Hashmi F, Siddiqui FG. Diltiazem (2%) versus glyceryl trinitrate cream (0.2%) in the management of chronic anal fissure. J Coll Physicians Surg Pak. 2009;19:750-753. [PubMed] [Cited in This Article: ] |
| 22. | Food and Drug Administration. New England Compounding Center (NECC) potentially contaminated medication: Fungal meningitis outbreak. October 6, 2012. Available from: http: //www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm322849.htm. [Cited in This Article: ] |
| 23. | Centers for Disease Control and Prevention (CDC). Multistate outbreak of fungal infection associated with injection of methylprednisolone acetate solution from a single compounding pharmacy - United States, 2012. MMWR Morb Mortal Wkly Rep. 2012;61:839-842. [PubMed] [Cited in This Article: ] |
| 24. | Sellers S, Utian WH. Pharmacy compounding primer for physicians: prescriber beware. Drugs. 2012;72:2043-2050. [PubMed] [Cited in This Article: ] |
| 25. | Gudeman J, Jozwiakowski M, Chollet J, Randell M. Potential risks of pharmacy compounding. Drugs R D. 2013;13:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 26. | Pergolizzi JV, Labhsetwar S, LeQuang JA. Compounding pharmacies: who is in charge? Pain Pract. 2013;13:253-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |