Published online Jul 7, 2013. doi: 10.3748/wjg.v19.i25.3931
Revised: April 18, 2013
Accepted: May 8, 2013
Published online: July 7, 2013
Inflammatory bowel diseases (IBD) such as Crohn’s disease (CD) or ulcerative colitis are chronic intestinal disorders, which are on the increase in “Westernised” countries. IBD can be caused by both genetic and environmental factors. Interleukin-10 (IL-10) is an immunoregulatory cytokine that has been identified as being involved in several diseases including IBD. Studies have shown that polymorphisms in the promoter region reduce serum levels of IL-10 and this reduction has been associated with some forms of IBD. Mouse models have shown promising results with IL-10 supplementation, as such IL-10 supplementation has been touted as being a possible alternative treatment for CD in humans. Clinical trials have shown that recombinant human IL-10 is safe and well tolerated up to a dose of 8 μg/kg. However, to date, the results of the clinical trials have been disappointing. Although CD activity was reduced as measured by the CD activity index, IL-10 supplementation did not result in significantly reduced remission rates or clinical improvements when compared to placebo. This review discusses why IL-10 supplementation is not effective in CD patients currently and what can be addressed to potentially make IL-10 supplementation a more viable treatment option in the future. Based on the current research we conclude that IL-10 supplementation is not a one size fits all treatment and if the correct population of patients is chosen then IL-10 supplementation could be of benefit.
Core tip: Inflammatory bowel disease (IBD) is a chronic condition with no known cure. This review addresses the current available treatments for IBD before discussing a potential new treatment strategy using the immunoregulatory cytokine interleukin-10 (IL-10). To date clinical trial results have been disappointing. We highlight the limitations of current IL-10 supplementation treatment and suggest how, with changes to IL-10 delivery and the correct choice of patient, IL-10 supplementation could become a viable treatment option.
- Citation: Marlow GJ, van Gent D, Ferguson LR. Why interleukin-10 supplementation does not work in Crohn’s disease patients. World J Gastroenterol 2013; 19(25): 3931-3941
- URL: https://www.wjgnet.com/1007-9327/full/v19/i25/3931.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i25.3931
Inflammatory bowel diseases (IBD) are chronic intestinal disorders that are typified by ulcerative colitis (UC) and Crohn’s disease (CD). They are considered to be caused by an aberrant intestinal immune response to commensal microbiota in genetically susceptible individuals[1-3]. IBD affects over 1.4 million people in the United States and over 2.2 million in Europe and is on the increase[4-7]. In New Zealand CD affects 16 per 100000 and UC 7 per 100000. Clinical symptoms include pain, diarrhoea, rectal bleeding and weight loss, which can have a debilitating effect on sufferers[8]. There are both environmental and genetic factors that have a role in the development and progression of IBD. IBD is more prevalent in “Westernised” countries, believed to be a result of diet and lifestyle and also an effect of improved sanitation[9-11].
Genome-wide association studies (GWAS) have highlighted the complexity of IBD. To date, 163 IBD susceptibility loci have been identified[12], 30 associated with CD, 23 with UC and 110 with both[10,12-14]. Some susceptibility genes have been identified, covering genes involved in autophagy (ATG16L1 and IRGM), pattern recognition receptors, intestinal epithelium maintenance and immune response[4].
The anti-inflammatory cytokine interleukin-10 (IL-10) has been identified as being involved in IBD[15]. Studies[16-18] have shown that polymorphisms in the IL-10 promoter alter IL-10 serum levels and have been linked to IBD. IL-10 supplementation has been tested as a potential therapy for CD[19-28]. This review will focus on the use of IL-10 supplementation explaining why it is currently ineffective at treating patients with CD and showing how that effectiveness could be improved.
IL-10 was first identified as a cytokine secreted by CD4+ Th2-cells that inhibits cytokine production in antigen presenting cells[29], and was described as a cytokine synthesis inhibitory factor. The gene for human IL-10 is located in the 1q32 band on chromosome 1 and encodes for 5 exons. The encoded protein is a homodimer with a mass of 37 kDa consisting of 160 amino acid monomers[16,19,30]. The structure of IL-10 resembles interferon gamma (IFN-γ) and both IL-10 receptor (IL-10R) subunits are members of the interferon receptor family[31,32].
IL-10 is a pluripotent cytokine and could be considered the most important anti-inflammatory cytokine found in the human immune response[4,19]. IL-10 is produced by different cell types including B- and T-lymphocytes, macrophages, monocytes, dendritic cells and mast cells[16,33]. IL-10 has the ability to differentially affect the function of different subsets of immune cells, affecting both the innate immune system and the adaptive immune system, and is therefore considered to have a broad effect in immunoregulation and host defense[34]. Broadly speaking, IL-10 inhibits pro-inflammatory mediator production while increasing the production of anti-inflammatory mediators[19,34].
Many of the pro-inflammatory cytokines suppressed by IL-10 are known to be regulated by nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). Dysregulation of NF-κB has been implicated in the pathogenesis of chronic inflammatory disease including IBD[35,36]. It has been shown that IL-10 can block IKK activation and directly inhibit the nuclear localisation ofthe NF-κB p65/p50 heterodimer[37,38]. It has also been shown that IL-10 can selectively induce nuclear translocation and DNA-binding of p50 homodimer, which has been shown to inhibit transcription[39].
IL-10 down-regulates major histocompatibility complex II (MHC class II) expression[40] and the expression of the co-stimulatory ligands CD80/CD86 (B7-1, B7-2) in monocytes[41], macrophages[42,43] and dendritic cells[44,45]. While both MHC class II and co-stimulatory ligands are needed to effectively activate CD4+ Th2 cells by antigen presentation, this results in decreased macrophage and T cell derived cytokine synthesis, e.g., IL-1, IL-6, IL-8, IFN-α, tumor necrosis factor-α[25,46-48]. However IL-10 also has immunostimulatory effects, by up-regulating MHC class II expression on B lymphocytes[49] and increasing the synthesis of several antibody isotypes e.g., immunoglobulins (IgM, IgA and IgG)[50].
Despite unanswered questions, our current knowledge credits IL-10 with having a significant critical role in regulating intestinal immune homeostasis, this is highlighted by the fact that impaired IL-10 signalling contributes to IBD[4,51,52]. Rare homozygous mutations in IL10RA and IL10RB, resulting in defective IL-10 signalling were identified in children with early-onset IBD[52] thereby confirming IL-10’s critical role in maintaining intestinal homeostasis.
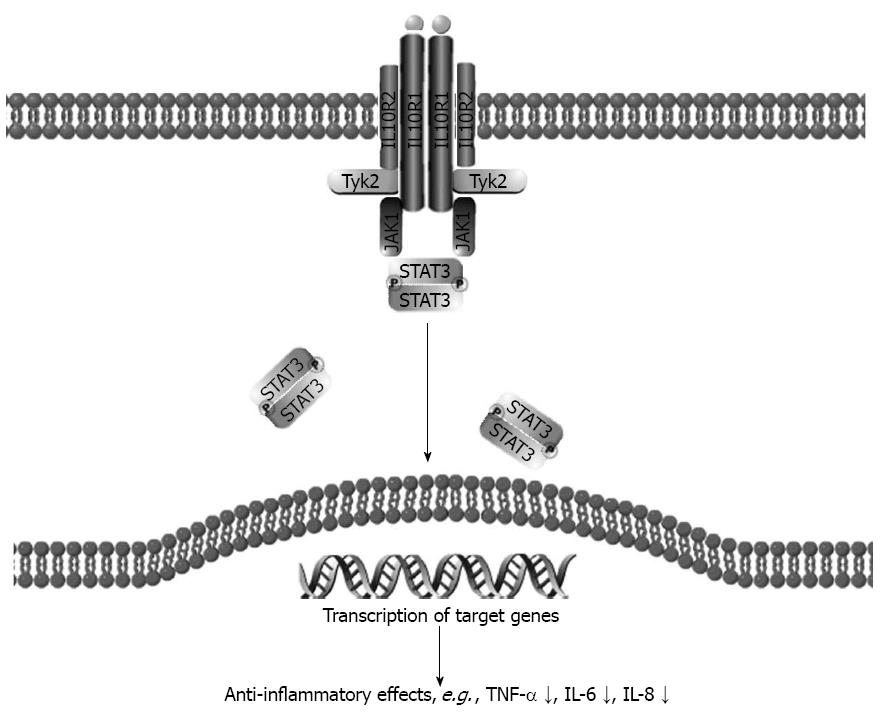
During IL-10 signalling the IL-10 homodimer binds to the tetrameric receptor IL-10R complex, consisting of 2 molecules of IL-10R α-chain (IL-10R1) and two molecules of the IL-10R β-chain (IL-10R2)[53-55]. This binding activates Janus Kinase 1 (JAK1) and tyrosine kinase 2 (Tyk2), which self-phosphorylate and subsequently phosphorylate IL-10R1 at tyrosine residues, 446 and 496, which recruits signal transducer and activator of transcription 3 (STAT3) via its SH2-domain. STAT3 is phosphorylated by JAK1 and Tyk2, causing dimerisation and translocation to the nucleus, where target genes are induced[2,4,20,53] (Figure 1).
There is contradictory evidence regarding the role of STAT3 in IBD[56], with studies showing that it can play both a pathogenic[57-60] or a regulatory[61-64] role in IBD depending on the specific activator and cell type[65]. STAT3 mediates mucosa-protective functions in epithelial and myeloid cells but can also contribute to inflammation if active in other cell types[66]. It has been shown that STAT3 is essential for all known functions of IL-10 and that STAT3 acts as a transcription factor for other genes within the anti-inflammatory response[67,68]. STAT3 is primarily recruited and activated in macrophages, and this activation is transient[69], which avoids the inflammation associated with an increase of activated STAT3 in IBD[70-72].
Genetic variants in IL-10, the IL-10 receptor and STAT3 genes are associated with IBD, highlighting the involvement of the IL-10 signalling cascade in the pathogenesis of CD and UC, further supporting the hypothesis that defective anti-inflammatory mechanisms may be key to IBD development[2,11,15,52,73-75].
The first evidence of a role of IL-10 in IBD, came from a GWAS study by Franke et al[15] that showed a significant (P = 1.35 × 10-12) association between a single nucleotide polymorphism (SNP) rs3024505 near the three-prime untranslated regions of the IL-10 gene and UC, there was modest association with CD.
IL-10 knockout mice develop chronic enterocolitis, which is similar to human CD, if they are not kept in germ-free conditions. Administration of IL-10 ameliorates inflammation in both animal and in vitro models[76], indicating a potential role for IL-10 in the down-regulation of Th1-mediated mucosal inflammation[16,77].
Because IL-10 mediated immune responses are so important in maintaining intestinal homeostasis and commensal flora tolerance, it has been hypothesized that a defect in IL-10 production may be involved in the pathogenesis of CD[78]. In fact impaired IL-10 production has been found in severe cases of both UC[79] and CD[78]. Studies show that CD patients have normal[80,81] or high IL-10 levels[18,82]. However, low IL-10 production in intestinal mucosa has been shown to be associated with increased postoperative recurrence[22,83] and it has been shown that administration of recombinant human IL-10 in low IL-10 producers significantly reduced recurrence after surgery[22].
It has been shown that intestinal epithelial cells from healthy and inflamed colonic tissue express IL-10 mRNA and protein to the same extent. However during inflammation and also in patients with CD, there are significantly increased numbers of mononuclear cells producing IL-10[20,25]. Circulating levels of IL-10, as determined by serum levels of IL-10[82] and mRNA levels[84], have been shown to correlate with disease activity.
It is believed that circulating levels of IL-10 are critical in immune regulation. Basal levels of IL-10 modulate production of other cytokines and thus minor changes can affect the cytokine network, which in turn affects inflammation.
Studies have been inconsistent regarding serum levels of IL-10 in IBD, as stated earlier some studies show higher IL-10 levels in CD, Wang et al[18] found that CD patients had significantly higher levels of IL-10 compared to controls. Kucharzik et al[82] reported increased serum IL-10 concentrations in patients with active CD or UC compared to controls. Mitsuyama et al[85] showed an increase in serum IL-10 in active UC patients but not CD. In contrast, Nielsen et al[81] reported that serum IL-10 concentrations did not differ among UC, CD and healthy control subjects. These inconsistencies could be the result of variations, e.g., age, severity of disease and ethnicity in the studied populations or in different methodological designs.
As IL-10 is an anti-inflammatory cytokine, we expect that high serum levels of IL-10 are likely to be good for patients with chronic inflammatory disease. In fact low IL-10 levels are known to increase disease severity in CD patients compared to high IL-10 levels[85,86]. From steroid treatment it has been shown that steroid non-responders have low IL-10 levels while steroid responders have sustainable high IL-10 levels during and after treatment[87]. Sufficient IL-10 levels seem to be required for recovery but do not offer a cure.
We can hypothesize that IL-10 has an optimal level to be beneficial to reduce chronic inflammatory diseases, and may prove detrimental at too high or too low levels. Diseases associated with IL-10 SNPs such as psoriasis and rheumatoid arthritis are known to have high IL-10 serum levels[18,88,89], while in other diseases like UC, IL-10 levels vary between individuals and studies, with a trend toward increased IL-10 production, though the big studies are lacking[90-92].
IL-10 serum level and disease severity is not restricted to IBD, other diseases including autoimmune diseases, such as systemic lupus erythematosis, Behçets, type 1 diabetes mellitus[14], psoriasis[93], atherosclerosis[94] and rheumatoid arthritis[89] have all been shown to be associated with IL-10 SNPs. Susceptibility to several cancers including prostate[95], breast[96], cervical[97] and more recently gastric[98,99] have been associated with IL-10 promoter polymorphisms.
SNPs are the most common form of genetic variation in humans. A SNP occurs at a location where more than one possible nucleotide occurs naturally within a population at a frequency >1%[100]. SNPs can be in both coding and non-coding regions of DNA. Due to the degeneracy of the genetic code. Even if the SNP is in a gene it may not change the amino acid and so has no effect on the protein (synonymous SNP), however non-synonymous SNPs do change the protein and are more commonly associated with disease. It is these variations that are most interesting to researchers as these can account for whether/how a person develops a disease, the severity of disease and how they respond to treatment.
Important variability in IL-10 secretion has been reported and is associated with SNPs in the IL-10 promoter at 3 locations -592, -819, -1082[78,101,102]. The IL-10 promoter polymorphisms C-592A (rs1800872), C-819T (rs1800871) and G-1082A (rs1800896) have been extensively studied. The most recent studies of Franke et al[15], Amre et al[17], Wang et al[18], Andersen et al[73], Fowler et al[103], Fernandez et al[104] and Tedde et al[105] reported a significant association between IL-10 rs1800896 and IBD.
The “A” allele of rs1800896 was found to be more common in IBD patients, especially in UC patients, individuals with the A/A genotype have lower IL-10 production than the G/G genotype[106], Koss et al[107] found that the -1082 AA is associated with decreased IL-10 production in both CD patients and controls. Wildtype -1082 (rs1800896) “G” and -592 (rs1800872) “C” are known to be associated with increased IL-10 levels; therefore we expect the GCC haplotype to show the highest IL-10 expression and ATA the lowest. This hypothesis was studied by Reuss et al[108] who showed in THP-1 monocyte cells that IL-10 expression was highest in the GCC haplotype compared to ACC and ATA (P = 0.042 and P = 0.0026). In the twin-study which followed, the haplotype showed no correlation with IL-10 serum levels. Wang et al[18] showed a significant (P = 0.001) increase in IL-10 production for TAT haplotype in healthy controls. The -592A allele was also shown to be associated with reduced transcription and decreased IL-10 secretion[16,102,109].
The current treatment options available for IBD, include: surgery, aminosalicylates, e.g., 5-aminosalicylic acid, corticosteroids, e.g., prednisone, immunosuppressants, e.g., azathioprine, cyclosporine or biologicals, e.g., infliximab[110,111]. The choice of treatment is dependent on phenotype, disease activity, characteristics of the drug and the patient.The choice should look to balance effectiveness with side effects and long term complications. As with any drug treatment there are side effects, these range from the usually well-tolerated upset stomach, nausea and headache to the more severe bone marrow and liver problems. As well as the associated side effects these treatments only work for some cases and can also result in a loss of response. Thus stronger treatments are required which have more severe side effects and long term consequences, and so alternative therapies are being investigated.
Environmental and dietary factors are thought to play a role in the development of CD[112,113] and so changes to diet and lifestyle can have beneficial effects. Studies[114-118] have shown that specific foods are associated with IBD and that avoiding certain foods can reduce both the severity and frequency of symptoms. There are several classes of new drugs being developed: monoclonal antibodies, small molecules, fusion proteins and recombinant growth factors, as well as stem cell based therapies; one of these new therapies is IL-10 supplementation.
Studies have suggested that IL-10 has huge therapeutic potential in intestinal inflammation, and that it should inhibit the up-regulated pro-inflammatory cytokines in CD and UC[25]. In most studies to date, Tenovil (Schering-Plough, Kenilworth, NJ, United States), has been used, which is the brand name of rhuIL-10. It is produced by a genetically engineered Escherichia coli strain, that expresses a 161 amino acid protein identical to human IL-10 with an additional amino-terminal methionine residue[119,120].
Based on the success of animal models[121-126] of intestinal inflammation, IL-10 therapy was heralded as a potential anti-inflammatory treatment in CD and several human trials have been undertaken. The first trial conducted by van Deventer et al[28] showed that IL-10 supplementation was safe and well tolerated. This was confirmed by subsequent studies[22,23,26]. van Deventer et al[28] showed a reduction in the average score of CD activity index (CDAI), but this was not significant. Fedorak et al[23] showed that 5 μg/kg of Tenovil given subcutaneously for 28 d to patients with mild to moderate CD activity resulted in improved clinical response (based on CDAI score) and improved endoscopic appearance of the disease. Schreiber et al[26] showed that 8 μg/kg of Tenovil given subcutaneously for 28 d to patients with mild to moderate CD activity resulted in a non-significant clinical improvement. However, Colombel et al[22] found no evidence that treatment with Tenovil for 12 wk in CD patients after intestinal resection prevented recurrence of CD. The key findings of these studies are summarised in Table 1.
| Ref. | Model | Intervention | Outcome |
| Human | |||
| Colombel et al[22] | 65 patients having recently undergone intestinal resection surgery | 4 μg/kg daily or 8 μg/kg twice weekly for 12 wk | No clear evidence of effect |
| Fedorak et al[23] | 95 mild to moderately active CD (CDAI 200-350) | 1, 5, 10 or 20 μg/kg of daily for 4, 20 wk follow up | Improved clinical response (based on CDAI score) and improved endoscopic appearance |
| Schreiber et al[26] | 329 therapy-refractory chronic active CD (CDAI 200-400) | 1, 4, 8 or 20 μg/kg of Tenovil subcutaneously for 28 d | Non-significant clinical improvements |
| van Deventer et al[28] | 46 patients with active steroid-resistant CD (CDAI 200-350) | 0.5, 1, 5, 10 or 25 μg/kg daily for 1, 3 wk follow up | Reduction in the average score of CDAI |
| Braat et al[128] | 10 patients with moderate to severe CD | 10 enteric-coated capsules containing 1010 cfu of LL-Thy12 twice daily for 7 d | Clinical benefit observed in 8 of 10 patients, including 5 showing complete remission |
| Animal | |||
| Barbara et al[121] | DNB induced colitis Spf Sprague-Dawley rats | Ad5IL-10 (5 × 108-1 × 1010 pfu) | Improved colitis macroscopically and histologically and decreased MPO activity and LTB4 levels |
| Grool et al[123] | 40 male NZ white rabbits formalin-immune complex induced colitis | 100 or 500 μg/kg single IV infusion of rIL-10 | Anti-inflammatory response as measured by decreased mucosal damage, leukocyte recruitment, MPO and LTB4 |
| Ribbons et al[124] | TNBS induced colitis in 74 Sprague-Dawley rats | 0.5, 5, 50, 500 μg/kg rIL-10 subcutaneous injection twice daily for 5 d | Mild anti-inflammatory effects Significant reduction in MPO |
| Sasaki et al[125] | 3% DSS induced C57B6 mice | Intra-peritoneal administration of adIL-10 | Significantly reduced disease activity and weight loss and completely prevented histopathologic injury to the colon |
| Tomoyose et al[126] | 4% DSS induced colitis BALB/c mice | Recombinant mouse rIL-10 (1, 100, 1000 unit/mL) | Marked improvement in intestinal inflammation Inhibition of tissue damage and production of pro-inflammatory cytokines |
| Steidler et al[127] | DSS induced and spontaneous IL10-/- mouse models of colitis | Daily intragastric inocula of 2 × 107 or 109 LL-mIL10 | Reduced histological score by 50% in DSS and prevented onset of colitis in IL-10-/- mice |
These data show that IL-10 treatment did not result in significantly reduced remission rates or clinical improvements when compared to placebo[21,24]. In fact a Cochrane review in 2010[21] concluded that “Interleukin 10 does not appear to provide any treatment of active Crohn’s disease. ...interleukin 10 does not increase the number of remissions (complete or clinical), but increases the rate of withdrawal due to adverse events relative to placebo.” This review only included three of the studies mentioned above[23,26,28] and although more patients receiving IL-10 withdrew from studies there was no significant difference in the number of patients reporting adverse reactions between treatment and control.
However this is not the whole story, as other studies not included in the Cochrane analysis have shown that patients respond differently to IL-10 supplementation. Colombel et al[22] reported that endoscopic recurrence in patients with low IL-10 levels at time of surgery reduced to 47% with Tenovil treatment compared to 80% in the placebo group. Schreiber et al[26] found that patients responded differently to IL-10 treatment, with patients suffering from high disease activity having a greater rate of clinical improvement. These data suggest that IL-10 levels and disease activity are factors in how a patient responds to IL-10 supplementation. Also, as previously stated, some CD patients already have raised levels of IL-10[18,82]. These patients will not benefit from IL-10 supplementation and may suffer detrimental effects as high doses of systemically administered IL-10 induce the pro-inflammatory cytokine IFN-γ[27].
The different response to IL-10 supplementation is not surprising given the heterogenous nature of CD. A therapy that targets one step within a complex immunological pathway may only benefit a small proportion of patients but if you select the correct sub-population of patients who under-produce IL-10, for example those that have a penetrating phenotype who have a greater deficiency in IL-10[78], you may see a significant beneficial response to IL-10 therapy[25].
There are five potential explanations as to why IL-10 treatment has not been effective as a therapeutic strategy: (1) the administered dose of IL-10 results in an intestinal concentration of IL-10 that is too low to elicit a response; (2) there are differences among individuals depending upon disease phenotype/severity; (3) IL-10 is only successful at preventing and not treating an established disease; (4) IL-10 alone fails to suppress all the pro-inflammatory mediators involved in chronic inflammation; or (5) IL-10’s immunostimulatory effects counterbalance its immunosuppressive properties.
Most of the potential explanations as to why IL-10 supplementation currently doesn’t work can be overcome.
The modest therapeutic benefits[23,26] and adverse effects can potentially be attributed to limited mucosal bioavailability of IL-10 and the fact that the trials so far have not separated patients by genotype or disease phenotype/severity. To address the low bioavailability of mucosal IL-10 without resorting to the detrimental high levels of IL-10 systemic administration, Lactococcus lactis (L. lactis) was engineered to secrete IL-10, and this was used to successfully prevent the onset of colitis in the IL-10 KO model and caused a 50% reduction in inflammation in the DSS mouse model[127]. This study was followed up with a small phase 1 human trial using L. lactis modified to contain the human IL-10 sequence (LL-Thy12)[128]. 10 capsules containing 1 × 1010 cfu of LL-Thy12 were given to 10 patients with moderate to severe CD twice daily for 7 d. The results showed this approach is both safe and biologically contained, avoiding the side effects associated with high systemic doses while still retaining the ability to reduce disease activity. This was a small trial in a controlled environment without a control comparison and so further studies are needed to confirm the effectiveness of this treatment. However based on these initial results this form of IL-10 supplementation is showing promise as a treatment for patients with chronic intestinal inflammation.
Alternative ways to improve local delivery of IL-10 include gene therapy using replication-deficient adenoviral vectors delivered directly to the gastrointestinal epithelial cells. This approach has proven successful in two mouse studies[121,129] showing an effect on colitis without the associated side-effects of systemic administration. Gelatine microspheres containing IL-10 (GM-IL-10) were developed by Nakase et al[130] to deliver sustained IL-10 release locally without losing bioactivity. Colonic inflammation in mice treated with GM-IL-10 was reduced compared to mice treated with IL-10 alone.
The second point of selecting patients based on disease phenotype and/or severity can be easily addressed based on clinical diagnosis. Selecting patients based on genotype is slightly more complicated and would require that potential candidates for treatment be screened. Genotyping has become relatively quick and easy to perform and the cost is reducing as the technology advances. However who actually performs the genotyping service, who pays for the service and gaining patient consent may be problematic. This could potentially be overcome by having the patients enrol onto a research study. However IL-10 serum levels are determined 50% by genetics and 50% by environment[108] and so just because a person has the low IL-10 producing SNP doesn’t necessarily mean they will have low IL-10 levels. Therefore a better measure to determine potential benefit of IL-10 supplementation would be to measure the serum level of IL-10, which can be done using commercially available ELISA kits. This should prove to be easier to conduct and in gaining patient consent.
As mouse models proved[121,131] IL-10 administration was only successful when administered prior to initiation of colitis and was unable to treat any established inflammation. Therefore IL-10 supplementation could be used to prevent relapses rather than to treat active inflammation.
If locally delivered IL-10 fails to have an effect, then it may be due to the fact IL-10 alone is unable to suppress all the pro-inflammatory mediators involved in chronic inflammation.Therefore it would be necessary to develop a combination treatment containing IL-10. However the evidence suggests IL-10 alone should have an effect and so it may be that IL-10 supplementation is not a suitable treatment for that disease phenotype.
Based on this knowledge, it is our opinion that a subpopulation of CD patients, who have lower expression of IL-10, and who have active disease could benefit from targeted IL-10 supplementation therapy. However further studies are needed to determine the exact population of patients who would benefit the most from this treatment and to determine if there are any long term detrimental effects of this treatment. Given that current treatments of IBD may not be beneficial to a patient or have severe side effects, we believe it is worth exploring this potential treatment avenue.
P- Reviewers Bamias GT, Cong YZ, Fitzpatrick LR, Swaminath A S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2894] [Cited by in F6Publishing: 3117] [Article Influence: 183.4] [Reference Citation Analysis (8)] |
| 2. | Paul G, Khare V, Gasche C. Inflamed gut mucosa: downstream of interleukin-10. Eur J Clin Invest. 2012;42:95-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Cooney R, Jewell D. The genetic basis of inflammatory bowel disease. Dig Dis. 2009;27:428-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Glocker EO, Kotlarz D, Klein C, Shah N, Grimbacher B. IL-10 and IL-10 receptor defects in humans. Ann N Y Acad Sci. 2011;1246:102-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 189] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 5. | Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590-1605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1347] [Cited by in F6Publishing: 1376] [Article Influence: 114.7] [Reference Citation Analysis (0)] |
| 6. | Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8:458-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 666] [Cited by in F6Publishing: 663] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 7. | Imielinski M, Baldassano RN, Griffiths A, Russell RK, Annese V, Dubinsky M, Kugathasan S, Bradfield JP, Walters TD, Sleiman P. Common variants at five new loci associated with early-onset inflammatory bowel disease. Nat Genet. 2009;41:1335-1340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 365] [Cited by in F6Publishing: 379] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 8. | Reiff C, Kelly D. Inflammatory bowel disease, gut bacteria and probiotic therapy. Int J Med Microbiol. 2010;300:25-33. [PubMed] [Cited in This Article: ] |
| 9. | Geier MS, Butler RN, Howarth GS. Inflammatory bowel disease: current insights into pathogenesis and new therapeutic options; probiotics, prebiotics and synbiotics. Int J Food Microbiol. 2007;115:1-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Loftus EV, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:1-20. [PubMed] [Cited in This Article: ] |
| 11. | Shanahan F. Probiotics in inflammatory bowel disease--therapeutic rationale and role. Adv Drug Deliv Rev. 2004;56:809-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3465] [Cited by in F6Publishing: 3323] [Article Influence: 276.9] [Reference Citation Analysis (0)] |
| 13. | Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42:1118-1125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1894] [Cited by in F6Publishing: 1924] [Article Influence: 137.4] [Reference Citation Analysis (0)] |
| 14. | Lees CW, Barrett JC, Parkes M, Satsangi J. New IBD genetics: common pathways with other diseases. Gut. 2011;60:1739-1753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 406] [Cited by in F6Publishing: 420] [Article Influence: 32.3] [Reference Citation Analysis (1)] |
| 15. | Franke A, Balschun T, Karlsen TH, Sventoraityte J, Nikolaus S, Mayr G, Domingues FS, Albrecht M, Nothnagel M, Ellinghaus D. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet. 2008;40:1319-1323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 434] [Cited by in F6Publishing: 452] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 16. | Aithal GP, Craggs A, Day CP, Welfare M, Daly AK, Mansfield JC, Hudson M. Role of polymorphisms in the interleukin-10 gene in determining disease susceptibility and phenotype in inflamatory bowel disease. Dig Dis Sci. 2001;46:1520-1525. [PubMed] [Cited in This Article: ] |
| 17. | Amre DK, Mack DR, Morgan K, Israel D, Lambrette P, Costea I, Krupoves A, Fegury H, Dong J, Grimard G. Interleukin 10 (IL-10) gene variants and susceptibility for paediatric onset Crohn’s disease. Aliment Pharmacol Ther. 2009;29:1025-1031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Wang AH, Lam WJ, Han DY, Ding Y, Hu R, Fraser AG, Ferguson LR, Morgan AR. The effect of IL-10 genetic variation and interleukin 10 serum levels on Crohn’s disease susceptibility in a New Zealand population. Hum Immunol. 2011;72:431-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy--review of a new approach. Pharmacol Rev. 2003;55:241-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 682] [Cited by in F6Publishing: 665] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 20. | Braat H, Peppelenbosch MP, Hommes DW. Interleukin-10-based therapy for inflammatory bowel disease. Expert Opin Biol Ther. 2003;3:725-731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Buruiana FE, Solà I, Alonso-Coello P. Recombinant human interleukin 10 for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2010;CD005109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Colombel JF, Rutgeerts P, Malchow H, Jacyna M, Nielsen OH, Rask-Madsen J, Van Deventer S, Ferguson A, Desreumaux P, Forbes A. Interleukin 10 (Tenovil) in the prevention of postoperative recurrence of Crohn’s disease. Gut. 2001;49:42-46. [PubMed] [Cited in This Article: ] |
| 23. | Fedorak RN, Gangl A, Elson CO, Rutgeerts P, Schreiber S, Wild G, Hanauer SB, Kilian A, Cohard M, LeBeaut A. Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn’s disease. The Interleukin 10 Inflammatory Bowel Disease Cooperative Study Group. Gastroenterology. 2000;119:1473-1482. [PubMed] [Cited in This Article: ] |
| 24. | Herfarth H, Schölmerich J. IL-10 therapy in Crohn’s disease: at the crossroads. Treatment of Crohn’s disease with the anti-inflammatory cytokine interleukin 10. Gut. 2002;50:146-147. [PubMed] [Cited in This Article: ] |
| 25. | Lindsay JO, Hodgson HJ. Review article: the immunoregulatory cytokine interleukin-10--a therapy for Crohn’s disease? Aliment Pharmacol Ther. 2001;15:1709-1716. [PubMed] [Cited in This Article: ] |
| 26. | Schreiber S, Fedorak RN, Nielsen OH, Wild G, Williams CN, Nikolaus S, Jacyna M, Lashner BA, Gangl A, Rutgeerts P. Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn’s disease. Crohn’s Disease IL-10 Cooperative Study Group. Gastroenterology. 2000;119:1461-1472. [PubMed] [Cited in This Article: ] |
| 27. | Tilg H, van Montfrans C, van den Ende A, Kaser A, van Deventer SJ, Schreiber S, Gregor M, Ludwiczek O, Rutgeerts P, Gasche C. Treatment of Crohn’s disease with recombinant human interleukin 10 induces the proinflammatory cytokine interferon gamma. Gut. 2002;50:191-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 182] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | van Deventer SJ, Elson CO, Fedorak RN. Multiple doses of intravenous interleukin 10 in steroid-refractory Crohn’s disease. Crohn’s Disease Study Group. Gastroenterology. 1997;113:383-389. [PubMed] [Cited in This Article: ] |
| 29. | Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081-2095. [PubMed] [Cited in This Article: ] |
| 30. | Mocellin S, Marincola F, Rossi CR, Nitti D, Lise M. The multifaceted relationship between IL-10 and adaptive immunity: putting together the pieces of a puzzle. Cytokine Growth Factor Rev. 2004;15:61-76. [PubMed] [Cited in This Article: ] |
| 31. | Zdanov A, Schalk-Hihi C, Gustchina A, Tsang M, Weatherbee J, Wlodawer A. Crystal structure of interleukin-10 reveals the functional dimer with an unexpected topological similarity to interferon gamma. Structure. 1995;3:591-601. [PubMed] [Cited in This Article: ] |
| 32. | Ho AS, Liu Y, Khan TA, Hsu DH, Bazan JF, Moore KW. A receptor for interleukin 10 is related to interferon receptors. Proc Natl Acad Sci USA. 1993;90:11267-11271. [PubMed] [Cited in This Article: ] |
| 33. | Mosmann TR. Properties and functions of interleukin-10. Adv Immunol. 1994;56:1-26. [PubMed] [Cited in This Article: ] |
| 34. | de Moreno de Leblanc A, Del Carmen S, Zurita-Turk M, Santos Rocha C, van de Guchte M, Azevedo V, Miyoshi A, Leblanc JG. Importance of IL-10 modulation by probiotic microorganisms in gastrointestinal inflammatory diseases. ISRN Gastroenterol. 2011;2011:892971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Schottelius AJ, Baldwin AS. A role for transcription factor NF-kappa B in intestinal inflammation. Int J Colorectal Dis. 1999;14:18-28. [PubMed] [Cited in This Article: ] |
| 36. | Dijkstra G, Moshage H, Jansen PL. Blockade of NF-kappaB activation and donation of nitric oxide: new treatment options in inflammatory bowel disease? Scand J Gastroenterol Suppl. 2002;37-41. [PubMed] [Cited in This Article: ] |
| 37. | Schottelius AJ, Mayo MW, Sartor RB, Baldwin AS. Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. J Biol Chem. 1999;274:31868-31874. [PubMed] [Cited in This Article: ] |
| 38. | Wang P, Wu P, Siegel MI, Egan RW, Billah MM. Interleukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J Biol Chem. 1995;270:9558-9563. [PubMed] [Cited in This Article: ] |
| 39. | Driessler F, Venstrom K, Sabat R, Asadullah K, Schottelius AJ. Molecular mechanisms of interleukin-10-mediated inhibition of NF-kappaB activity: a role for p50. Clin Exp Immunol. 2004;135:64-73. [PubMed] [Cited in This Article: ] |
| 40. | Koppelman B, Neefjes JJ, de Vries JE, de Waal Malefyt R. Interleukin-10 down-regulates MHC class II alphabeta peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity. 1997;7:861-871. [PubMed] [Cited in This Article: ] |
| 41. | Willems F, Marchant A, Delville JP, Gérard C, Delvaux A, Velu T, de Boer M, Goldman M. Interleukin-10 inhibits B7 and intercellular adhesion molecule-1 expression on human monocytes. Eur J Immunol. 1994;24:1007-1009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 289] [Cited by in F6Publishing: 302] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 42. | Chan LL, Cheung BK, Li JC, Lau AS. A role for STAT3 and cathepsin S in IL-10 down-regulation of IFN-gamma-induced MHC class II molecule on primary human blood macrophages. J Leukoc Biol. 2010;88:303-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224-1234. [PubMed] [Cited in This Article: ] |
| 44. | Buelens C, Willems F, Delvaux A, Piérard G, Delville JP, Velu T, Goldman M. Interleukin-10 differentially regulates B7-1 (CD80) and B7-2 (CD86) expression on human peripheral blood dendritic cells. Eur J Immunol. 1995;25:2668-2672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 196] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 45. | McBride JM, Jung T, de Vries JE, Aversa G. IL-10 alters DC function via modulation of cell surface molecules resulting in impaired T-cell responses. Cell Immunol. 2002;215:162-172. [PubMed] [Cited in This Article: ] |
| 46. | de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209-1220. [PubMed] [Cited in This Article: ] |
| 47. | Del Prete G, De Carli M, Almerigogna F, Giudizi MG, Biagiotti R, Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993;150:353-360. [PubMed] [Cited in This Article: ] |
| 48. | Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815-3822. [PubMed] [Cited in This Article: ] |
| 49. | Galbas T, Steimle V, Lapointe R, Ishido S, Thibodeau J. MARCH1 down-regulation in IL-10-activated B cells increases MHC class II expression. Cytokine. 2012;59:27-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 50. | Rousset F, Garcia E, Defrance T, Péronne C, Vezzio N, Hsu DH, Kastelein R, Moore KW, Banchereau J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci USA. 1992;89:1890-1893. [PubMed] [Cited in This Article: ] |
| 51. | Glocker EO, Frede N, Perro M, Sebire N, Elawad M, Shah N, Grimbacher B. Infant colitis--it’s in the genes. Lancet. 2010;376:1272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 52. | Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schäffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033-2045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1121] [Cited by in F6Publishing: 1015] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 53. | Donnelly RP, Dickensheets H, Finbloom DS. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J Interferon Cytokine Res. 1999;19:563-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 320] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 54. | Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4725] [Cited by in F6Publishing: 4743] [Article Influence: 206.2] [Reference Citation Analysis (0)] |
| 55. | Williams LM, Ricchetti G, Sarma U, Smallie T, Foxwell BM. Interleukin-10 suppression of myeloid cell activation--a continuing puzzle. Immunology. 2004;113:281-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 151] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 56. | Li Y, de Haar C, Peppelenbosch MP, van der Woude CJ. New insights into the role of STAT3 in IBD. Inflamm Bowel Dis. 2012;18:1177-1183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 57. | Atreya R, Mudter J, Finotto S, Müllberg J, Jostock T, Wirtz S, Schütz M, Bartsch B, Holtmann M, Becker C. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 945] [Cited by in F6Publishing: 981] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 58. | Ohta N, Hiroi T, Kweon MN, Kinoshita N, Jang MH, Mashimo T, Miyazaki J, Kiyono H. IL-15-dependent activation-induced cell death-resistant Th1 type CD8 alpha beta+NK1.1+ T cells for the development of small intestinal inflammation. J Immunol. 2002;169:460-468. [PubMed] [Cited in This Article: ] |
| 59. | Siegmund B, Lehr HA, Fantuzzi G. Leptin: a pivotal mediator of intestinal inflammation in mice. Gastroenterology. 2002;122:2011-2025. [PubMed] [Cited in This Article: ] |
| 60. | Fitzpatrick LR. Novel Pharmacological Approaches for Inflammatory Bowel Disease: Targeting Key Intracellular Pathways and the IL-23/IL-17 Axis. Int J Inflam. 2012;2012:389404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Sands BE, Bank S, Sninsky CA, Robinson M, Katz S, Singleton JW, Miner PB, Safdi MA, Galandiuk S, Hanauer SB, Varilek GW, Buchman AL, Rodgers VD, Salzberg B, Cai B, Loewy J, DeBruin MF, Rogge H, Shapiro M, Schwertschlag US. Preliminary evaluation of safety and activity of recombinant human interleukin 11 in patients with active Crohn’s disease. Gastroenterology. 1999;117:58-64. [PubMed] [Cited in This Article: ] |
| 62. | Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534-544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 525] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 63. | Williams KL, Fuller CR, Dieleman LA, DaCosta CM, Haldeman KM, Sartor RB, Lund PK. Enhanced survival and mucosal repair after dextran sodium sulfate-induced colitis in transgenic mice that overexpress growth hormone. Gastroenterology. 2001;120:925-937. [PubMed] [Cited in This Article: ] |
| 64. | Williams L, Bradley L, Smith A, Foxwell B. Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. J Immunol. 2004;172:567-576. [PubMed] [Cited in This Article: ] |
| 65. | Sugimoto K. Role of STAT3 in inflammatory bowel disease. World J Gastroenterol. 2008;14:5110-5114. [PubMed] [Cited in This Article: ] |
| 66. | Hruz P, Dann SM, Eckmann L. STAT3 and its activators in intestinal defense and mucosal homeostasis. Curr Opin Gastroenterol. 2010;26:109-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | El Kasmi KC, Holst J, Coffre M, Mielke L, de Pauw A, Lhocine N, Smith AM, Rutschman R, Kaushal D, Shen Y. General nature of the STAT3-activated anti-inflammatory response. J Immunol. 2006;177:7880-7888. [PubMed] [Cited in This Article: ] |
| 68. | Murray PJ. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr Opin Pharmacol. 2006;6:379-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 320] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 69. | Niemand C, Nimmesgern A, Haan S, Fischer P, Schaper F, Rossaint R, Heinrich PC, Müller-Newen G. Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. J Immunol. 2003;170:3263-3272. [PubMed] [Cited in This Article: ] |
| 70. | Lovato P, Brender C, Agnholt J, Kelsen J, Kaltoft K, Svejgaard A, Eriksen KW, Woetmann A, Ødum N. Constitutive STAT3 activation in intestinal T cells from patients with Crohn’s disease. J Biol Chem. 2003;278:16777-16781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 71. | Mudter J, Weigmann B, Bartsch B, Kiesslich R, Strand D, Galle PR, Lehr HA, Schmidt J, Neurath MF. Activation pattern of signal transducers and activators of transcription (STAT) factors in inflammatory bowel diseases. Am J Gastroenterol. 2005;100:64-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 182] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 72. | Musso A, Dentelli P, Carlino A, Chiusa L, Repici A, Sturm A, Fiocchi C, Rizzetto M, Pegoraro L, Sategna-Guidetti C. Signal transducers and activators of transcription 3 signaling pathway: an essential mediator of inflammatory bowel disease and other forms of intestinal inflammation. Inflamm Bowel Dis. 2005;11:91-98. [PubMed] [Cited in This Article: ] |
| 73. | Andersen V, Ernst A, Christensen J, Ostergaard M, Jacobsen B, Tjonneland A, Krarup H, Vogel U. The polymorphism rs3024505 proximal to IL-10 is associated with risk of ulcerative colitis and Crohns disease in a Danish case-control study. BMC Medical Genetics. 2010;11:82. [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 74. | Sanchez R, Levy E, Costea F, Sinnett D. IL-10 and TNF-alpha promoter haplotypes are associated with childhood Crohn’s disease location. World J Gastroenterol. 2009;15:3776-3782. [PubMed] [Cited in This Article: ] |
| 75. | Begue B, Verdier J, Rieux-Laucat F, Goulet O, Morali A, Canioni D, Hugot JP, Daussy C, Verkarre V, Pigneur B. Defective IL10 signaling defining a subgroup of patients with inflammatory bowel disease. Am J Gastroenterol. 2011;106:1544-1555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 203] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 76. | Ishizuka K, Sugimura K, Homma T, Matsuzawa J, Mochizuki T, Kobayashi M, Suzuki K, Otsuka K, Tashiro K, Yamaguchi O. Influence of interleukin-10 on the interleukin-1 receptor antagonist/interleukin-1 beta ratio in the colonic mucosa of ulcerative colitis. Digestion. 2001;63 Suppl 1:22-27. [PubMed] [Cited in This Article: ] |
| 77. | Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3189] [Cited by in F6Publishing: 3127] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 78. | Correa I, Veny M, Esteller M, Piqué JM, Yagüe J, Panés J, Salas A. Defective IL-10 production in severe phenotypes of Crohn’s disease. J Leukoc Biol. 2009;85:896-903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 79. | Schreiber S, Heinig T, Thiele HG, Raedler A. Immunoregulatory role of interleukin 10 in patients with inflammatory bowel disease. Gastroenterology. 1995;108:1434-1444. [PubMed] [Cited in This Article: ] |
| 80. | Gasche C, Bakos S, Dejaco C, Tillinger W, Zakeri S, Reinisch W. IL-10 secretion and sensitivity in normal human intestine and inflammatory bowel disease. J Clin Immunol. 2000;20:362-370. [PubMed] [Cited in This Article: ] |
| 81. | Nielsen OH, Køppen T, Rüdiger N, Horn T, Eriksen J, Kirman I. Involvement of interleukin-4 and -10 in inflammatory bowel disease. Dig Dis Sci. 1996;41:1786-1793. [PubMed] [Cited in This Article: ] |
| 82. | Kucharzik T, Stoll R, Lügering N, Domschke W. Circulating antiinflammatory cytokine IL-10 in patients with inflammatory bowel disease (IBD). Clin Exp Immunol. 1995;100:452-456. [PubMed] [Cited in This Article: ] |
| 83. | Meresse B, Rutgeerts P, Malchow H, Dubucquoi S, Dessaint JP, Cohard M, Colombel JF, Desreumaux P. Low ileal interleukin 10 concentrations are predictive of endoscopic recurrence in patients with Crohn’s disease. Gut. 2002;50:25-28. [PubMed] [Cited in This Article: ] |
| 84. | Melgar S, Yeung MM, Bas A, Forsberg G, Suhr O, Oberg A, Hammarstrom S, Danielsson A, Hammarstrom ML. Over-expression of interleukin 10 in mucosal T cells of patients with active ulcerative colitis. Clin Exp Immunol. 2003;134:127-137. [PubMed] [Cited in This Article: ] |
| 85. | Mitsuyama K, Tomiyasu N, Takaki K, Masuda J, Yamasaki H, Kuwaki K, Takeda T, Kitazaki S, Tsuruta O, Sata M. Interleukin-10 in the pathophysiology of inflammatory bowel disease: increased serum concentrations during the recovery phase. Mediators Inflamm. 2006;2006:26875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 86. | Ljuca F, Gegic A, Salkic NN, Pavlovic-Calic N. Circulating cytokines reflect mucosal inflammatory status in patients with Crohn’s disease. Dig Dis Sci. 2010;55:2316-2326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 87. | Santaolalla R, Mañé J, Pedrosa E, Lorén V, Fernández-Bañares F, Mallolas J, Carrasco A, Salas A, Rosinach M, Forné M. Apoptosis resistance of mucosal lymphocytes and IL-10 deficiency in patients with steroid-refractory Crohn’s disease. Inflamm Bowel Dis. 2011;17:1490-1500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 88. | Borska L, Andrys C, Krejsek J, Hamakova K, Kremlacek J, Ettler K, Fiala Z. Serum levels of the pro-inflammatory cytokine interleukin-12 and the anti-inflammatory cytokine interleukin-10 in patients with psoriasis treated by the Goeckerman regimen. Int J Dermatol. 2008;47:800-805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 89. | Ying B, Shi Y, Pan X, Song X, Huang Z, Niu Q, Cai B, Wang L. Association of polymorphisms in the human IL-10 and IL-18 genes with rheumatoid arthritis. Mol Biol Rep. 2011;38:379-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 90. | Szkaradkiewicz A, Marciniak R, Chudzicka-Strugała I, Wasilewska A, Drews M, Majewski P, Karpiński T, Zwoździak B. Proinflammatory cytokines and IL-10 in inflammatory bowel disease and colorectal cancer patients. Arch Immunol Ther Exp (Warsz). 2009;57:291-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 91. | Roda G, Marocchi M, Sartini A, Roda E. Cytokine Networks in Ulcerative Colitis. Ulcers. 2011;2011:391787. [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 92. | Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008;14:4280-4288. [PubMed] [Cited in This Article: ] |
| 93. | Settin AA, Hassan HA, El-Baz RA, Hassan TA. Association of cytokine gene polymorphisms with psoriasis in cases from the nile delta of egypt. Indian J Dermatol. 2011;56:272-277. [PubMed] [Cited in This Article: ] |
| 94. | Heiskanen M, Kähönen M, Hurme M, Lehtimäki T, Mononen N, Juonala M, Hutri-Kähönen N, Viikari J, Raitakari O, Hulkkonen J. Polymorphism in the IL10 promoter region and early markers of atherosclerosis: the Cardiovascular Risk in Young Finns Study. Atherosclerosis. 2010;208:190-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 95. | McCarron SL, Edwards S, Evans PR, Gibbs R, Dearnaley DP, Dowe A, Southgate C, Easton DF, Eeles RA, Howell WM. Influence of cytokine gene polymorphisms on the development of prostate cancer. Cancer Res. 2002;62:3369-3372. [PubMed] [Cited in This Article: ] |
| 96. | Giordani L, Bruzzi P, Lasalandra C, Quaranta M, Schittulli F, Della Ragione F, Iolascon A. Association of breast cancer and polymorphisms of interleukin-10 and tumor necrosis factor-alpha genes. Clin Chem. 2003;49:1664-1667. [PubMed] [Cited in This Article: ] |
| 97. | Stanczuk GA, Sibanda EN, Perrey C, Chirara M, Pravica V, Hutchinson IV, Tswana SA. Cancer of the uterine cervix may be significantly associated with a gene polymorphism coding for increased IL-10 production. Int J Cancer. 2001;94:792-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 98. | Ni P, Xu H, Xue H, Lin B, Lu Y. A meta-analysis of interleukin-10-1082 promoter polymorphism associated with gastric cancer risk. DNA Cell Biol. 2012;31:582-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 99. | Xue H, Lin B, An J, Zhu Y, Huang G. Interleukin-10-819 promoter polymorphism in association with gastric cancer risk. BMC Cancer. 2012;12:102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 100. | Wang DG, Fan JB, Siao CJ, Berno A, Young P, Sapolsky R, Ghandour G, Perkins N, Winchester E, Spencer J. Large-scale identification, mapping, and genotyping of single-nucleotide polymorphisms in the human genome. Science. 1998;280:1077-1082. [PubMed] [Cited in This Article: ] |
| 101. | Eskdale J, Gallagher G, Verweij CL, Keijsers V, Westendorp RG, Huizinga TW. Interleukin 10 secretion in relation to human IL-10 locus haplotypes. Proc Natl Acad Sci USA. 1998;95:9465-9470. [PubMed] [Cited in This Article: ] |
| 102. | Turner DM, Williams DM, Sankaran D, Lazarus M, Sinnott PJ, Hutchinson IV. An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immunogenet. 1997;24:1-8. [PubMed] [Cited in This Article: ] |
| 103. | Fowler EV, Eri R, Hume G, Johnstone S, Pandeya N, Lincoln D, Templeton D, Radford-Smith GL. TNFalpha and IL10 SNPs act together to predict disease behaviour in Crohn’s disease. J Med Genet. 2005;42:523-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 104. | Fernandez L, Martinez A, Mendoza JL, Urcelay E, Fernandez-Arquero M, Garcia-Paredes J, Diaz-Rubio M, de la Concha EG. Interleukin-10 polymorphisms in Spanish patients with IBD. Inflamm Bowel Dis. 2005;11:739-743. [PubMed] [Cited in This Article: ] |
| 105. | Tedde A, Laura Putignano A, Bagnoli S, Congregati C, Milla M, Sorbi S, Genuardi M, Papi L. Interleukin-10 promoter polymorphisms influence susceptibility to ulcerative colitis in a gender-specific manner. Scand J Gastroenterol. 2008;43:712-718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 106. | Tagore A, Gonsalkorale WM, Pravica V, Hajeer AH, McMahon R, Whorwell PJ, Sinnott PJ, Hutchinson IV. Interleukin-10 (IL-10) genotypes in inflammatory bowel disease. Tissue Antigens. 1999;54:386-390. [PubMed] [Cited in This Article: ] |
| 107. | Koss K, Satsangi J, Fanning GC, Welsh KI, Jewell DP. Cytokine (TNF alpha, LT alpha and IL-10) polymorphisms in inflammatory bowel diseases and normal controls: differential effects on production and allele frequencies. Genes Immun. 2000;1:185-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 164] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 108. | Reuss E, Fimmers R, Kruger A, Becker C, Rittner C, Höhler T. Differential regulation of interleukin-10 production by genetic and environmental factors--a twin study. Genes Immun. 2002;3:407-413. [PubMed] [Cited in This Article: ] |
| 109. | Crawley E, Kay R, Sillibourne J, Patel P, Hutchinson I, Woo P. Polymorphic haplotypes of the interleukin-10 5’ flanking region determine variable interleukin-10 transcription and are associated with particular phenotypes of juvenile rheumatoid arthritis. Arthritis Rheum. 1999;42:1101-1108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 110. | Looijer-van Langen MA, Dieleman LA. Prebiotics in chronic intestinal inflammation. Inflamm Bowel Dis. 2009;15:454-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 111. | Pithadia AB, Jain S. Treatment of inflammatory bowel disease (IBD). Pharmacol Rep. 2011;63:629-642. [PubMed] [Cited in This Article: ] |
| 112. | Ferguson LR, Philpott M, Dryland P. Nutrigenomics in the whole-genome scanning era: Crohn’s disease as example. Cell Mol Life Sci. 2007;64:3105-3118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 113. | Ferguson LR, Shelling AN, Browning BL, Huebner C, Petermann I. Genes, diet and inflammatory bowel disease. Mutat Res. 2007;622:70-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 114. | Triggs CM, Munday K, Hu R, Fraser AG, Gearry RB, Barclay ML, Ferguson LR. Dietary factors in chronic inflammation: food tolerances and intolerances of a New Zealand Caucasian Crohn’s disease population. Mutat Res. 2010;690:123-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 115. | Joachim G. Responses of people with inflammatory bowel disease to foods consumed. Gastroenterol Nurs. 2000;23:160-167. [PubMed] [Cited in This Article: ] |
| 116. | Jowett SL, Seal CJ, Pearce MS, Phillips E, Gregory W, Barton JR, Welfare MR. Influence of dietary factors on the clinical course of ulcerative colitis: a prospective cohort study. Gut. 2004;53:1479-1484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 307] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 117. | Jowett SL, Seal CJ, Phillips E, Gregory W, Barton JR, Welfare MR. Dietary beliefs of people with ulcerative colitis and their effect on relapse and nutrient intake. Clin Nutr. 2004;23:161-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 118. | Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106:563-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 590] [Cited by in F6Publishing: 607] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 119. | McHutchison JG, Giannelli G, Nyberg L, Blatt LM, Waite K, Mischkot P, Pianko S, Conrad A, Grint P. A pilot study of daily subcutaneous interleukin-10 in patients with chronic hepatitis C infection. J Interferon Cytokine Res. 1999;19:1265-1270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 120. | Rosenblum IY, Johnson RC, Schmahai TJ. Preclinical safety evaluation of recombinant human interleukin-10. Regul Toxicol Pharmacol. 2002;35:56-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 121. | Barbara G, Xing Z, Hogaboam CM, Gauldie J, Collins SM. Interleukin 10 gene transfer prevents experimental colitis in rats. Gut. 2000;46:344-349. [PubMed] [Cited in This Article: ] |
| 122. | Duchmann R, Schmitt E, Knolle P, Meyer zum Büschenfelde KH, Neurath M. Tolerance towards resident intestinal flora in mice is abrogated in experimental colitis and restored by treatment with interleukin-10 or antibodies to interleukin-12. Eur J Immunol. 1996;26:934-938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 273] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 123. | Grool TA, van Dullemen H, Meenan J, Koster F, ten Kate FJ, Lebeaut A, Tytgat GN, van Deventer SJ. Anti-inflammatory effect of interleukin-10 in rabbit immune complex-induced colitis. Scand J Gastroenterol. 1998;33:754-758. [PubMed] [Cited in This Article: ] |
| 124. | Ribbons KA, Thompson JH, Liu X, Pennline K, Clark DA, Miller MJ. Anti-inflammatory properties of interleukin-10 administration in hapten-induced colitis. Eur J Pharmacol. 1997;323:245-254. [PubMed] [Cited in This Article: ] |
| 125. | Sasaki M, Mathis JM, Jennings MH, Jordan P, Wang Y, Ando T, Joh T, Alexander JS. Reversal of experimental colitis disease activity in mice following administration of an adenoviral IL-10 vector. J Inflamm (Lond). 2005;2:13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 126. | Tomoyose M, Mitsuyama K, Ishida H, Toyonaga A, Tanikawa K. Role of interleukin-10 in a murine model of dextran sulfate sodium-induced colitis. Scand J Gastroenterol. 1998;33:435-440. [PubMed] [Cited in This Article: ] |
| 127. | Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, Fiers W, Remaut E. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352-1355. [PubMed] [Cited in This Article: ] |
| 128. | Braat H, Rottiers P, Hommes DW, Huyghebaert N, Remaut E, Remon JP, van Deventer SJ, Neirynck S, Peppelenbosch MP, Steidler L. A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:754-759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 531] [Cited by in F6Publishing: 550] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 129. | Lindsay JO, Ciesielski CJ, Scheinin T, Brennan FM, Hodgson HJ. Local delivery of adenoviral vectors encoding murine interleukin 10 induces colonic interleukin 10 production and is therapeutic for murine colitis. Gut. 2003;52:363-369. [PubMed] [Cited in This Article: ] |
| 130. | Nakase H, Okazaki K, Tabata Y, Ozeki M, Watanabe N, Ohana M, Uose S, Uchida K, Nishi T, Mastuura M. New cytokine delivery system using gelatin microspheres containing interleukin-10 for experimental inflammatory bowel disease. J Pharmacol Exp Ther. 2002;301:59-65. [PubMed] [Cited in This Article: ] |
| 131. | Herfarth HH, Böcker U, Janardhanam R, Sartor RB. Subtherapeutic corticosteroids potentiate the ability of interleukin 10 to prevent chronic inflammation in rats. Gastroenterology. 1998;115:856-865. [PubMed] [Cited in This Article: ] |