Published online Jul 14, 2013. doi: 10.3748/wjg.v19.i26.4192
Revised: June 21, 2013
Accepted: July 5, 2013
Published online: July 14, 2013
AIM: To assess the technical safety and efficacy of transcatheter arterial chemoembolization (TACE) combined with immediate radiofrequency ablation (RFA) for large hepatocellular carcinomas (HCC) (maximum diameter ≥ 5 cm).
METHODS: Individual lesions in 18 patients with HCCs (mean maximum diameter: 7.5 cm; range: 5.1-15.5 cm) were treated by TACE combined with percutaneous RFA between January 2010 and June 2012. All of the patients had previously undergone one to four cycles of TACE treatment. Regular imaging and laboratory tests were performed to evaluate the rate of technical success, technique-related complications, local-regional tumor responses, recurrence-free survival time and survival rate after treatment.
RESULTS: Technical success was achieved for all 18 visible HCCs. Complete response (CR) was observed in 17 cases, and partial response was observed in 1 case 1 mo after intervention. The CR rate was 94.4%. Local tumors were mainly characterized by coagulative necrosis. During follow-up (2-29 mo), the mean recurrence-free survival time was 16.8 ± 4.0 mo in 17 cases of CR. The estimated overall survival rate at 6, 12, and 18 mo was 100%. No major complications were observed. Levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in the blood of 17 patients transiently increased on the third day after treatment (ALT 200.4 ± 63.4 U/L vs 24.7 ± 9.3 U/L, P < 0.05; AST 228.1 ± 25.4 U/L vs 32.7 ± 6.8 U/L, P < 0.05). Severe pain occurred in three patients, which was controlled with morphine and fentanyl.
CONCLUSION: TACE combined with immediate RFA is a safe and effective treatment for large solitary HCCs. Severe pain is a major side effect, but can be controlled by morphine.
Core tip: Transcatheter arterial chemoembolization (TACE) immediately followed by radiofrequency ablation (RFA) under digital subtraction angiography-computed tomography is used to treat large hepatocellular carcinomas. This technology can improve the synergistic treatment effects of TACE and RFA, as well as reduce the need for repeated treatments and amount of radiation exposure. Furthermore, different treatment technologies are fused into one machine, thereby simplifying the operational process. TACE immediately followed by RFA enhances tumor inactivation ability, decreases recurrence rates, prolongs patient survival time and improves prognosis.
- Citation: Wang ZJ, Wang MQ, Duan F, Song P, Liu FY, Chang ZF, Wang Y, Yan JY, Li K. Transcatheter arterial chemoembolization followed by immediate radiofrequency ablation for large solitary hepatocellular carcinomas. World J Gastroenterol 2013; 19(26): 4192-4199
- URL: https://www.wjgnet.com/1007-9327/full/v19/i26/4192.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i26.4192
Hepatocellular carcinoma (HCC), the sixth most malignant tumor worldwide, is the third most common tumor leading to death; unfortunately, only 10%-54% of all patients with HCC are suitable for surgery[1-3]. Transcatheter arterial chemoembolisation (TACE) is one of the modalities used to treat unresectable HCC; however, its low complete tumor necrosis rate results in tumor recurrence and metastasis and influences long-term efficacy[1,3-6]. In addition, the effect of TACE is influenced by tumor size which decreases inactivation ability, especially for HCCs with diameters larger than 5 cm[7,8]. Compared with TACE, the combination of TACE with radiofrequency ablation (RFA) shows enhanced efficacy against HCC and prolonged survival in patients[7-11]. RFA is usually performed 1-4 wk after TACE[3,11-13]. However, the combination of TACE with immediate synchronous RFA for unresectable and large HCCs has not yet been reported. We retrospectively summarized 18 patients treated between January 2010 and June 2012 to assess the technical safety and efficacy of TACE combined with immediate synchronous RFA as a treatment modality for HCC.
This retrospective study was approved by the Ethics Committee of the People’s Liberation Army (PLA) General Hospital, and all patients signed informed consent forms. A total of 18 patients were admitted to the Department of Interventional Radiology of the PLA General Hospital between January 2010 and June 2012 and were diagnosed with HCC by ultrasound, computed tomography (CT), magnetic resonance imaging (MRI) and α-fetoprotein (AFP) blood test or pathological examination according to the diagnostic criteria for HCC established by the National Association for the Study of Liver Cancer. All patients, including 16 males and 2 females, with an average age of 55 ± 8.0 years (47-63 years) underwent TACE followed immediately by synchronous RFA. The AFP level was higher than 20 ng/mL in 10 cases. On the basis of the Child-Pugh score, 13 cases were classified as Grade A and 5 cases were classified as Grade B.
Patients were allowed to receive the combination therapy if: (1) various imaging examinations (ultrasound, CT, MRI and intra-procedure imaging in TACE) showed one lesion in the liver with a maximum diameter larger than 5 cm and the patients had no surgical indications or refusal to surgery, and (2) the Child-Pugh score was Grade A or B. Patients were excluded from the treatment if they had: (1) cancer embolus in the main portal vein and its left and right portal veins, arteriovenous fistula formation, biliary invasion and extrahepatic metastases and (2) severe coagulation disorders, such as prothrombin activity < 40% and platelet concentration < 30 × 109/L. All the RFA lesions were located 1 cm away from the gall bladder, intestinal canal, bile duct and major blood vessels. All patients underwent regular physical examination and tests (routine blood test, hepatorenal function, electrolytes, blood coagulation and tumor markers), as well as other relevant examinations (liver CT, ultrasound or MRI, lung and brain CT and bone ECT). The maximum diameter of the lesion was determined on enhanced CT or MRI. TACE was performed in these 18 patients one to three times prior to the procedure.
TACE: All the interventional procedures were performed via INNOVA4100 IQ digital subtraction angiography (DSA) (GE Company, United States) by an interventional radiologist with 8-10 years of experience at the Department of Interventional Radiology. After the right femoral artery was punctured by Seldinger’s technique, a 4F catheter (RH, Terumo Corporation, Japan) was used for celiac artery and superior mesenteric artery angiography, as well as selective hepatic arteriography if necessary. Chemoembolisation was then conducted with a 3F microcatheter (Progreat, Terumo Corporation, Japan) on the feeding arteries of the tumor. Three to four of the following drugs were administered during the procedure: epirubicin (30-50 mg), cisplatin (40-60 mg) or oxaliplatin (100-150 mg), mitomycin (10-14 mg), 5-FU (500-750 mg), calcium folinate (200-300 mg) and hydroxycamptothecine (10-14 mg). Each drug powder was mixed with lipiodol to form an emulsion and liquid chemotherapy drugs for target vessel perfusion through a microcatheter. When the branch of the portal vein had developed or blood flow had obviously slowed down after chemoembolisation induced by the lipiodol emulsion, gelatine sponge particles were used to perform embolisation. Patients with large lesions or a large number of blood vessels around the tumor were administered the same drugs, but with the addition of 500-700 μmol/L polyvinyl alcohol (Cook Medical, Bloomington, IN, United States). Collateral artery embolisation was conducted if branches such as the phrenic artery and internal thoracic artery were involved in the tumor blood supply.
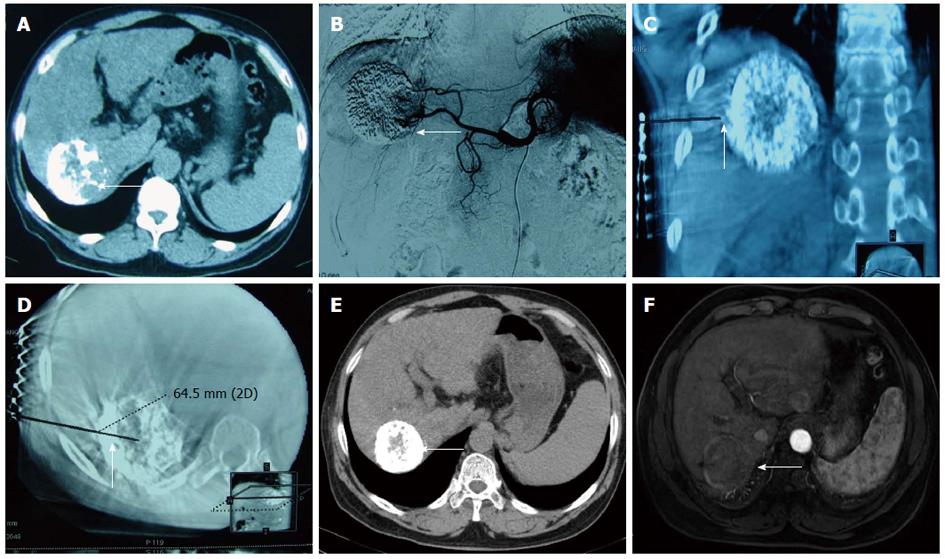
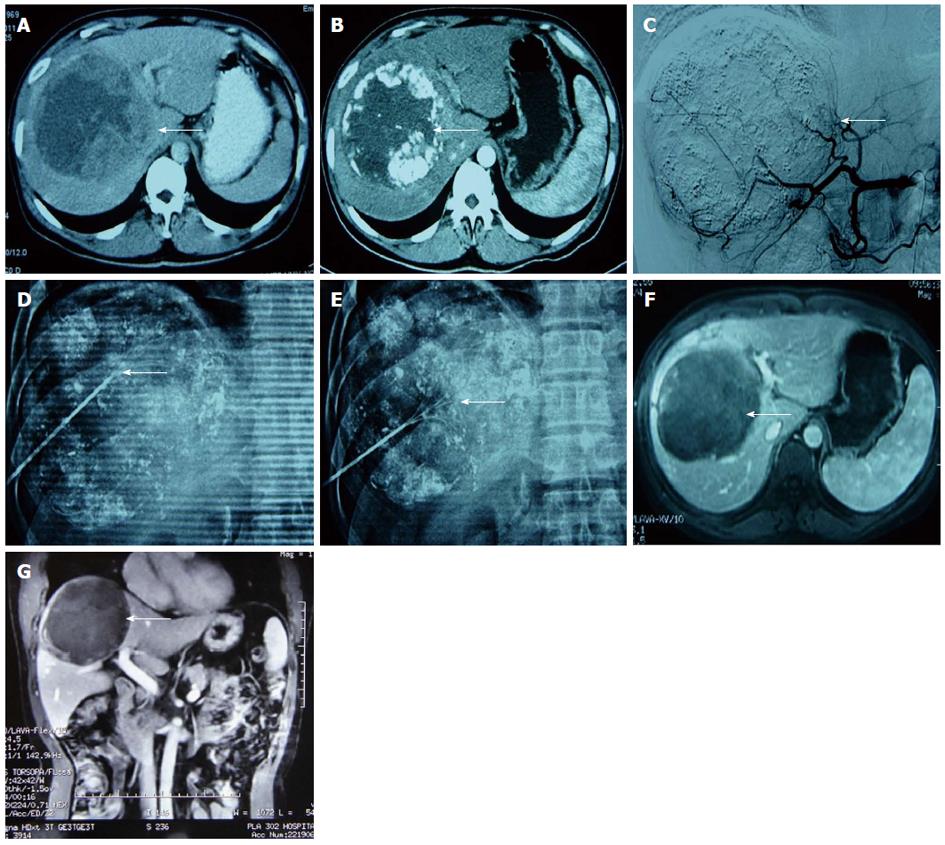
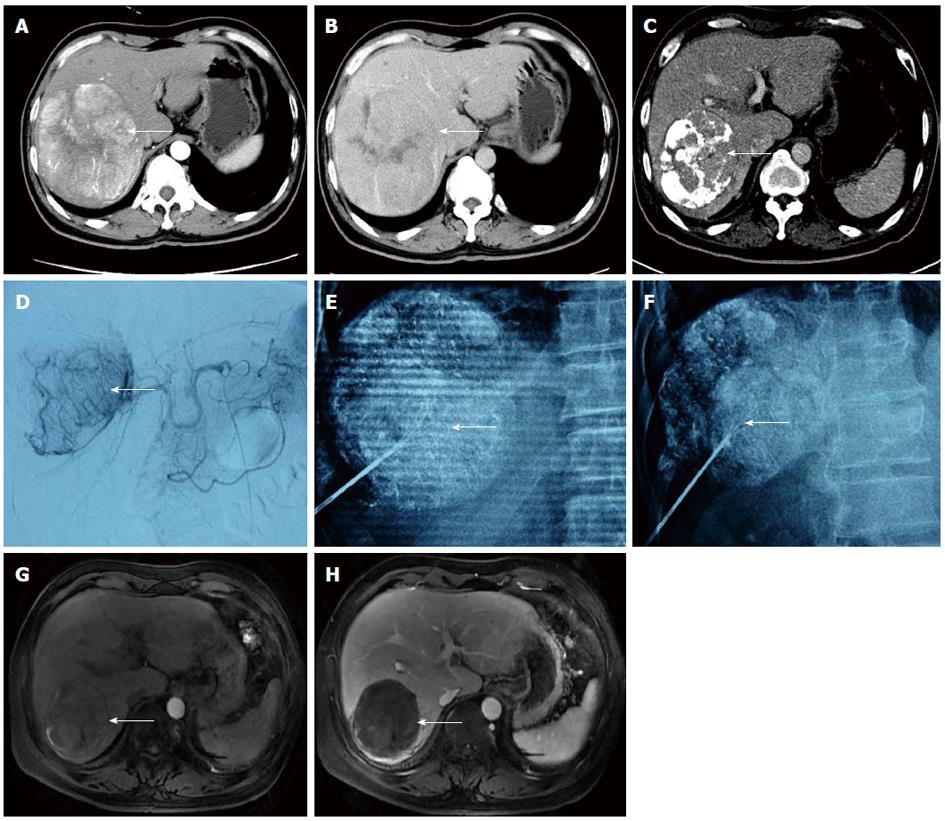
Immediate synchronous RFA: Percutaneous RFA was immediately performed under general anaesthesia after TACE with the guidance of a C-arm cone beam CT and DSA. Three-dimensional (3D) CT navigation with INNOVA4100 IQ DSA was used. To establish 3D CT images, the radiofrequency (RF) puncture path and its parameters, 6 × 6 square metal grid lines (diameter: 1 cm) were placed in parallel on the right side of the 8th-10th costal margin or below the xiphoid. Then, a 3D CT scan of the target lesion and image reconstruction (Figure 1) were conducted. The corresponding site was located on the body surface instead of the ribs, and the puncture path was identified through the surface point. The target lesion was determined to avoid important organs, such as the intestinal canal, gallbladder and lung. The puncture path was calibrated to one (i.e., bull’s eye configuration), and various parameters, such as the angle of the head, lateral position of the tube ball and needle depth, were determined. The device was then switched on to automatically set the system, which adjusted the tube ball to the correct position. To puncture and localize the target lesion under the perspective, the RF needle was inserted toward the target, and the bull’s eye graphic converged on the target. When the puncture needle was in the correct position, the multipolar RF needle was switched on and the tube ball was rotated 70°-90° toward the right and left lateral positions to verify whether the RFA needle was located in the target lesion. For the RFA parameter setting, different RFA needles and RFA parameters were selected according to the tumor position, size and shape. A multipolar RF needle (RITA Company, Cristal Lake, IL, United States) with a maximum ablation diameter of 5 cm and needle length of 15 to 25 cm was used in all cases due to the presence of large HCCs in some patients. The following settings were used: power, 150 to 200 W; ablation time, 6 min (3 cm), 8 min (4 cm) and 15 min (5 cm) and target temperature, 105 °C. RFA was performed twice as routine and three times if necessary. After ablation, final solidification of the puncture path and inactivation of tumor tissue were conducted to avoid bleeding and tumor implantation metastasis.
Post-procedure treatment and follow-up: Local pressure (RF puncture site and puncture site in right femoral artery) was applied after RFA. To alleviate pain, an analgesic pump was used continuously for 3 d which injected 0.2 mg/kg fentanyl with 10 mg of tropisetron hydrochloride and normal saline at a total volume of 80 mL. Electrocardiographic monitoring was performed for 24 h. The following procedures were also conducted: anti-infection, improvement of damaged liver function, nutritional support and defaecation. About 3 d to 1 wk after the procedure, routine blood examination, hepatorenal function and electrolytes were examined. Exactly 1 mo after the procedure, the patients underwent CT, ultrasound or enhanced MRI scan, and examinations for hepatorenal function and tumor markers (AFP, CA199 and carcinoembryonic antigen). If the tumor was well controlled, subsequent reviews were arranged at 2-3 mo intervals. All the images were analysed by a radiologist with over 8 years of experience. The efficacy of the combination therapy on local tumors was assessed according to the evaluation method recommended by the European Association for the Study of the Liver. Complete response (CR) was defined as the absence of signs of intensified lesions in and around the tumor. Partial response (PR) referred to a minimum of 50% reduction in size of the enhancing tumor. Progressive disease (PD) described the presence of new lesions or at least one lesion with 25% reduction in the size of the enhancing tumor. Stable disease (SD) referred to the presence of a stable lesion between PR and SD. During follow-up, the complete inactivation rate, duration and necrosis characteristics of the local tumor and survival condition of the patients were assessed. The appropriate treatment (combination therapy, close observation or single RFA or TACE) of the patients was determined on the basis of clinical conditions, such as the characteristics of new or residual lesions. Major complications were evaluated on the basis of bleeding and injuries of the intestinal canal, bile duct and gallbladder. Minor complications were assessed using several indicators, including changes in hepatorenal function after combination therapy, and changes in syndrome symptoms after embolisation, such as pain.
Quantitative data were analysed using CHISS2004 software. The t test was employed to compare liver function (Child-Pugh Grade) before and after intervention.
All patients were tolerant of the concurrent combination therapy. A total of 18 lesions were confirmed by previous images and successfully labelled with lipiodol deposit in TACE. One-off RFA puncture was successfully conducted with two to three RFA needles per lesion (Figures 2 and 3). The success rate of the combination therapy was 100%. For 9 cases with lesions near the diaphragm, the puncture avoided normal lung tissue under the perspective and entered the lesions for RFA treatment.
One month after the intervention, all 18 patients underwent routine imaging (ultrasound, enhanced CT or MRI of liver) and AFP examination. Local lesions were mainly characterized by coagulative necrosis without liquefactive necrosis. Increased AFP was discovered in 10 patients before intervention, which significantly decreased 1 mo after surgery. CR was observed in 17 cases, and PR was observed in 1 case. During follow-up, the mean recurrence-free survival time of the 18 cases was 16.8 ± 4.0 mo (2-29 mo). The estimated overall survival rate at 6, 12 and 18 mo was 100%.
No TACE and percutaneous RFA-related complications, such as bleeding or necrosis of the gallbladder, bile duct, intestinal canal, pneumothorax or hepatapostema or liver/kidney failure, occurred after the intervention. Pain was completely alleviated by injection of the fentanyl mixture in 16 patients using the analgesic pump, and by 3-4 mg of morphine once every 24 h in 3 patients in addition to the analgesic pump. All patients received oral central analgesics (such as oxycodone hydrochloride) 4 d after treatment to relieve pain. Different degrees of constipation, low fever (37.3-38.4 °C) and nausea and vomiting were observed in 5, 7 and 5 cases, respectively, and relieved with medication. The ALT (200.4 ± 63.4 U/L) and AST (228.1 U/L ± 25.4 U/L) levels in all patients transiently increased (P = 0.00 < 0.05) on the third day after treatment relative to levels before surgery (24.7 ± 9.3 and 32.7 ± 6.8 U/L, respectively). ALT and AST levels decreased to 29.8 ± 11.5 U/L and 36.8 ± 10.2 U/L (P = 0.15, P = 0.16 > 0.05), respectively, on the 7th day after treatment. No statistical differences in bilirubin and albumin were found before and after treatment.
Large solitary HCCs are a special type of liver cancer, the prognosis of which is better than that of the multinodular type after complete inactivation by chemoembolisation or resection[14-17]. Although surgery is still the primary treatment mode for HCC, it has several disadvantages resulting from the large size or location of the carcinoma, such as difficulty in complete resection, heavy bleeding, high incidence of complications and high recurrence rates after surgery[16,17]. Aside from surgery, many other non-surgical treatments such as TACE and RFA are used to cure solitary HCCs. However, TACE or RAF can only inactivate local carcinomas with diameters smaller than a specific value. For instance, TACE treatment requires repetition and a large dose of lipiodol, involves a high likelihood of collateral formation after multiple embolisations and has low inactivation rates after the procedure and adverse effects on long-term liver function and prognosis[17]. In our study, all the patients underwent one to three cycles of TACE before the combination therapy and were reviewed for the presence of residual or new tumors. The results indicate that a single technique cannot completely inactivate HCC and that recurrence rate of the cancer was high after the procedure.
The combination of TACE and RFA is one of the major treatments used to enhance the inactivation rate of local tumors, decreasing short- or long-term recurrence rates and extending patient survival[3-12,17-20]. However, the current combination therapy is mainly performed separately or several times and the local recurrence rate increases as tumor diameter increases[21,22]. The interval between TACE and RFA is longer than 1 or 4 wk, during which recanalization after embolisation, collateral formation and elimination of lipiodol-chemotherapeutant may occur. Therefore, this method is not strictly concurrent, and the effects of TACE and RFA are not fully synergistic.
To increase local tumor inactivation rates, preliminary studies on the safety and effectiveness of TACE combined with immediate RFA in the treatment of liver tumors (diameter, ≤ 5 cm) have been conducted. Gadaleta et al[23] used immediate combination therapy to treat HCC and liver metastatic tumors of different sizes and achieved a success rate of 100% with an 88% CR. Kang et al[18] treated HCC (diameter, ≤ 5 cm) by TACE combined with immediate RFA. Approximately 1 mo later, the complete necrosis rate of tumors successfully labelled by TACE was 100%, and the cumulative incidence of local tumor progression at 1 year and 3 years was 1.8% and 9.4%. These results demonstrate the positive effects of immediate combination therapy. We studied the application of immediate combination therapy for large solitary HCCs (diameter, > 5 cm) and showed the favorable clinical efficacy of this method. The clinical efficiency was 100% in 18 patients, with 17 cases of CR and 1 case of PR. Compared with single TACE, combination therapy increased the local inactivation rate and prolonged recurrence-free survival. During follow-up, the estimated overall survival rate at 6, 12 and 18 mo was 100%. Based on the literature and clinical practice, as well as comparisons with conventional sequential therapy, the advantages of immediate combination therapy are as follows: (1) Single TACE or RFA cannot completely inactivate the tumor, especially the tissue on the tumor border, resulting in recurrence. In immediate combination therapy, lipiodol precipitation in the lesion wraps around and inactivates the surrounding tissue of the tumor, thereby preventing recurrence from residual tumors[3,24]; (2) Immediate combination therapy also fully enhances the synergistic effects of chemotherapeutics and thermal ablation. In TACE, lipiodol, which is the carrier of chemotherapeutics, is uncontrolled and unstable. Lipiodol cannot be released slowly with sustained high concentrations. Chemotherapeutics in lipiodol are eliminated over time if no other embolisation agents (such as sponge) are added to the drug. However, in TACE with immediate RFA, chemotherapeutics, such as adriamycin, can inhibit tumors due to their high accumulative concentration in and around the lesions[25,26]; and (3) TACE accurately locates and labels new and residual lesions, as well as lesions that cannot be observed by conventional CT and B ultrasound, through lipiodol precipitation. Thus, RFA targets are more specific. The labelling of lesions by lipiodol precipitation is more effective, especially in lesions that have become relatively complicated after several cycles of TACE because previous necrotic and new or residual lesions are labelled[27,28].
TACE combined with RFA is a safe treatment with 4%-6% incidence of various complications; severe complications are rare[3,18,23,28]. Kang et al[18] reported two cases of serious liver damage and one case of hemorrhage from a ruptured hepatic artery in combination therapy. Only one case of hepatic function damage without other complications, such as severe liver failure, was found in our series; this complication may have resulted from the application of a super-selective embolisation technique and bypassing of normal liver tissue by the RFA needle. Most patients only experienced post-embolisation syndrome (pain, low fever, etc.), transient liver dysfunction and constipation after surgery, all of which were relieved with medication. Constipation may be associated with the continuous application of analgesics, and low fever may be related to absorption after tumor necrosis. Transient liver dysfunction is related to combination therapy. Given that concurrent TACE with RFA increases pain in patients, RFA was performed immediately after TACE under general anaesthesia and tracheal intubation. An analgesic pump was utilized for 3 d to relieve pain. However, three patients still experienced intense local pain, which was alleviated by the addition of analgesics. Therefore, instead of the conventional combination of TACE and RFA, we recommend the application of an analgesic pump for 3 d after immediate combination therapy. Compared with the liquefactive necrosis induced by single TACE or RFA, the tumors were characterized by coagulative necrosis, which has a lower risk for local secondary hepatapostema[5].
Our study has several limitations: (1) The small sample size in this retrospective research may have influenced the results to some extent; and (2) the follow-up period was too short to accurately evaluate long-term efficacy. More patients should be included in comparative studies and further assessment of the advantages of combination therapy.
In conclusion, TACE immediately followed by RFA is a safe and effective treatment for large HCCs. To prevent pain after the procedure, an analgesic pump should be used for 3 d. The long-term efficacy of this combination therapy requires further assessment.
Transcatheter arterial chemoembolisation (TACE) is the main treatment method for unresectable primary hepatocellular carcinomas (HCC). However, simple TACE has low tumor inactivation and high recurrence rates. In addition, with increasing tumor size, the tumor inactivation rate significantly decreases.
Combination therapy [mainly TACE combined with radiofrequency ablation (RFA)] is one of the main modalities for treating unresectable HCC. However, RFA is often performed 1 to 2 wk after TACE, which considerably reduces the synergistic effects of the combination therapy, especially for large HCCs.
The combination of TACE with immediate RFA under digital subtraction angiography-computed tomography (CT) guidance is applied to treat large single HCCs. With this technique, different treatment technologies are fused into one angiographic machine, which improves their synergistic effects (e.g., heat treatment with epirubicin and embolisation with thermal ablation), thereby enhancing the inactivation rate of large HCCs and reducing the radiation dose applied to patients and the risk of repeated treatment. Preliminary results show that combination therapy has obvious advantages and can help improve the long-term survival of patients. This technique also has significance in the treatment of other metastatic and hypervascular HCCs.
TACE immediately followed by RFA is a safe and effective treatment for large HCCs. This technology can improve the synergistic treatment effects of TACE and RFA, as well as reduce the need for repeated treatments and amount of radiation exposure. Furthermore, different treatment technologies are fused into one machine, thereby simplifying the operational process. TACE immediately followed by RFA enhances tumor inactivation ability, decreases recurrence rates, prolongs patient survival time and improves prognosis.
TACE: Transcatheter arterial chemoembolisation with lipiodol and chemical drugs. Immediate RFA: RFA procedure is performed immediately after TACE. A three-dimensional CT image, radiofrequency puncture path and parameters are first established to target the lesion and avoid non-target lesions. This combination therapeutic modality requires general anesthesia. Post-procedure pain management is required for 2 to 3 d. The combined therapeutic modality can be used for HCCs and single hypervascular metastatic tumors.
In this manuscript, the authors summarised 18 patients treated between January 2010 and June 2012 to assess the technical safety and efficacy of TACE combined with immediate synchronous RFA as a means of treating HCC. The manuscript is very interesting. It is a very good study about the technical safety and efficacy of the combined therapy for large hepatocellular carcinomas.
P- Reviewers Masmoudi K, Minor DR S- Editor Song XX L- Editor A E- Editor Zhang DN
| 1. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3249] [Cited by in F6Publishing: 3477] [Article Influence: 289.8] [Reference Citation Analysis (3)] |
| 2. | Marín-Hargreaves G, Azoulay D, Bismuth H. Hepatocellular carcinoma: surgical indications and results. Crit Rev Oncol Hematol. 2003;47:13-27. [PubMed] [Cited in This Article: ] |
| 3. | Takaki H, Yamakado K, Uraki J, Nakatsuka A, Fuke H, Yamamoto N, Shiraki K, Yamada T, Takeda K. Radiofrequency ablation combined with chemoembolization for the treatment of hepatocellular carcinomas larger than 5 cm. J Vasc Interv Radiol. 2009;20:217-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Georgiades CS, Hong K, Geschwind JF. Radiofrequency ablation and chemoembolization for hepatocellular carcinoma. Cancer J. 2008;14:117-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Miyayama S, Yamashiro M, Okuda M, Yoshie Y, Sugimori N, Igarashi S, Nakashima Y, Notsumata K, Toya D, Tanaka N. Chemoembolization for the treatment of large hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21:1226-1234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Kasai K, Ushio A, Sawara K, Miyamoto Y, Kasai Y, Oikawa K, Kuroda H, Takikawa Y, Suzuki K. Transcatheter arterial chemoembolization with a fine-powder formulation of cisplatin for hepatocellular carcinoma. World J Gastroenterol. 2010;16:3437-3444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Fan WZ, Yang JY, Lü MD, Xie XY, Yin XY, Huang YH, Kuang M, Li HP, Xu HX, Li JP. [Transcatheter arterial chemoembolization plus percutaneous thermal ablation in large hepatocellular carcinoma: clinical observation of efficacy and predictors of prognostic factors]. Zhonghua Yi Xue Zazhi. 2011;91:2190-2194. [PubMed] [Cited in This Article: ] |
| 8. | Yamakado K, Nakatsuka A, Ohmori S, Shiraki K, Nakano T, Ikoma J, Adachi Y, Takeda K. Radiofrequency ablation combined with chemoembolization in hepatocellular carcinoma: treatment response based on tumor size and morphology. J Vasc Interv Radiol. 2002;13:1225-1232. [PubMed] [Cited in This Article: ] |
| 9. | Wang W, Shi J, Xie WF. Transarterial chemoembolization in combination with percutaneous ablation therapy in unresectable hepatocellular carcinoma: a meta-analysis. Liver Int. 2010;30:741-749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Yamanaka T, Yamakado K, Takaki H, Nakatsuka A, Shiraki K, Hasegawa H, Takei Y, Takeda K. Ablative zone size created by radiofrequency ablation with and without chemoembolization in small hepatocellular carcinomas. Jpn J Radiol. 2012;30:553-559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Peng ZW, Zhang YJ, Liang HH, Lin XJ, Guo RP, Chen MS. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology. 2012;262:689-700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 12. | Yamakado K, Nakatsuka A, Takaki H, Sakurai H, Isaji S, Yamamoto N, Shiraki K, Takeda K. Subphrenic versus nonsubphrenic hepatocellular carcinoma: combined therapy with chemoembolization and radiofrequency ablation. AJR Am J Roentgenol. 2010;194:530-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Zhao M, Wang JP, Wu PH, Zhang FJ, Huang ZL, Li W, Zhang L, Pan CC, Li CX, Jiang Y. [Comparative analysis of TACE alone or plus RFA in the treatment of 167 cases of intermediate and advanced staged primary hepatocellular carcinoma]. Zhonghua Yi Xue Zazhi. 2010;90:2916-2921. [PubMed] [Cited in This Article: ] |
| 14. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [PubMed] [Cited in This Article: ] |
| 15. | Yang LY, Fang F, Ou DP, Wu W, Zeng ZJ, Wu F. Solitary large hepatocellular carcinoma: a specific subtype of hepatocellular carcinoma with good outcome after hepatic resection. Ann Surg. 2009;249:118-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 158] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 16. | Shimada K, Sakamoto Y, Esaki M, Kosuge T. Role of a hepatectomy for the treatment of large hepatocellular carcinomas measuring 10 cm or larger in diameter. Langenbecks Arch Surg. 2008;393:521-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Zangos S, Eichler K, Balzer JO, Straub R, Hammerstingl R, Herzog C, Lehnert T, Heller M, Thalhammer A, Mack MG. Large-sized hepatocellular carcinoma (HCC): a neoadjuvant treatment protocol with repetitive transarterial chemoembolization (TACE) before percutaneous MR-guided laser-induced thermotherapy (LITT). Eur Radiol. 2007;17:553-563. [PubMed] [Cited in This Article: ] |
| 18. | Kang SG, Yoon CJ, Jeong SH, Kim JW, Lee SH, Lee KH, Kim YH. Single-session combined therapy with chemoembolization and radiofrequency ablation in hepatocellular carcinoma less than or equal to 5 cm: a preliminary study. J Vasc Interv Radiol. 2009;20:1570-1577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Peng ZW, Chen MS. Transcatheter arterial chemoembolization combined with radiofrequency ablation for the treatment of hepatocellular carcinoma. Oncology. 2013;84 Suppl 1:40-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Nishikawa H, Osaki Y, Inuzuka T, Takeda H, Nakajima J, Matsuda F, Henmi S, Sakamoto A, Ishikawa T, Saito S. Branched-chain amino acid treatment before transcatheter arterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2012;18:1379-1384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 26] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Takaki H, Yamakado K, Nakatsuka A, Fuke H, Murata K, Shiraki K, Takeda K. Radiofrequency ablation combined with chemoembolization for the treatment of hepatocellular carcinomas 5 cm or smaller: risk factors for local tumor progression. J Vasc Interv Radiol. 2007;18:856-861. [PubMed] [Cited in This Article: ] |
| 22. | Murakami T, Ishimaru H, Sakamoto I, Uetani M, Matsuoka Y, Daikoku M, Honda S, Koshiishi T, Fujimoto T. Percutaneous radiofrequency ablation and transcatheter arterial chemoembolization for hypervascular hepatocellular carcinoma: rate and risk factors for local recurrence. Cardiovasc Intervent Radiol. 2007;30:696-704. [PubMed] [Cited in This Article: ] |
| 23. | Gadaleta C, Catino A, Ranieri G, Fazio V, Gadaleta-Caldarola G, Cramarossa A, Armenise F, Canniello E, Vinciarelli G, Laricchia G. Single-step therapy -- feasibility and safety of simultaneous transarterial chemoembolization and radiofrequency ablation for hepatic malignancies. In Vivo. 2009;23:813-820. [PubMed] [Cited in This Article: ] |
| 24. | Shiraishi R, Yamasaki T, Saeki I, Okita K, Yamaguchi Y, Uchida K, Terai S, Sakaida I. Pilot study of combination therapy with transcatheter arterial infusion chemotherapy using iodized oil and percutaneous radiofrequency ablation during occlusion of hepatic blood flow for hepatocellular carcinoma. Am J Clin Oncol. 2008;31:311-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Dhanasekaran R, Kooby DA, Staley CA, Kauh JS, Khanna V, Kim HS. Comparison of conventional transarterial chemoembolization (TACE) and chemoembolization with doxorubicin drug eluting beads (DEB) for unresectable hepatocelluar carcinoma (HCC). J Surg Oncol. 2010;101:476-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 26. | Head HW, Dodd GD, Bao A, Soundararajan A, Garcia-Rojas X, Prihoda TJ, McManus LM, Goins BA, Santoyo CA, Phillips WT. Combination radiofrequency ablation and intravenous radiolabeled liposomal Doxorubicin: imaging and quantification of increased drug delivery to tumors. Radiology. 2010;255:405-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Lee MW, Kim YJ, Park SW, Hwang JH, Jung SI, Jeon HJ, Kwon WK. Percutaneous radiofrequency ablation of small hepatocellular carcinoma invisible on both ultrasonography and unenhanced CT: a preliminary study of combined treatment with transarterial chemoembolisation. Br J Radiol. 2009;82:908-915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Lee MW, Kim YJ, Park SW, Yu NC, Choe WH, Kwon SY, Lee CH. Biplane fluoroscopy-guided radiofrequency ablation combined with chemoembolisation for hepatocellular carcinoma: initial experience. Br J Radiol. 2011;84:691-697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |