Published online Sep 7, 2013. doi: 10.3748/wjg.v19.i33.5565
Revised: May 30, 2013
Accepted: July 4, 2013
Published online: September 7, 2013
Processing time: 194 Days and 13.9 Hours
AIM: To perform a meta-analysis of palliative stent placement vs palliative surgical decompression for management of incurable malignant colorectal obstructions.
METHODS: The databases of Medline, Web of Science, Embase, and the Cochrane Central Register of Controlled Trials were searched from their inception to July 2012 for studies (prospective, retrospective, randomized controlled trials, and case-control trials) designed as comparative analyses of patients with incurable malignant colorectal obstructions treated by self-expanding metallic stents (SEMS) or palliative surgery. No language restrictions were imposed. The main outcome measures were hospital stay, intensive care unit admission, clinical success rate, 30-d mortality, stoma formation, complications, and overall survival time. The data extraction was conducted by two investigators working independently and using a standardized form. The Mantel-Haenszel χ2 method was used to estimate the pooled risk ratios with 95%CI under a fixed-effects model; when statistical heterogeneity existed in the pooled data (as evaluated by Q test and I2 statistics, where P < 0.10 and I2 < 25% indicated heterogeneity), a random-effects model was used.
RESULTS: Thirteen relevant articles, representing 837 patients (SEMS group, n = 404; surgery group, n = 433), were selected for analysis. Compared to the surgery group, the SEMS group showed lower clinical success (99.8% vs 93.1%, P = 0.0009) but shorter durations of hospital stay (18.84 d vs 9.55 d, P < 0.00001) and time to initiation of chemotherapy (33.36 d vs 15.53 d, P < 0.00001), and lower rate of stoma formation (54.0% vs 12.7%, P < 0.00001). Additionally, the SEMS group experienced a significantly lower rate of 30-d mortality (4.2% vs 10.5%, P = 0.01). Stent-related complications were not uncommon and included perforation (10.1%), migration (9.2%), and occlusion (18.3%). Surgery-related complications were slightly less common and included wound infection (5.0%) and anastomotic leak (4.7%). The rate of total complications was similar between these two groups (SEMS: 34.0% vs surgery: 38.1%, P = 0.60), but the surgery-related complications occurred earlier than stent-related complications (rate of early complications: 33.7% vs 13.7%, P = 0.03; rate of late complications: 32.3% vs 12.7%, P < 0.0001). The overall survival time of SEMS- and surgery-treated patients was not significantly different (7.64 mo vs 7.88 mo).
CONCLUSION: SEMS is less effective than surgery for palliation of incurable malignant colorectal obstructions, but is associated with a shorter time to chemotherapy and lower 30-d mortality.
Core tip: This meta-analysis demonstrates the advantages of self-expandable metal stent (SEMS) placement as palliative therapy for incurable malignant colorectal obstructions. Specifically, when compared to the outcomes of surgical treatment, the SEMS treatment is associated with shorter hospital stay and interval to chemotherapy initiation, as well as lower early morbidity and 30-d mortality rates. These advantageous features may surmount the overall lower rate of palliative efficacy when considering treatment options for cases with extensive metastatic disease or severe comorbid medical illness that disqualify a patient from operative candidacy; regardless, SEMS application should be performed as an alternative to surgery with caution.
- Citation: Zhao XD, Cai BB, Cao RS, Shi RH. Palliative treatment for incurable malignant colorectal obstructions: A meta-analysis. World J Gastroenterol 2013; 19(33): 5565-5574
- URL: https://www.wjgnet.com/1007-9327/full/v19/i33/5565.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i33.5565
Malignant colorectal obstruction, a type of large bowel obstruction (LBO), is a frequent and serious complication of advanced cancers, including colorectal cancer or those with near organ (e.g., ovary, vagina, and prostate) or distant metastases[1]. LBO initially manifests non-specific gastrointestinal symptoms, such as vomiting, abdominal distention and abdominal pain; however, if left untreated, the condition may progress to a life-threatening status, as the weak necrotic areas of the bowel become more susceptible to tears and a risk for rapid onset infection and septicemia.
The traditional therapeutic approach for LBO is surgical, and the Hartmann’s pouch procedure and loop colostomy are the most widely applied surgical methods used for treating obstruction of incurable advanced cancer. Unfortunately, these procedures are associated with substantial drawbacks, including high mortality and morbidity[2-4], as well as detrimental impacts on a patient’s quality of life when irreversible ostomies necessitate a colostomy bag[1,5,6]. The alternative method of colonic stent insertion was introduced by Dohmoto[7] to overcome the risks associated with open surgery. Since then, self-expanding metallic stents (SEMS) have been widely applied to patients with incurable malignant obstructions as palliative treatment or as a bridge to elective primary resection and anastomosis.
SEMS placement is achieved by feeding the metal tube in a collapsed state to the site of obstruction by using a guidewire and visualization by fluoroscopy and/or endoscopy. The inserted stents then undergo passive expansion to create a strong, passable space and relieve the obstruction. Numerous stents of various lengths and maximal expanded diameter have been designed specifically for treating lower gastrointestinal obstructions, so that the appropriate stent can be chosen for each patient based on location and length of the lesion and severity of the obstruction.
Despite the widespread availability and application of SEMS, its efficacy and safety for treating incurable malignant colorectal obstruction, as compared to that of the traditional surgical approach, has been addressed in relatively few studies with small populations. Thus, this meta-analysis was designed to provide stronger evidence of the outcomes, benefits, and risks of these two palliative treatments through the increased statistical power afforded by pooling data of the previously studied patient populations.
Two investigators (Zhao XD and Cai BB) performed independent searches of the Medline, Web of Science, Embase, and Cochran Central Register of Controlled Trials databases. These literature collections were queried from inception to July 2012 using the following keywords and medical subject heading terms: stents, colonic stent, colorectal stent, Hartmann’s procedures, Hartmann’s, colostomy, palliative surgery, intestinal obstruction, large bowel obstruction, colorectal obstruction, comparative study, treatment outcomes, and human. The search strategy was widened or narrowed by applying Boolean operators (NOT, AND, and OR), and no language restriction was applied. All potentially relevant abstracts, studies, and citations were retrieved for review, and the references cited in each were further searched to identify any additional potentially relevant publications.
The two investigators also performed the data extraction (inclusion and exclusion criteria described below), working independently and using pre-determined forms to record first author, year of publication, study design including inclusion and exclusion criteria, and study population characteristics. The extracted datasets were compared and any disagreements were resolved by discussion and consensus.
Potentially relevant studies were selected for inclusion in the meta-analysis according to the following criteria: (1) comparative analysis of palliative SEMS and palliative surgery for treating malignant colorectal obstructions that were unresectable and had negative margins; (2) patients lacked signs of peritonitis and perforation; (3) reporting of at least one of the outcomes measures listed below; (4) designed as randomized controlled trials (RCTs) or other case-control study; and (5) performed with human patients.
Studies were excluded from the meta-analysis according to the following criteria: (1) evaluation of SEMS as a bridge to surgery (SBTS) or as a treatment for benign strictures, or comprehensive studies in which the data could not be clearly separated for exclusion; and (2) missing or unclear data for the outcomes of interest.
The Newcastle-Ottawa scale[8] was employed to assess the quality of non-randomized studies, with scores of ≥ 5 indicating high quality. The modified Jadad score[9] was employed to assess the quality of randomized studies, with the cumulative scores of 4 to 7 indicating high quality.
The meta-analysis was performed by the RevMan 5.0.25 software (The Cochran Collaboration, Oxford, England) and the statistical analysis was carried out by the Stata 12.0 software (StataCorp, College Station, TX, United States). The risk ratios (RRs, with 95%CI) of dichotomous data were estimated by the Mantel-Haenszel χ2 method; P values of < 0.05 were considered to indicate statistically significant differences between groups. Between-study heterogeneity was evaluated by the Q test and I2 statistic, for which P values > 0.10 and I2 < 25% indicated a lack of heterogeneity, respectively. In order to broaden the effect estimate in the presence of heterogeneity, the random-effects model was applied for evaluation of the pooled data. Finally, publication bias was estimated by Egger’s and Begg’s funnel plots, for which P values > 0.05 indicated a lack of publication bias.
Thirteen studies, including 10 nonrandomized controlled studies[10-19] and three RCTs[20-22], met the criteria for inclusion in the meta-analysis. The studies’ characteristics and quality assessment scores are presented in Tables 1 and 2, respectively. Eleven (84.62%) of the studies were categorized as high-quality. The total number of included patients was 837, of which 404 were treated by SEMS (48.3%) and 433 (51.7%) by palliative surgery. Eleven of the studies[10-12,14-19,21,22] focused solely on cases with colorectal cancer etiology, and the remaining two studies[13,20] also included etiologies of ovarian cancer and disseminated upper gastrointestinal malignancy. The studies also used different definitions of palliative surgery, with four of the studies[12,13,20,21] specifically reporting the colostomy procedure and the others reporting primary resection with anastomosis, primary resection without anastomosis, bypass, or Hartmann’s procedure, as well. Complications reported for the total case population were categorized as early (occurring ≤ 30 d post-treatment) or late (occurring > 30 d post-treatment).
| Ref. | Design | Diagnosis | Palliative SEMS (n) | Palliative surgery (n) | Matching | Female | Study quality (NOS score) |
| Law et al[10] | P | a | 30 | 31 | 1, 2, 3 | 21 (34.4) | 8 |
| Carne et al[11] | R | a | 25 | 19 | 3 | 19 (43.2) | 4 |
| Johnson et al[12] | M | a | 20 | 18 | 2, 3 | 17 (47.2) | 6 |
| Tomiki et al[13] | P | a, b, c | 18 | 17 | 4 | 15 (42.9) | 4 |
| Ptok et al[14] | P | a | 40 | 38 | 2, 3, 4 | 34 (44.7) | 7 |
| Faragher et al[15] | R | a | 29 | 26 | 1, 2, 4 | 22 (40.0) | 6 |
| Vemulapalli et al[16] | R | a | 53 | 70 | 1, 2, 4 | 49 (41.2) | 5 |
| Súarez et al[17] | P | a | 45 | 53 | 1, 4, 6 | 31 (31.6) | 7 |
| Lee et al[18] | P | a | 71 | 73 | 1, 2, 6 | 50 (34.7) | 7 |
| Lee et al[19] | R | a | 36 | 52 | 1, 2, 4 | 39 (44.3) | 6 |
Length of hospital stay: The mean length of hospital stay for the pooled SEMS group was significantly lower than that of the pooled surgery group (9.6 d vs 18.8 d, P < 0.00001).
Intensive care unit admission: Three studies[10,12,18] reported cases requiring intensive care unit (ICU) admission after treatment. Analysis of the 241 patients, including 119 treated with SEMS and 122 treated with surgery, indicated that the rate of ICU usage was significantly lower in the SEMS group than in the surgery group (0.8% vs 18.0%, P = 0.001; Figure 1A).
Time to chemotherapy initiation: Three studies[17-19] reported cases receiving chemotherapy after treatment. Analysis of the 330 patients, including 152 treated with SEMS and 178 treated with surgery, indicated that the mean time to chemotherapy initiation following treatment was significantly lower in the SEMS group than in the surgery group (15.5 d vs 33.4 d).
Clinical relief of obstructions: Data of treatment efficacy were available for all cases from all 13 studies. The surgery-treated patients showed a significantly higher rate of clinical relief of obstructions than the SEMS-treated patients (93.1% vs 99.8%, P = 0.0009; Figure 1B).
30-d mortality or in-hospital mortality: Two studies[19,21] reported zero mortalities during both the in-hospital stay period and the 30-d follow-up. Meta-analysis of the 688 patients in the remaining ten studies, including 334 treated with SEMS and 354 treated with surgery, indicated that the SEMS group experienced fewer overall deaths than the surgery group (4.2% vs 10.5%, P = 0.01; Figure 1C).
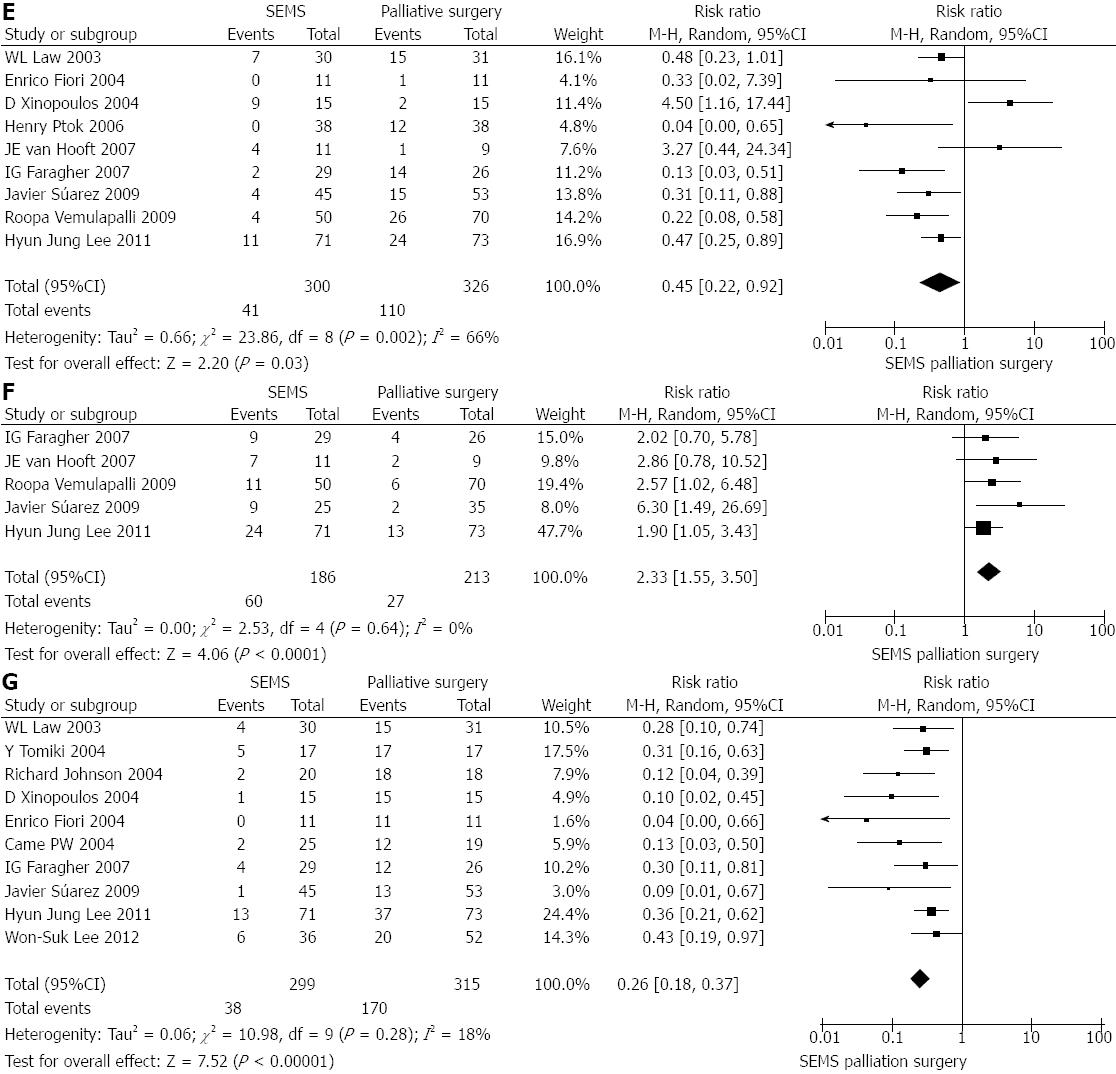
Overall, early- and late-onset complications: Data of treatment-related complications were available for all cases from all 13 studies. Although a slightly lower percentage of the SEMS-treated patients experienced complications, the amount was not significantly different from that in the surgery-treated patients (34.0% vs 38.1%, P = 0.60; Figure 1D). Nine of the studies[10,14-18,20-22] reported data sub-categorized as early complications; while five studies[15-18,22] reported data as late complications. Compared to the surgery group (n = 326), the SEMS-treated patients (n = 300) experienced significantly less early complications (13.7% vs 33.7%, P = 0.03; Figure 1E) but significantly more late complications (32.3% vs 12.7%, P < 0.0001; Figure 1F).
Eleven studies[10-19,22] reported stent-related complications. The overall rate of perforation was 10.1% (for 367 patients), of stent migration was 9.2% (for 361 patients), of stent obstruction was 18.3% (for 331 patients).
Surgery-related complications: Seven studies[10,11,15-19] reported surgery-related complications. Six of those studies[10,15-19] reported wound infection, and the rate was 5.0% (for 15 patients). Three of those studies[11,17,19] reported anastomotic leak, and the rate was 4.7% (for 95 patients).
Overall survive time: Data of survival time were available for all cases from all 13 studies. The overall survival time was similar between the SEMS-treated and surgery-treated patients (7.6 mo vs 7.9 mo; P > 0.05).
Stoma formation: Ten studies[10-13,15,17-21] reported stoma formation. Among the 299 patients for whom colonic stent insertion was attempted, 12.7% (n = 38) ultimately required a stoma. Among the 315 surgery-treated patients, 54.0% (n = 170) required stoma formation. The amount of patients with stoma formation was significantly lower in the SEMS group (vs surgery group, P < 0.00001; Figure 1G).
Therapeutic efficacy and outcomes of SEMS and surgery for colorectal cancer-related obstructions: Comparative analysis of the therapeutic efficacies of SEMS and surgery for resolving colorectal cancer-related obstructions[10-12,14-19,21] and obstructions caused by other advanced cancers[13,20] revealed no differences between the two treatment approaches. However, among the subset of patients with colorectal cancer-related obstructions (n = 772), the SEMS-treated patients (n = 370) showed significantly lower rates of 30-d mortality (3.79% vs surgery-treated patients: 10.4%, P = 0.008), early complications (11.2% vs 34.7%, P = 0.0002), and stoma formation (12.0% vs 48.8%, P < 0.00001). Unfortunately, these SEMS-treated patients also showed a significantly lower rate of clinical relief of the colorectal cancer-related obstructions (94.6% vs 99.8%, P = 0.002). No significant difference was observed between the two treatments for total complications (SEMS: 32.1% vs surgery: 37.9%, P = 0.34) (Table 3).
| Studies (n) | Patients (n) | RR (95%CI) | P value | |
| Studies including colorectal cancer only | ||||
| Clinical success rate | 11 | 772 | 0.96 (0.93, 0.98) | 0.002 |
| 30-d mortality | 9 | 654 | 0.42 (0.22,0.80) | 0.008 |
| Total complications | 12 | 821 | 0.84 (0.59, 1.20) | 0.340 |
| Early complications | 8 | 596 | 0.35 (0.20, 0.60) | 0.0002 |
| Stoma formation | 8 | 550 | 0.26 (0.17, 0.39) | < 0.00001 |
| Studies including colostomy only | ||||
| Clinical success rate | 4 | 127 | 0.89 (0.76,1.05) | 0.18 |
| 30-d mortality | 2 | 70 | 1.00 (0.27, 3.68) | 1.00 |
| Total complications | 3 | 92 | 1.79 (1.03, 3.09) | 0.04 |
| Early complications | 2 | 52 | 1.80 (0.16, 20.79) | 0.64 |
| Stoma formation | 4 | 124 | 0.16 (0.07, 0.38) | < 0.00001 |
Therapeutic efficacy and outcomes of SEMS vs the colostomy surgical treatment: Four studies[12,13,20,21] compared outcomes of SEMS against the colostomy surgical approach. In contrast to the results of SEMS compared to all types of surgeries for treating incurable malignant colorectal obstructions, there was no significant difference found between clinical relief attained by SEMS and colostomy (84.4% vs 100%, P = 0.18). The SEMS-treated patients, however, did require significantly less stoma formation than the colostomy-treated patients (12.7% vs 100%, P < 0.00001), and experienced significantly less total complications (23.9% vs 41.3%, P = 0.04). The rates of 30-d mortality and early complications were not significantly different between the SEMS-treated group and the colostomy-treated group (P = 1.00 and P = 0.64, respectively) (Table 3).
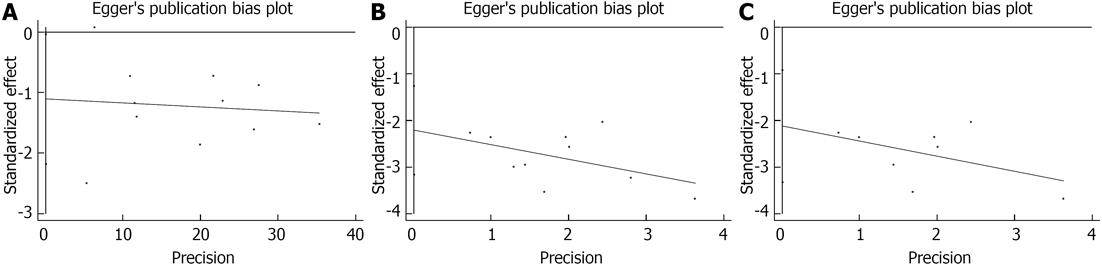
As shown in Figure 2, three comparisons showed potential bias: clinical relief of incurable malignant LBO (Egger’s test P = 0.04 and Begg’s test, P = 0.12) and stoma formation (incurable malignant LBO: Egger’s test, P = 0.001 and Begg’s test, P = 0.03; incurable malignant colorectal cancer-related obstructions: Egger’s test, P = 0.005 and Begg’s test, P = 0.04). However, the statistical analysis revealed no evidence of publication bias among any of these comparisons.
Previous studies have demonstrated the risks associated with the traditional surgical approach for treating malignant LBO, namely high rates of morbidity, mortality, and stoma formation[23,24]. The less invasive alternative approach of colonic stent insertion, particularly of SEMS, promised to overcome the high hospitalization costs and poor quality of life related to these outcomes. While subsequent meta-analyses have been conducted to investigate the benefit and risk of endoscopic SBTS[25-28], no study to date had performed a focused comparison of palliative SEMS and palliative surgery for treating incurable malignant LBO-as is described herein.
In the current meta-analysis, palliative surgery was found to be superior to SEMS for decompressing incurable malignant LBO; while this finding is contrary to the majority of individual studies of this subject[29-32], it is consistent with the investigations by Cirocchi et al[28] and Sagar[33]. An important distinguishing feature among these collective studies is the variable definitions of palliative surgery that were used as the basis of analysis; in addition, these studies have yet to address whether and to what extent primary tumor resection affects the mean survival time of those patients suffering from advanced cancer[34,35]. In our meta-analysis of eleven studies, the overall clinical success rate of SEMS treatment ranged from 70%-95%. A previous multicenter study[36] of SEMS with long-term follow-up revealed that the clinical success rate increased gradually over time (87.8% at 30 d, 89.7% at 3 mo, 92.8% at 6 mo, and 96% at 12 mo). The follow-up period in our included studies are different but all within 12 mo and the clinical success rate was approximately similar. In addition, our meta-analysis revealed that obstructions caused by colorectal cancer benefited more from the surgical approach. Fernández-Esparrach et al[37] have reported a similar finding and hypothesized that the severe complications associated with the SEMS procedure, such as migration, obstruction and perforation, limited its long-term clinical efficacy. Moreover, the authors advised that adjunct palliative chemotherapy may help to promote the life expectancy of SEMS-treated patients. A retrospective study conducted in Korean patients advanced gastric cancer[38] also indicated that SEMS insertion was less effective than emergency surgery for the palliative treatment for colorectal obstructions. In light of these previous findings, and in agreement with the opinions expressed by other interested groups in this field[39,40], it is possible that the clinical stent success rate observed in our current meta-analysis had nothing to do with the stent placement or the etiology of the obstructions. Indeed, Sebastian et al[31] suggested that the clinical success rate of stenting is mainly associated with the site and extent of the obstruction.
Our meta-analysis also indicated that SEMS treatment is associated with shorter lengths of hospital stay, reduced ICU admissions, fewer stoma formation, and shorter time to initiation of adjunct chemotherapy; These findings are consistent with results from other relevant studies[30,32,33,41] and suggest that the less trauma endured produced by the SEMS approach eliminates delay of post-procedure chemotherapy, thereby promoting beneficial patient outcome. It was unfortunate that the current meta-analysis was limited by a lack of comparative data concerning quality of life outcome and cost-effectiveness between these two palliative treatments; analysis of such data will be necessary for comprehensively assessing the feasibility of these palliative management approaches for advanced disease. Only one of the studies included in the meta-analysis, a RCT[20] comprised of 30 patients, attempted to address the monetary expense of stent placement, as compared to colostomy treatment; however, the analysis was abandoned due to the high rate of colonic perforation that occurred in the nonsurgical arm. However, some studies[32,42] that did not meet the criteria for inclusion in our meta-analysis have suggested that SEMS may be less costly than the conventional surgical approach for treating colonic cancer obstructions; but, we cannot comment on the quality or appropriateness of these data or the implications related to our findings.
The safety of stent placement was also evaluated in the current meta-analysis. Although SEMS insertion is considered a less invasive method than surgery, and advanced procedure-related devices, such as hydrophilic elastic guidewires and stent delivery systems, have improved the ease and successful application of this method, complications still occur. Fortunately, the majority of complications are minor, such as low fever and abdominal discomfort, and resolved easily by medication. While less frequent, the major complications of the stent procedure, such as bleeding, colonic perforation, stent migration and stent occlusion, can be life-threatening[43]. In a systematic review[30] of 88 articles reporting on stent-related complications in cases of LBO, the median rates of stent migration, perforation, and reconstruction were reported as 11%, 4.5% and 12%, respectively. In the current meta-analysis, the rates of perforation and reconstruction were slightly higher; we believe this finding reflects the fact that data on perforations caused by tumor infiltration were included in the analysis and that the data on reconstructions included not only the etiologies of tumor ingrowth/overgrowth and stent migration, but also of fecal implant.
The contributing factors to complications of stent insertion have been extensively studied. Factors related to stent type have been particularly well studied, and it is believed that covered stents provide the optimal resistance to tumor ingrowth, thereby helping to reduce reconstruction events, while uncovered stents are believed to minimize stent migration[30,39,44]. The type of stent, however, does not appear to be related to perforation events[30], nor to have a significant effect on the safety of stent placement[45]. Furthermore, a retrospective analysis of uncovered SEMS for treating primary colorectal cancer vs non-colorectal extrinsic cancer found no significant difference in migration or occlusion events[46]. That study also suggested that insufficient stent expansion (< 70%) at 48 h after insertion may be a predictor of subsequent stent occlusion. Another retrospective analysis of 168 SEMS-treated LBO patients[47] identified five risk factors of therapeutic inefficacy, including male sex, complete obstruction, stent diameter ≤ 22 cm, premature dilation of the stent, and operators’ experience. In addition, subsequent chemotherapy, especially Bevacizumab therapy, was demonstrated to nearly triple the risk of perforation. This latter finding was not supported by the study by Kim et al[39], who demonstrated that chemotherapy had no affect on migration or reconstruction and that stent length had no relationship with complications, but showed that stent diameter < 24 cm had negative impact on palliative SEMS migration. In another study, stent migration was shown to occur more frequently in the distal colon[31].
Despite significant improvements in the surgical procedures used for managing incurable malignant colorectal obstructions, the perioperative morbidity and mortality rates have remained high. Similarly, the patients treated with surgery in the current meta-analysis experienced appreciable levels of anastomotic dehiscence, wound infection, and death. The former two complications may have a negative influence on tumor recurrence, metastasis, and long-term survival. In the current meta-analysis, a greater number of surgery-treated patients died within 30 d after treatment, as compared to those treated with SEMS. While this result is contrary to those obtained with other similar patient series[30,33] and meta-analyses comparing SBTS[25-28], it may be explained by the lower amount of total complications that were experienced by the overall SEMS-treated group. Another study also found significantly lower complications in a stent-treated group, but we cannot comment on the related implications for our findings as the previous data had significant heterogeneity[25]. In an attempt to address this issue, we performed sub-group analysis of the complications, independently assessing the early- and late-onset complications; the results indicated that surgery had a higher risk of early complications, while SEMS insertion had a higher risk of late complications. Future studies should further investigate the roles of early and late complications in therapeutic efficacy and overall survival.
Two of the studies[13,20] included in the overall meta-analysis were excluded from the focused comparison of SEMS and surgery outcomes for incurable colorectal-related obstructions. The results were not impacted by their removal and were in accordance with the findings reported by Kim et al[40]. Then, we investigated the comparison between SEMS and colostomy for incurable malignant LBO (using four studies). Unlike the previous results, these results suggested that, compared to colostomy, SEMS could be an effective palliative treatment for incurable malignant LBO; no significant difference was found for the clinical success rates between groups with fewer stoma, but the 30-d mortality and the complications should be taken into account. Unfortunately, the current meta-analysis was underpowered to investigate the differences in overall survival time between these two groups.
Other limitations of our meta-analysis design may have impacted our results and their interpretation. First, only three of the 13 included studies are RCTs. Second, the pooled sample size was still relatively small and the data from the included studies was not uniform for the outcome measures. Third, publication bias existed among four of the studies; indeed, a general limitation of all meta-analyses is publication bias introduced by the fact that positive results are more likely to be published. To overcome these limitations, long-term RCTs should be conducted with large numbers of patients to achieve a sufficient level of statistical power for accurately estimating the optimal palliative treatment for incurable malignant LBO.
In summary, palliative SEMS does not appear to have a significant advantage over palliative surgery for decompressing incurable malignant colorectal obstructions, regardless of etiology; however, the use of colonic stents is safe. The shorter interval to chemotherapy and significantly lower rates of 30-d mortality and short-term complications suggest that SEMS may be a reasonable alternative for treating patients with extensive metastatic disease or who are poor operative candidates due to severe comorbid medical illnesses.
Malignant colorectal obstruction is a common and serious complication of advanced cancer. The traditional treatment approach, surgery, is associated with high risks of morbidity and mortality. The more recently developed approach of stent insertion is less invasive and has been widely applied, especially using self-expanding metallic stents (SEMS), but its risks and benefits in patients with incurable malignant large bowel obstruction (LBO) remain to be definitively established.
The current meta-analysis was carried out to comparatively assess the outcomes of palliative surgery and palliative SEMS insertion in patients with incurable malignant LBO; the main outcome measure included length of hospital stay, intensive care unit admission, clinical success rate, 30-d mortality, complications, stoma formation, and overall survival time.
The current meta-analysis demonstrated the advantages of SEMS as a palliative therapy for incurable malignant LBO, in terms of shortened durations of hospital stay and time to chemotherapy initiation, and decreased rates of 30-d mortality and early-onset complications. However, SEMS failed to show a greater efficacy than palliative surgery for resolving obstructions.
The results from this meta-analysis suggests that colonic stent insertion may be a safe and feasible alternative palliative treatment for patients who are otherwise poor candidates for the traditional surgical treatment, such as those with extensive metastatic disease or severe comorbid medical illnesses. SEMS is not absolutely recommended, however, since it is associated with significant late-onset complications and mortality. Until further randomized controlled trials, with large patient populations, are carried out, application should be considered on a case-by-case basis.
SEMS: expandable metal tubes that are placed in the collapsed state at the site of obstruction by means of a guidewire and fluoroscopy and/or endoscopy visualization; gradual, automatic expansion to the maximum diameter of the stent serves to relive the obstruction and create a strong and passable space. Meta-analysis: the collection, combination, and analysis of data from multiple previously completed studies on a particular topic of interest that is carried out with the aim of increasing statistical power to draw stronger conclusions about a controversial subject.
The current meta-analysis was designed to evaluate the risks and benefits of SEMS treatment for incurable malignant colorectal obstructions, as compared to surgical treatment. The analysis included a total of 13 studies, nine of which scored high upon established quality assessment systems. The research design is solid, and its results have clinical relevancy as they demonstrate that, in patients with incurable malignant colorectal obstruction, stent placement improves treatment outcome, specifically by shortening the time to chemotherapy initiation and lowering the 30-d mortality rate.
P- Reviewer Hassan M S- Editor Gou SX L- Editor A E- Editor Ma S
| 1. | Deans GT, Krukowski ZH, Irwin ST. Malignant obstruction of the left colon. Br J Surg. 1994;81:1270-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 355] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 2. | Liu SK, Church JM, Lavery IC, Fazio VW. Operation in patients with incurable colon cancer--is it worthwhile. Dis Colon Rectum. 1997;40:11-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Riedl S, Wiebelt H, Bergmann U, Hermanek P. [Postoperative complications and fatalities in surgical therapy of colon carcinoma. Results of the German multicenter study by the Colorectal Carcinoma Study Group]. Chirurg. 1995;66:597-606. [PubMed] |
| 4. | Bhardwaj R, Parker MC. Palliative therapy of colorectal carcinoma: stent or surgery. Colorectal Dis. 2003;5:518-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Wong RW, Rappaport WD, Witzke DB, Putnam CW, Hunter GC. Factors influencing the safety of colostomy closure in the elderly. J Surg Res. 1994;57:289-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Nagula S, Ishill N, Nash C, Markowitz AJ, Schattner MA, Temple L, Weiser MR, Thaler HT, Zauber A, Gerdes H. Quality of life and symptom control after stent placement or surgical palliation of malignant colorectal obstruction. J Am Coll Surg. 2010;210:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Dohmoto M. New method: endoscopic implantation of rectal stent in palliative treatment of malignant stenosis. Endosc Dig. 1991;3:1507-1512. |
| 8. | Wells GA, Shea B, O’Connell D, Robertson J, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. 2009-10-19. Available from: http: //www.ohri.ca/programs/clinical epidemiology/oxford.htm. |
| 9. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary. Control Clin Trials. 1996;17:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12275] [Cited by in RCA: 12863] [Article Influence: 443.6] [Reference Citation Analysis (1)] |
| 10. | Law WL, Choi HK, Chu KW. Comparison of stenting with emergency surgery as palliative treatment for obstructing primary left-sided colorectal cancer. Br J Surg. 2003;90:1429-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 114] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Carne PW, Frye JN, Robertson GM, Frizelle FA. Stents or open operation for palliation of colorectal cancer: a retrospective, cohort study of perioperative outcome and long-term survival. Dis Colon Rectum. 2004;47:1455-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Johnson R, Marsh R, Corson J, Seymour K. A comparison of two methods of palliation of large bowel obstruction due to irremovable colon cancer. Ann R Coll Surg Engl. 2004;86:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Tomiki Y, Watanabe T, Ishibiki Y, Tanaka M, Suda S, Yamamoto T, Sakamoto K, Kamano T. Comparison of stent placement and colostomy as palliative treatment for inoperable malignant colorectal obstruction. Surg Endosc. 2004;18:1572-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Ptok H, Marusch F, Steinert R, Meyer L, Lippert H, Gastinger I. Incurable stenosing colorectal carcinoma: endoscopic stent implantation or palliative surgery. World J Surg. 2006;30:1481-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Faragher IG, Chaitowitz IM, Stupart DA. Long-term results of palliative stenting or surgery for incurable obstructing colon cancer. Colorectal Dis. 2008;10:668-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Vemulapalli R, Lara LF, Sreenarasimhaiah J, Harford WV, Siddiqui AA. A comparison of palliative stenting or emergent surgery for obstructing incurable colon cancer. Dig Dis Sci. 2010;55:1732-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Súarez J, Jiménez J, Vera R, Tarifa A, Balén E, Arrazubi V, Vila J, Lera JM. Stent or surgery for incurable obstructive colorectal cancer: an individualized decision. Int J Colorectal Dis. 2010;25:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Lee HJ, Hong SP, Cheon JH, Kim TI, Min BS, Kim NK, Kim WH. Long-term outcome of palliative therapy for malignant colorectal obstruction in patients with unresectable metastatic colorectal cancers: endoscopic stenting versus surgery. Gastrointest Endosc. 2011;73:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Lee WS, Baek JH, Kang JM, Choi S, Kwon KA. The outcome after stent placement or surgery as the initial treatment for obstructive primary tumor in patients with stage IV colon cancer. Am J Surg. 2012;203:715-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Xinopoulos D, Dimitroulopoulos D, Theodosopoulos T, Tsamakidis K, Bitsakou G, Plataniotis G, Gontikakis M, Kontis M, Paraskevas I, Vassilobpoulos P. Stenting or stoma creation for patients with inoperable malignant colonic obstructions Results of a study and cost-effectiveness analysis. Surg Endosc. 2004;18:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 142] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Fiori E, Lamazza A, De Cesare A, Bononi M, Volpino P, Schillaci A, Cavallaro A, Cangemi V. Palliative management of malignant rectosigmoidal obstruction. Colostomy vs. endoscopic stenting. A randomized prospective trial. Anticancer Res. 2004;24:265-268. [PubMed] |
| 22. | van Hooft JE, Fockens P, Marinelli AW, Timmer R, van Berkel AM, Bossuyt PM, Bemelman WA. Early closure of a multicenter randomized clinical trial of endoscopic stenting versus surgery for stage IV left-sided colorectal cancer. Endoscopy. 2008;40:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 212] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 23. | Nugent KP, Daniels P, Stewart B, Patankar R, Johnson CD. Quality of life in stoma patients. Dis Colon Rectum. 1999;42:1569-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 286] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Park JJ, Del Pino A, Orsay CP, Nelson RL, Pearl RK, Cintron JR, Abcarian H. Stoma complications: the Cook County Hospital experience. Dis Colon Rectum. 1999;42:1575-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 211] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 25. | Zhang Y, Shi J, Shi B, Song CY, Xie WF, Chen YX. Self-expanding metallic stent as a bridge to surgery versus emergency surgery for obstructive colorectal cancer: a meta-analysis. Surg Endosc. 2012;26:110-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 26. | Ye GY, Cui Z, Chen L, Zhong M. Colonic stenting vs emergent surgery for acute left-sided malignant colonic obstruction: a systematic review and meta-analysis. World J Gastroenterol. 2012;18:5608-5615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Cennamo V, Luigiano C, Coccolini F, Fabbri C, Bassi M, De Caro G, Ceroni L, Maimone A, Ravelli P, Ansaloni L. Meta-analysis of randomized trials comparing endoscopic stenting and surgical decompression for colorectal cancer obstruction. Int J Colorectal Dis. 2013;28:855-863. [PubMed] |
| 28. | Cirocchi R, Farinella E, Trastulli S, Desiderio J, Listorti C, Boselli C, Parisi A, Noya G, Sagar J. Safety and efficacy of endoscopic colonic stenting as a bridge to surgery in the management of intestinal obstruction due to left colon and rectal cancer: a systematic review and meta-analysis. Surg Oncol. 2013;22:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 29. | Khot UP, Lang AW, Murali K, Parker MC. Systematic review of the efficacy and safety of colorectal stents. Br J Surg. 2002;89:1096-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 417] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 30. | Watt AM, Faragher IG, Griffin TT, Rieger NA, Maddern GJ. Self-expanding metallic stents for relieving malignant colorectal obstruction: a systematic review. Ann Surg. 2007;246:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 297] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 31. | Sebastian S, Johnston S, Geoghegan T, Torreggiani W, Buckley M. Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol. 2004;99:2051-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 411] [Article Influence: 19.6] [Reference Citation Analysis (1)] |
| 32. | Siddiqui A, Khandelwal N, Anthony T, Huerta S. Colonic stent versus surgery for the management of acute malignant colonic obstruction: a decision analysis. Aliment Pharmacol Ther. 2007;26:1379-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Sagar J. Colorectal stents for the management of malignant colonic obstructions. Cochrane Database Syst Rev. 2011;CD007378. [PubMed] |
| 34. | Michel P, Roque I, Di Fiore F, Langlois S, Scotte M, Tenière P, Paillot B. Colorectal cancer with non-resectable synchronous metastases: should the primary tumor be resected. Gastroenterol Clin Biol. 2004;28:434-437. [PubMed] |
| 35. | Eisenberger A, Whelan RL, Neugut AI. Survival and symptomatic benefit from palliative primary tumor resection in patients with metastatic colorectal cancer: a review. Int J Colorectal Dis. 2008;23:559-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Meisner S, González-Huix F, Vandervoort JG, Repici A, Xinopoulos D, Grund KE, Goldberg P, Registry Group TW. Self-Expanding Metal Stenting for Palliation of Patients with Malignant Colonic Obstruction: Effectiveness and Efficacy on 255 Patients with 12-Month’s Follow-up. Gastroenterol Res Pract. 2012;2012:296347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Fernández-Esparrach G, Bordas JM, Giráldez MD, Ginès A, Pellisé M, Sendino O, Martínez-Pallí G, Castells A, Llach J. Severe complications limit long-term clinical success of self-expanding metal stents in patients with obstructive colorectal cancer. Am J Gastroenterol. 2010;105:1087-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 38. | Kim BK, Hong SP, Heo HM, Kim JY, Hur H, Lee KY, Cheon JH, Kim TI, Kim WH. Endoscopic stenting is not as effective for palliation of colorectal obstruction in patients with advanced gastric cancer as emergency surgery. Gastrointest Endosc. 2012;75:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Kim BC, Han KS, Hong CW, Sohn DK, Park JW, Park SC, Kim SY, Baek JY, Choi HS, Chang HJ. Clinical outcomes of palliative self-expanding metallic stents in patients with malignant colorectal obstruction. J Dig Dis. 2012;13:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Kim JY, Kim SG, Im JP, Kim JS, Jung HC. Comparison of treatment outcomes of endoscopic stenting for colonic and extracolonic malignant obstruction. Surg Endosc. 2013;27:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Tilney HS, Lovegrove RE, Purkayastha S, Sains PS, Weston-Petrides GK, Darzi AW, Tekkis PP, Heriot AG. Comparison of colonic stenting and open surgery for malignant large bowel obstruction. Surg Endosc. 2007;21:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 42. | Osman HS, Rashid HI, Sathananthan N, Parker MC. The cost effectiveness of self-expanding metal stents in the management of malignant left-sided large bowel obstruction. Colorectal Dis. 2000;2:233-237. [RCA] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Athreya S, Moss J, Urquhart G, Edwards R, Downie A, Poon FW. Colorectal stenting for colonic obstruction: the indications, complications, effectiveness and outcome--5 year review. Eur J Radiol. 2006;60:91-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Park S, Cheon JH, Park JJ, Moon CM, Hong SP, Lee SK, Kim TI, Kim WH. Comparison of efficacies between stents for malignant colorectal obstruction: a randomized, prospective study. Gastrointest Endosc. 2010;72:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 45. | Park JK, Lee MS, Ko BM, Kim HK, Kim YJ, Choi HJ, Hong SJ, Ryu CB, Moon JH, Kim JO. Outcome of palliative self-expanding metal stent placement in malignant colorectal obstruction according to stent type and manufacturer. Surg Endosc. 2011;25:1293-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Suh JP, Kim SW, Cho YK, Park JM, Lee IS, Choi MG, Chung IS, Kim HJ, Kang WK, Oh ST. Effectiveness of stent placement for palliative treatment in malignant colorectal obstruction and predictive factors for stent occlusion. Surg Endosc. 2010;24:400-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Small AJ, Coelho-Prabhu N, Baron TH. Endoscopic placement of self-expandable metal stents for malignant colonic obstruction: long-term outcomes and complication factors. Gastrointest Endosc. 2010;71:560-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |