Published online May 28, 2013. doi: 10.3748/wjg.v19.i20.3043
Revised: March 26, 2013
Accepted: April 9, 2013
Published online: May 28, 2013
AIM: To evaluate the association between Helicobacter pylori (H. pylori) infection and MLH1 and MGMT methylation and its relationship with microsatellite instability (MSI).
METHODS: The methylation status of the MLH1 and MGMT promoter region was analysed by methylation specific methylation-polymerase chain reaction (MSP-PCR) in gastric biopsy samples from uninfected or H. pylori-infected children (n = 50), from adults with chronic gastritis (n = 97) and from adults with gastric cancer (n = 92). MLH1 and MGMT mRNA expression were measured by real-time PCR and normalised to a constitutive gene (β actin). MSI analysis was performed by screening MSI markers at 4 loci (Bat-25, Bat-26, D17S250 and D2S123) with PCR; PCR products were analysed by single strand conformation polymorphism followed by silver staining. Statistical analyses were performed with either the χ2 test with Yates continuity correction or Fisher’s exact test, and statistical significance for expression analysis was assessed using an unpaired Student’s t-test.
RESULTS: Methylation was not detected in the promoter regions of MLH1 and MGMT in gastric biopsy samples from children, regardless of H. pylori infection status. The MGMT promoter was methylated in 51% of chronic gastritis adult patients and was associated with H. pylori infection (P < 0.05); this region was methylated in 66% of gastric cancer patients, and the difference in the percentage of methylated samples between these patients and those from H. pylori-infected chronic gastritis patients was statistically significant (P < 0.05). MLH1 methylation frequencies among H. pylori-infected and non-infected chronic gastritis adult patients were 13% and 7%, respectively. We observed methylation of the MLH1 promoter (39%) and increased MSI levels (68%) in samples from gastric cancer patients in comparison to samples from H. pylori-infected adult chronic gastritis patients (P < 0.001 and P < 0.01, respectively). The frequency of promoter methylation for both genes was higher in gastric cancer samples than in H. pylori-positive chronic gastritis samples (P < 0.05). The levels of MLH1 and MGMT mRNA were significantly reduced in chronic gastritis samples that were also hypermethylated (P < 0.01).
CONCLUSION: In summary, MGMT and MLH1 methylation did not occur in earlier-stage H. pylori infections and thus might depend on the duration of infection.
Core tip: Gastric carcinogenesis is a multistep process that is triggered by Helicobacter pylori (H. pylori) infection and characterised by multiple genetic and epigenetic alterations, including the DNA repair genes. To date, few advances have been made to determine the time duration required for to H. pylori infection to induce such epigenetics alteration and thus potentially induce gastric carcinogenesis. The results presented in this study indicate that the methylation of the MGMT and MLH1 promoter regions might depend on the duration of infection because these methylation events were not observed in children.
-
Citation: Alvarez MC, Santos JC, Maniezzo N, Ladeira MS, da Silva AL, Scaletsky IC, Pedrazzoli Jr J, Ribeiro ML.
MGMT andMLH1 methylation inHelicobacter pylori -infected children and adults. World J Gastroenterol 2013; 19(20): 3043-3051 - URL: https://www.wjgnet.com/1007-9327/full/v19/i20/3043.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i20.3043
Helicobacter pylori (H. pylori) is an important pathogen in the human stomach. The natural acquisition of H. pylori infection occurs mainly during childhood. Once established within the gastric mucosa, the infection persists for life if left untreated. The epidemiological evidence and the rare occurrence of peptic ulcers or gastric atrophy in children[1,2] suggest that H. pylori-related gastric mucosal damage might be progressive through childhood into adulthood. H. pylori infection elicits a host inflammatory response, including mucosal infiltration by polymorphonuclear leukocytes, macrophages, and T and B lymphocytes. The inflammatory response has a slow onset and becomes chronic after a long period of infection. Although the symptoms of chronic infection are not as severe as those of acute inflammation, the condition is persistent[3]. The activated inflammatory cells release reactive oxygen and nitrogen species that can induce DNA injury and cellular apoptosis[4]. The chronic colonisation of the stomach with H. pylori causes inflammation within the gastric mucosa and activates multiple oncogenic pathways[5].
The interaction of H. pylori with the surface mucosa results in the increased release of pro-inflammatory cytokines[6] that exacerbate the inflammatory response. The persistence of this immune response leads to chronic inflammation, one of the factors associated with DNA methylation. DNA methylation is one of the most important epigenetic modifications and primarily occurs on the cytosine residues of CpG dinucleotides, which are frequently clustered into CpG islands within the promoter regions of certain genes[7]. DNA methylation of these promoter regions inhibits transcription through chromatin structural changes that are mediated by the interactions of the methyl-cytosines with the protein complexes that recruit histone-modifying enzymes[8,9].
Globally, gastric cancer is the fourth most common type of cancer and the second leading cause of cancer death, and 930000 new cases of gastric cancer are projected per year. South Korea, Japan and Eastern Asia have the highest incidences of gastric cancer, followed by Eastern Europe and Latin America[10]. Since the discovery of H. pylori by Warren et al in 1982[11], many studies have demonstrated a strong association between H. pylori infection and the development of gastric cancer[12,13]. Moreover, in 1994, the International Agency for Research on Cancer recognised H. pylori as a definitive carcinogenic agent based on several epidemiological reports[14]. The primary mechanism by which H. pylori induces gastric cancer is thought to include the upregulation of several genes, including cytokines, oncogenes and growth factors, as well as the downregulation of tumour suppressor genes. These alterations in gene expression are believed to result from mutations and microsatellite instability[15]. Additionally, several studies have demonstrated a close association between H. pylori infection and aberrant CpG island methylation[16-18].
Many critical genes are silenced by DNA methylation during cancer development. Recent studies have shown that the silencing of certain DNA repair genes by DNA methylation might be related to the occurrence of tumorigenic mutations. The expression levels of MLH1, a mismatch repair gene, are frequently altered in gastric cancers, and changes in MLH1 expression can promote tumour development[19]. Additionally, O6-methylguanine DNA methyltransferase (MGMT) is a protein required for the repair of alkylated guanines in DNA that arise from exposures to environmental alkylation mutagens or through endogenous mechanisms. It has been reported that the loss of MGMT expression might increase the occurrence of genetic mutations and thus lead to gastric cancer development[20]. Additionally, Kitajima et al[21] reported that the loss of MGMT along with mismatch repair (MMR) proteins in gastric cancer patients is an important event in tumour progression.
Microsatellite instability (MSI) is one hallmark of DNA MMR deficiency that is observed in gastric carcinogenesis. Microsatellites are short DNA sequence repeats that are scattered throughout the human genome. Errors in the DNA MMR mechanisms of tumour cells can result in the expansion or contraction of these repeated sequences and thus MSI[22]. MSI occurs in nearly every case of gastric cancer that is associated with germline mutations of the MMR genes.
The inactivation of the MMR genes MSH2 and MLH1, either through a mutation or an epigenetic mechanism, is responsible for the development of MSI in gastric cancer. The aberrant loss of expression of either the MLH1 or MSH2 proteins has been observed in patients exhibiting gastric cancer with MSI. In particular, altered MLH1 expression is associated with self-gene inactivation through the hypermethylation of its promoter regions[23,24].
It is well established that H. pylori infection leads to chronic inflammation in the gastric mucosa, which is a condition associated with DNA methylation. Because such epigenetic alterations play an important role in the regulation of gene expression and the maintenance of DNA integrity and stability, the aim of this study was to analyse the effects of H. pylori infection on the methylation statuses of MLH1 and MGMT and the relationship between the methylation of these promoters and microsatellite instability in H. pylori-infected or uninfected children as well as infected or uninfected adults with chronic gastritis or gastric cancer.
This study was approved by the following institutions: the Ethical Committee of the Paulista Medical School, State University (UNESP), Botucatu, SP, Brazil; the Sao Francisco University, Braganca Paulista, SP, Brazil; the School of Medicine of the São Paulo University, SP, Brazil; and by the National Committee of Ethics in Research, Brasília, DF, Brazil. Informed consent to participate in the study was obtained from all patients.
This study included 239 patients, of which 50 were children between the ages of 2 and 18 years old (average age = 8 ± 4 years; 47% male, 53% female) who suffered from dyspepsia. Of the remaining patients, 97 suffered from chronic gastritis (average age = 35 ± 13 years; 33% male, 67% female), and 92 suffered from gastric cancer (average age = 60 ± 12 years; 82% male, 18% female). All of the patients were non-smokers, did not abuse alcohol and were not using prescription or recreational drugs.
Biopsies from patients with gastric complaints were obtained from the lesser curvature of the antrum (the distal region of the stomach) within 2 cm of the pyloric ring during endoscopies. For patients with gastric cancer, biopsies were obtained during gastric surgeries (to remove gastric carcinoma). One biopsy was used for a rapid urease test[25], 2 were used for histopathological evaluations, and 1 was used for bacterial genotyping by polymerase chain reaction (PCR) . H. pylori infection was considered present when positive results were obtained from all of the following tests: rapid urease test, histological analysis and gastric biopsy PCR. The patients were considered uninfected when negative results were obtained for all tests.
Gastric mucosal samples were fixed in 10% formalin for 24 h, dehydrated in alcohol and xylene, and embedded in paraffin. Sequential 3-5-μm sections were cut and stained with haematoxylin-eosin for routine histology. Gastritis was classified according to Sydney’s system[26], and the presence of H. pylori was determined by carbolfuchsin staining of the sections.
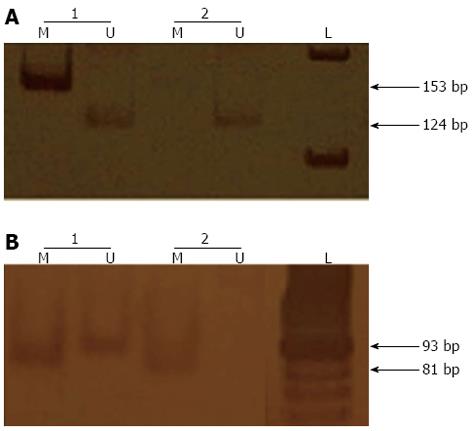
Bisulphite treatment was performed on 1 μg of DNA with the EpiTect Bisulfite kit (Qiagen, Valencia, CA, United States). Methylation-specific PCR (MSP-PCR) was performed with a primer set specific to the methylated or unmethylated sequences (M or U sets, respectively)[27]. The PCR reactions were performed in a final volume of 25 μL with approximately 200 ng of sodium bisulphite-treated DNA and 25 pmol of each primer. The PCR amplifications were performed for 40 cycles that each consisted of a denaturation step at 95 °C for 5 min, a primer-annealing step at 58 °C for 35 s and an extension step at 72 °C for 40 s, with a single final extension step at 72 °C for 7 min. The reaction products were separated by electrophoresis on 8% polyacrylamide gels and visualised by silver staining (Figure 1).
The gastric biopsies were collected, snap frozen, and stored at -80 °C in RNAlater® (Qiagen, Valencia, CA, United States). Total RNA was isolated with the RNeasy tissue kit® (Qiagen). The gastric cancer biopsies were microdissected prior to RNA extraction. The PixCell® IIe Laser Capture Microdissection (LCM) System (Arcturus Engineering, Mountain View, CA, United States) was used to obtain laser captures using an amplitude of 50 mW, a duration of 800 ms and a 7.5-mm beam. RNA from the capture lids (Arcturus) that contained the microdissected tissue was extracted with the PicoPure RNA isolation kit (Arcturus). Single-stranded cDNA was synthesised from the RNA using the high capacity cDNA archive kit® (Applied Biosystems, Foster City, CA, United States) according to the manufacturer’s protocol.
Quantitative PCR was performed on a 7300 real-time PCR system (Applied Biosystems) and the threshold cycle numbers were determined with the RQ Study software (Applied Biosystems). The reactions were run in triplicate and the threshold cycle numbers were averaged. The 50-μL reaction mixture was prepared as follows: 25 μL of Sybr Green® Quantitative PCR SuperMix-UDG (Invitrogen Life Technologies, Alemeda, CA, United States), 10 mmol/L of each primer (Table 1) and 10 μL of cDNA (100 ng). The reactions was were performed with a preliminary UDG treatment for 2 min at 50 °C and denaturation for 2 min at 95 °C, followed by 45 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 15 s, and primer extension at 72 °C for 15 s. This treatment was followed by a melting-point analysis of the double-stranded amplicons that consisted of 40 cycles of 1 °C decrement (15 s each), beginning at 95 °C. The first derivative of this plot, dF/dT, is the rate of change of fluorescence in the reaction. A significant change in fluorescence occurs at the melting point of the double-stranded PCR products. A plot of dF/dT versus temperature displays these changes as distinct peaks.
| Gene | Primer | Sequence (5’-3’) |
| β-actin | Sense | ACACTGGCTCGTGTGACAAGG |
| Antisense | CGGCTAATACACACTCCAAGGC | |
| MGMT | Sense | CACCACACTGGACAGCCCTTT |
| Antisense | CGAACTTGCCCAGGAGCTTTATTT | |
| MLH1 | Sense | CGGTTAACTACCCAATGCCTCAAC |
| Antisense | TTCTCGACTAACAGCATTTCCAA |
MLH1 and MGMT gene expression was measured and normalised to a constitutive gene (β-actin). The relative expression was calculated according to the formula 2(-∆∆Ct)[28], and the results are expressed as the average gene expression ± SD.
All samples were analysed for 4 markers (BAT-25, BAT-26, D17S250 and D2S123) as recommended by the American National Cancer Institute (NCI) workshop on MSI[29].
PCR was performed in a final volume of 25 μL with approximately 200 ng of template genomic DNA and 25 pmol of each primer. PCR amplifications was performed for 35 cycles that consisted of a denaturation step at 95 °C for 30 s, a primer-annealing step at 55-58 °C for 30 s and an extension step at 72 °C for 30 min, followed by a final single extension step at 72 °C for 10 min.
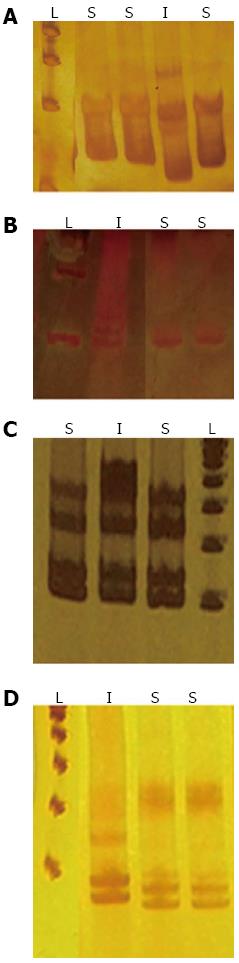
Single strand conformation polymorphism (SSCP) analysis was performed on each sample. Briefly, 12 μL of each PCR product were mixed with 12 μL of denaturing buffer (95% Formamide, 0.05% Bromophenol blue, and 0.05% xylenocianol), denatured at 95 °C for 5 min and separated by electrophoresis on a non-denaturing 7% polyacrylamide gel for 3 h at room temperature. The single strands of the PCR products were visualised as bands by silver staining.
MSI was defined as a shift in the mobility of the DNA band from either allele or by the appearance of a new band (Figure 2). The MSI status was also confirmed by direct sequencing (data not shown). High-level instability (MSI-H) was defined by the presence of more than one instability marker. Low-level instability (MSI-L) was defined as the presence of a single instability marker. Finally, if no instability markers were present, the sample was classified as displaying microsatellite stability (MSS)[29].
The association between microsatellite instability and the methylation pattern was evaluated with either the χ2 test with Yates continuity correction or Fisher’s exact test. Statistical significance for expression analysis was assessed by an unpaired Student’s t-test. A P value of < 0.05 was considered statistically significant.
The presence of H. pylori infection was analysed in biopsy specimens from 50 paediatric patients and 189 adult patients who underwent endoscopies. Infections were detected in 22 of the 50 (44%) children, 83 of the 97 (86%) adults with chronic gastritis and all (100%) of the 92 adult gastric cancer patients.
Methylation was not detected in the promoter regions of MLH1 and MGMT in the paediatric samples. The samples from the chronic gastritis patients showed hypermethylation in the MHL1 gene promoter region in 11 of the 83 (13%) samples from the H. pylori-infected subjects and in 1 of the 14 (7%) samples from the uninfected subjects (P > 0.05; Table 2). Methylation of the MHL1 promoter region was observed in the samples from 36 of the 92 (39%) gastric cancer patients. The differences observed in the percentages of methylated samples are statistically significant when the patients with gastric cancer are compared to those with chronic gastritis, regardless of H. pylori infection status (P < 0.001; P < 0.05, respectively; Table 2).
| Subjects | MLH1 | MGMT | ||
| Methylated | Unmethylated | Methylated | Unmethylated | |
| Chronic gastritis | ||||
| Child H. pylori negative | - | 28 (100%) | - | 28 (100%) |
| Child H. pylori positive | - | 22 (100%) | - | 22 (100%) |
| Adults H. pylori negative | 1 (7%) | 13 (93%) | 3 (21%) | 11 (79%) |
| Adults H. pylori positive | 11 (13%) | 72 (87%) | 42 (51%)c | 41 (49%) |
| Gastric cancer | 36 (39%)ab | 56 (61%) | 61 (66%)de | 31 (34%) |
In patients with chronic gastritis, MGMT promoter region methylation was observed in the biopsies from 42 of the 83 (51%) H. pylori-positive patients and 3 of the 14 (21%) H. pylori-negative patients (Table 2); this difference was statistically significant (P < 0.05). In the gastric cancer patients, MGMT promoter region methylation was observed in the biopsies from 61 of the 92 (66%) patients. The difference in the percentages of samples with MGMT promoter region methylation between the gastric cancer patients and the non-infected chronic gastritis patients was statistically significant (P < 0.01). Additionally, there was a statistically significant difference between the percentages of methylated samples from the gastric cancer patients and the H. pylori-infected chronic gastritis patients (P < 0.05). Furthermore, the frequency of promoter methylation for both genes was higher in the gastric cancer samples than in the H. pylori-positive chronic gastritis samples; this difference was statistically significant (P < 0.05).
The MLH1 and MGMT gene expression levels were measured and evaluated in the context of promoter methylation. The levels of both MLH1 and MGMT mRNA were significantly reduced in the methylated samples compared to the unmethylated samples in both the H. pylori-positive and uninfected adult chronic gastritis samples (Table 3). Overall, gastric cancer patients and paediatric patients had low levels of MLH1 and MGMT mRNA expression.
| Subjects | MLH1 | MGMT | ||
| Methylated | Unmethylated | Methylated | Unmethylated | |
| Chronic gastritis | ||||
| Child H. pylori negative | - | 0.25 ± 0.03 | - | 0.13 ± 0.08 |
| Child H. pylori positive | - | 0.27 ± 0.02 | - | 0.12 ± 0.01 |
| Adults H. pylori negative | 0.35 ± 0.01b | 0.85 ± 0.07 | 0.40 ± 0.16b | 0.99 ± 0.21 |
| Adults H. pylori positive | 0.88 ± 0.10b | 1.29 ± 0.27 | 0.79 ± 0.19b | 1.78 ± 0.47 |
| Gastric cancer | 0.27 ± 0.07 | 0.25 ± 0.08 | 0.49 ± 0.10 | 0.46 ± 0.10 |
Biopsy samples from children with chronic gastritis were relatively stable for the tested microsatellite markers. Only 2 samples (both H. pylori-negative) were scored as MSI-H, and the remaining paediatric samples were scored as MSS (stable; Table 4). MSI was present in 51 of the 83 (61%) H. pylori-positive chronic gastritis samples from adults. Of these, 37 (73%) samples were scored as MSI-L and 14 (27%) samples as MSI-H. MSI was observed in 9 of the 14 (64%) uninfected patients with gastritis; 6 (67%) samples were scored as MSI-L and 3 (33%) as MSI-H. MSI was observed in 63 of the 92 (68%) gastric cancer patients; 38 (60%) were scored as MSI-L and 25 (40%) as MSI-H. No significant association was found between H. pylori infection and MSI in the samples from chronic gastritis adult patients. However, there was a significant difference (P = 0.03) in the percentages of samples with MSI for between the gastric cancer patients and the H. pylori-infected chronic gastritis patients.
| Subjects | Microsatellite instability status | |||
| MSI-L | MSI-H | MSI | MSS | |
| Chronic gastritis | ||||
| Child H. pylori negative | - | 2 (7) | 2 (7) | 26 (93) |
| Child H. pylori positive | - | - | - | 22 (100) |
| Adults H. pylori negative | 6 (67) | 3 (33) | 9 (64) | 5 (36) |
| Adults H. pylori positive | 37 (73) | 14 (27) | 51 (61) | 32 (39) |
| Gastric cancer | 38 (60) | 25 (40) | 63 (68)a | 29 (32) |
Various authors have postulated that the methylation of the MHL1 promoter region leads to the downregulated expression of the MHL1 gene, an effect that is strongly associated with MSI[23,24,30]. Therefore, we evaluated whether there was any association between the MLH1 methylation status and MSI in our study population. The data presented in this study show a strong association between the MLH1 methylation status and MSI in patients with gastric cancer (P < 0.01). However, we did not find any significant association between the MLH1 methylation status and MSI in the samples from chronic gastritis adult patients, regardless of H. pylori infection status. Additionally, a multivariate analysis did not show that the H. pylori infection was associated with gene promoter methylation and MSI.
Genomic DNA methylation is one of the most important epigenetic modifications in eukaryotes. DNA methylation is essential for life, and alterations of the methylation process are often associated with carcinogenesis related to chronic inflammation or persistent infections of viruses or other pathogenic microorganisms. In this setting, we evaluated the effects of H. pylori infection on the methylation patterns of the MLH1 and MGMT promoter regions as well as the MSI statuses of paediatric and adult patients.
Children are an interesting natural model for H. pylori infection studies not only because they are not usually exposed to gastric mucosal irritants such as alcohol, tobacco, and anti-inflammatory medications but also because the gastric mucosal changes in children might represent an earlier stage of the inflammatory response when compared to those in adult hosts, due to the shorter duration of H. pylori infections in children. To our knowledge, this is the first study to evaluate the methylation patterns of DNA repair genes in paediatric samples. Our data showed an absence of the methylation in the MLH1 and MGMT promoter regions and that the mRNA levels of both genes were similar in infected and uninfected children. Although our data did not indicate an effect of H. pylori infection on the methylation patterns of two DNA repair genes, a recent study reported an association between H. pylori infection and the methylation patterns of 7 genes in paediatric samples; however, none of these genes were involved with DNA repair[31]. The methylation levels in these susceptible loci were increased in the adult samples when compared to the paediatric samples, suggesting that the altered methylation patterns might be related to the duration exposure to of H. pylori[31]. Accordingly, our data showed that the methylation rates were significantly higher in the samples from adults with chronic gastritis than in the samples from children with chronic gastritis.
Recently, it has been shown that prolonged bacterial infections lead to saturation of the repair capabilities of the host cells and thus to an ineffective and mutagenic DNA repair system[32]. Accordingly, it is believed that H. pylori-associated gastric mucosal damage might be progressive through childhood and into adulthood[1,2]. Taking this into account, our data suggest that H. pylori infection-mediated DNA methylation in adults may depend not only on the level of the inflammatory response but also on the persistence and duration of the infection.
Our data showed high levels of MGMT promoter region methylation in patients with H. pylori-positive chronic gastritis when compared to those with H. pylori-negative chronic gastritis, indicating that MGMT promoter methylation is significantly associated with H. pylori infection in chronic gastritis cases. Previous studies reported that MGMT CpG methylation is more frequent and extensive in H. pylori-infected versus uninfected patients[33]. Additionally, it has been suggested that the MGMT promoter methylation in H. pylori-infected patients is related to tumour progression[34,35].
Our data showed no differences in MLH1 promoter region methylation between patients with H. pylori-positive chronic gastritis and those with H. pylori-negative chronic gastritis. However, the methylation levels were higher in patients with gastric cancer than in patients with chronic gastritis. Similarly, it has been reported that MLH1 promoter methylation occurs late in intestinal metaplasia development[36]. Taken together, our data suggest that MLH1 methylation occurs late in the progression of gastric carcinoma and that methylation depends partly on the persistence of the H. pylori infection.
The data presented herein also showed that the methylation of the promoter regions significantly reduced the mRNA levels of both MGMT and MLH1 in patients with chronic gastritis, regardless of H. pylori infection status. Conversely, no differences in mRNA levels were observed in gastric cancer samples, regardless of methylation status; this is likely consequent to other epigenetic and genetic mechanisms. Recently, it was reported that H. pylori infections in patients with gastritis were associated with MGMT hypermethylation and reduced levels of MGMT mRNA in the gastric epithelium[33]. Similar results were reported for MLH1 in gastric carcinoma samples[19]. Therefore, it is possible that the hypermethylation of the MLH1 promoter region leads to the reduced expression of its protein product. This phenomenon could permit the accumulation of mutations due to the lack of surveillance and repair that are consequent to this deficiency in the DNA repair system. Ultimately, the deficient DNA repair process can be detected by the appearance of microsatellite instability[37].
In this study, we screened a group of H. pylori-infected and uninfected paediatric patients as well as a group of adult patients who were divided as follows: H. pylori-infected chronic gastritis patients, uninfected chronic gastritis patients and gastric cancer patients (all of whom were infected with H. pylori). MSI instability was found to be a very rare event in the paediatric population. Our data showed that H. pylori infection was not associated with MSI among patients with chronic gastritis. The incidence of MSI in gastric cancer patients was 64%, which was consistent with results reported previously by others (incidences ranging from 58% to 76%)[38,39]. Additionally, several studies have reported the presence of MSI in patients with intestinal metaplasia and gastric cancer, suggesting that the development of MSI may be an early event in the multi-step progression of gastric carcinogenesis[40,41].
The presence of MSI-H in sporadic carcinomas has been significantly associated with the loss of MLH1 expression[42,43]. This phenomenon was associated with the hypermethylation of the MLH1 promoter, which is the underlying mechanism that causes MSI in gastric adenomas and early gastric cancers[24,44]. It is well known that H. pylori infection causes an increased rate of cell turnover in the gastric mucosa and thus overwhelms the DNA repair system. This process might allow for the accumulation of mutations that are consequent to H. pylori infection and other environmental risk factors[45].
Previously, we reported that H. pylori infection leads to decreased MLH1 expression in patients with gastric cancer[46]. This result correlated with the high levels of MSI that were detected in these samples because the downregulation of MLH1 can lead to DNA repair system failures. Moreover, when the methylation patterns were compared with the MLH1 expression levels and the MSI levels in gastric cancer samples, we found higher methylation levels and a consequent downregulation of MLH1 in samples that were characterised as MSI-H versus those characterised as MSI-L samples (P < 0.03). Similarly, Mizoshita et al[47] identified a strong association between the MSI phenotype and the loss of MLH1 expression in advanced gastric cancers.
We did not find an association between MGMT promoter region methylation and MSI status. Furthermore, it was observed that the methylation of both the MLH1 and MGMT promoter regions is a frequent event in gastric cancers. Similarly, Zou et al[34] reported an increase in MGMT methylation during the progression from intestinal metaplasia to early gastric carcinoma.
H. pylori infections are generally acquired during childhood and, if left untreated, will persist indefinitely. These infections lead to chronic inflammation, one of the factors associated with epigenetic alterations and possibly with the development of gastric cancer. The results presented in this study indicate that the methylation of the MGMT and MLH1 promoter regions might be considered to be dependent on the duration of infection because similar methylation patterns had not been observed in children. Moreover, in gastric cancer patients it was shown that the MLH1 expression levels, the hypermethylation pattern of the MLH1 promoter region and the consequent increase in MSI frequency are all related events. The results of this study are in accordance with the results presented in previous studies; taken together, these findings provide a better understanding of gastric carcinogenesis in the Brazilian population. Although other authors had previously studied these genes in gastric cancers or in chronic gastritis samples from adult patients, this is the first study that included samples from children with chronic gastritis to represent the earlier stages of the inflammatory response, which supported the idea that the methylation of these genes might depend on the duration of the H. pylori infection, among other factors.
Another interesting point of this study is that it addresses a new concept described by Ogino et al[48], termed “Molecular Pathological Epidemiology”, a new field of epidemiology based on the molecular classification of cancer, in which a known or suspected etiologic factor is examined in relation to a specific molecular change to gain insights into the carcinogenic mechanisms. It is well known that gastric carcinogenesis is a multifactorial process that results from the interactions of factors related to diet, environment, individual genetic susceptibility and H. pylori infection. In this manner, this study provides insights into the carcinogenic mechanisms that are induced by H. pylori infection, one of the etiologic factors involved in gastric carcinogenesis, and the induction of epigenetic alterations in the early stages of this process. The chronic gastric mucosal inflammation induced by H. pylori infection, which is characterised by mucosal infiltration by polymorphonuclear leukocytes, macrophages and T and B lymphocytes, leads to the release of reactive oxygen species (ROS) from activated inflammatory cells. ROS can induce DNA damage and the lack of a proper MMR repair system, which is partly due to the persistence of the organisms and the associated inflammation, and thus can lead to the accumulation of DNA mutations in gastric epithelial cells that contribute to gastric carcinogenesis. Additionally, recent studies have shown that specific types of inflammation that are characterised by the expression of specific inflammation-related genes, as well as increased cell proliferation, are necessary for methylation induction. In a previous study, the authors showed that H. pylori-induced inflammation was able to induce methylation, unlike alcohol or saturated NaCl-induced inflammation[49]. Despite all of the evidence for H. pylori infection-induced methylation, we cannot exclude the presence of potential confounding factors such as differences in individual host genetics, different bacterial strains and the local microenviroment, all of which might affect an association study. Further studies of H. pylori-induced molecular pathogenesis will be useful to our understanding of gastric carcinogenesis.
Gastric carcinogenesis is a multistep process that is triggered by Helicobacter pylori (H. pylori) infection and characterised by multiple genetic and epigenetic alterations, including the DNA repair genes. The action of H. pylori through inflammatory mediators might play a key role in the epigenetic silencing of these genes.
Impairment of the DNA MMR system is a known mechanism of carcinogenesis and tumour progression. H. pylori infection leads to chronic inflammation in the gastric mucosa, which is associated with DNA methylation; this epigenetic alteration plays an important role in the regulation of gene expression and the maintenance of DNA integrity and stability. To date, few advances have been made to determine the time duration required for to H. pylori infection to induce such epigenetics alteration and thus potentially induce gastric carcinogenesis.
To the knowledge, this is the first study to evaluate the methylation patterns of DNA repair genes in paediatric samples. Children can be considered interesting natural models for the study of H. pylori infection not only because they are not usually submitted to gastric mucosal irritants such as alcohol, tobacco, and anti-inflammatory medications but also because the gastric mucosal changes in children might represent an earlier stage of the inflammatory response compared to those in adult hosts, due to the shorter duration of H. pylori infections in children. The results presented in this study indicate that the methylation of the MGMT and MLH1 promoter regions might depend on the duration of infection because these methylation events were not observed in children.
This study indicates that the impairment of the DNA MMR system through DNA promoter methylation is an infrequent event in the early stages of H. pylori-induced inflammation.
This is overall an interesting study that analyses the relationship between H. pylori infection and molecular changes in the gastric mucosa, diseases, and cancers. In this respect, this is a very unique study in the field of MPE research in non-neoplastic diseases (gastritis).
P- Reviewers Ogino S, Wang K S- Editor Song XX L- Editor A E- Editor Zhang DN
| 1. | Drumm B. Helicobacter pylori in the pediatric patient. Gastroenterol Clin North Am. 1993;22:169-182. [PubMed] [Cited in This Article: ] |
| 2. | Drumm B, Day AS, Gold B, Gottrand F, Kato S, Kawakami E, Madrazo A, Snyder J, Thomas J. Helicobacter pylori and peptic ulcer: Working Group Report of the second World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2004;39 Suppl 2:S626-S631. [PubMed] [Cited in This Article: ] |
| 3. | Bayarsaihan D. Epigenetic mechanisms in inflammation. J Dent Res. 2011;90:9-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 4. | Shimada T, Watanabe N, Hiraishi H, Terano A. Redox regulation of interleukin-8 expression in MKN28 cells. Dig Dis Sci. 1999;44:266-273. [PubMed] [Cited in This Article: ] |
| 5. | Ding SZ, Goldberg JB, Hatakeyama M. Helicobacter pylori infection, oncogenic pathways and epigenetic mechanisms in gastric carcinogenesis. Future Oncol. 2010;6:851-862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 6. | Yamaoka Y, Kita M, Kodama T, Sawai N, Kashima K, Imanishi J. Expression of cytokine mRNA in gastric mucosa with Helicobacter pylori infection. Scand J Gastroenterol. 1995;30:1153-1159. [PubMed] [Cited in This Article: ] |
| 7. | Roa S JC, García M P, Melo A A, Tapia E O, Villaseca H M, Araya O JC, Guzmán G P. [Gene methylation patterns in digestive tumors]. Rev Med Chil. 2008;136:451-458. [PubMed] [Cited in This Article: ] |
| 8. | Esteller M. CpG island hypermethylation and tumor suppressor genes: a booming present, a brighter future. Oncogene. 2002;21:5427-5440. [PubMed] [Cited in This Article: ] |
| 9. | Esteller M. CpG island methylation and histone modifications: biology and clinical significance. Ernst Schering Res Found Workshop. 2006;115-126. [PubMed] [Cited in This Article: ] |
| 10. | Hatakeyama M. Helicobacter pylori and gastric carcinogenesis. J Gastroenterol. 2009;44:239-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 11. | Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273-1275. [PubMed] [Cited in This Article: ] |
| 12. | Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131. [PubMed] [Cited in This Article: ] |
| 13. | Yamagata H, Kiyohara Y, Aoyagi K, Kato I, Iwamoto H, Nakayama K, Shimizu H, Tanizaki Y, Arima H, Shinohara N. Impact of Helicobacter pylori infection on gastric cancer incidence in a general Japanese population: the Hisayama study. Arch Intern Med. 2000;160:1962-1968. [PubMed] [Cited in This Article: ] |
| 14. | Schistosomes , liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1-241. [PubMed] [Cited in This Article: ] |
| 15. | Teh BT, Larsson C, Nordenskjöld M. Tumor suppressor genes (TSG). Anticancer Res. 1999;19:4715-4728. [PubMed] [Cited in This Article: ] |
| 16. | Chan AO, Lam SK, Wong BC, Wong WM, Yuen MF, Yeung YH, Hui WM, Rashid A, Kwong YL. Promoter methylation of E-cadherin gene in gastric mucosa associated with Helicobacter pylori infection and in gastric cancer. Gut. 2003;52:502-506. [PubMed] [Cited in This Article: ] |
| 17. | Maekita T, Nakazawa K, Mihara M, Nakajima T, Yanaoka K, Iguchi M, Arii K, Kaneda A, Tsukamoto T, Tatematsu M. High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin Cancer Res. 2006;12:989-995. [PubMed] [Cited in This Article: ] |
| 18. | Nakajima T, Yamashita S, Maekita T, Niwa T, Nakazawa K, Ushijima T. The presence of a methylation fingerprint of Helicobacter pylori infection in human gastric mucosae. Int J Cancer. 2009;124:905-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Kim TY, Jong HS, Jung Y, Kim TY, Kang GH, Bang YJ. DNA hypermethylation in gastric cancer. Aliment Pharmacol Ther. 2004;20 Suppl 1:131-142. [PubMed] [Cited in This Article: ] |
| 20. | Engelbergs J, Thomale J, Rajewsky MF. Role of DNA repair in carcinogen-induced ras mutation. Mutat Res. 2000;450:139-153. [PubMed] [Cited in This Article: ] |
| 21. | Kitajima Y, Miyazaki K, Matsukura S, Tanaka M, Sekiguchi M. Loss of expression of DNA repair enzymes MGMT, hMLH1, and hMSH2 during tumor progression in gastric cancer. Gastric Cancer. 2003;6:86-95. [PubMed] [Cited in This Article: ] |
| 22. | Smith MG, Hold GL, Tahara E, El-Omar EM. Cellular and molecular aspects of gastric cancer. World J Gastroenterol. 2006;12:2979-2990. [PubMed] [Cited in This Article: ] |
| 23. | Leung SY, Yuen ST, Chung LP, Chu KM, Chan AS, Ho JC. hMLH1 promoter methylation and lack of hMLH1 expression in sporadic gastric carcinomas with high-frequency microsatellite instability. Cancer Res. 1999;59:159-164. [PubMed] [Cited in This Article: ] |
| 24. | Baek MJ, Kang H, Kim SE, Park JH, Lee JS, Paik YK, Kim H. Expression of hMLH1 is inactivated in the gastric adenomas with enhanced microsatellite instability. Br J Cancer. 2001;85:1147-1152. [PubMed] [Cited in This Article: ] |
| 25. | Coelho LGV, Barros CA, Lima DCA. National consensus on “H. pylori”. GED. 1996;15:53-58. [Cited in This Article: ] |
| 26. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [PubMed] [Cited in This Article: ] |
| 27. | Kang GH, Shim YH, Jung HY, Kim WH, Ro JY, Rhyu MG. CpG island methylation in premalignant stages of gastric carcinoma. Cancer Res. 2001;61:2847-2851. [PubMed] [Cited in This Article: ] |
| 28. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [PubMed] [Cited in This Article: ] |
| 29. | Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248-5257. [PubMed] [Cited in This Article: ] |
| 30. | Kang GH, Lee S, Cho NY, Gandamihardja T, Long TI, Weisenberger DJ, Campan M, Laird PW. DNA methylation profiles of gastric carcinoma characterized by quantitative DNA methylation analysis. Lab Invest. 2008;88:161-170. [PubMed] [Cited in This Article: ] |
| 31. | Shin SH, Park SY, Ko JS, Kim N, Kang GH. Aberrant CpG island hypermethylation in pediatric gastric mucosa in association with Helicobacter pylori infection. Arch Pathol Lab Med. 2011;135:759-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 32. | Toller IM, Neelsen KJ, Steger M, Hartung ML, Hottiger MO, Stucki M, Kalali B, Gerhard M, Sartori AA, Lopes M. Carcinogenic bacterial pathogen Helicobacter pylori triggers DNA double-strand breaks and a DNA damage response in its host cells. Proc Natl Acad Sci U S A. 2011;108:14944-14949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 33. | Sepulveda AR, Yao Y, Yan W, Park DI, Kim JJ, Gooding W, Abudayyeh S, Graham DY. CpG methylation and reduced expression of O6-methylguanine DNA methyltransferase is associated with Helicobacter pylori infection. Gastroenterology. 2010;138:1836-1844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 34. | Zou XP, Zhang B, Zhang XQ, Chen M, Cao J, Liu WJ. Promoter hypermethylation of multiple genes in early gastric adenocarcinoma and precancerous lesions. Hum Pathol. 2009;40:1534-1542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Schneider BG, Peng DF, Camargo MC, Piazuelo MB, Sicinschi LA, Mera R, Romero-Gallo J, Delgado AG, Bravo LE, Wilson KT. Promoter DNA hypermethylation in gastric biopsies from subjects at high and low risk for gastric cancer. Int J Cancer. 2010;127:2588-2597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Perri F, Cotugno R, Piepoli A, Merla A, Quitadamo M, Gentile A, Pilotto A, Annese V, Andriulli A. Aberrant DNA methylation in non-neoplastic gastric mucosa of H. Pylori infected patients and effect of eradication. Am J Gastroenterol. 2007;102:1361-1371. [PubMed] [Cited in This Article: ] |
| 37. | Kolodner RD, Marsischky GT. Eukaryotic DNA mismatch repair. Curr Opin Genet Dev. 1999;9:89-96. [PubMed] [Cited in This Article: ] |
| 38. | Leung WK, Kim JJ, Kim JG, Graham DY, Sepulveda AR. Microsatellite instability in gastric intestinal metaplasia in patients with and without gastric cancer. Am J Pathol. 2000;156:537-543. [PubMed] [Cited in This Article: ] |
| 39. | Liu P, Zhang XY, Shao Y, Zhang DF. Microsatellite instability in gastric cancer and pre-cancerous lesions. World J Gastroenterol. 2005;11:4904-4907. [PubMed] [Cited in This Article: ] |
| 40. | Semba S, Yokozaki H, Yamamoto S, Yasui W, Tahara E. Microsatellite instability in precancerous lesions and adenocarcinomas of the stomach. Cancer. 1996;77:1620-1627. [PubMed] [Cited in This Article: ] |
| 41. | Ottini L, Palli D, Falchetti M, D’Amico C, Amorosi A, Saieva C, Calzolari A, Cimoli F, Tatarelli C, De Marchis L. Microsatellite instability in gastric cancer is associated with tumor location and family history in a high-risk population from Tuscany. Cancer Res. 1997;57:4523-4529. [PubMed] [Cited in This Article: ] |
| 42. | Edmonston TB, Cuesta KH, Burkholder S, Barusevicius A, Rose D, Kovatich AJ, Boman B, Fry R, Fishel R, Palazzo JP. Colorectal carcinomas with high microsatellite instability: defining a distinct immunologic and molecular entity with respect to prognostic markers. Hum Pathol. 2000;31:1506-1514. [PubMed] [Cited in This Article: ] |
| 43. | Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Rüschoff J, Fishel R, Lindor NM, Burgart LJ, Hamelin R. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261-268. [PubMed] [Cited in This Article: ] |
| 44. | Fleisher AS, Esteller M, Tamura G, Rashid A, Stine OC, Yin J, Zou TT, Abraham JM, Kong D, Nishizuka S. Hypermethylation of the hMLH1 gene promoter is associated with microsatellite instability in early human gastric neoplasia. Oncogene. 2001;20:329-335. [PubMed] [Cited in This Article: ] |
| 45. | Gologan A, Graham DY, Sepulveda AR. Molecular markers in Helicobacter pylori-associated gastric carcinogenesis. Clin Lab Med. 2005;25:197-222. [PubMed] [Cited in This Article: ] |
| 46. | Bartchewsky W, Martini MR, Squassoni AC, Alvarez MC, Ladeira MS, Salvatore DM, Trevisan MA, Pedrazzoli J, Ribeiro ML. Influence of Helicobacter pylori infection on the expression of MLH1 and MGMT in patients with chronic gastritis and gastric cancer. Eur J Clin Microbiol Infect Dis. 2009;28:591-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 47. | Mizoshita T, Tsukamoto T, Cao X, Otsuka T, Ito S, Takahashi E, Nakamura S, Nakamura T, Yamamura Y, Tatematsu M. Microsatellite instability is linked to loss of hMLH1 expression in advanced gastric cancers: lack of a relationship with the histological type and phenotype. Gastric Cancer. 2005;8:164-172. [PubMed] [Cited in This Article: ] |
| 48. | Ogino S, Chan AT, Fuchs CS, Giovannucci E. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011;60:397-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 403] [Cited by in F6Publishing: 436] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 49. | Hur K, Niwa T, Toyoda T, Tsukamoto T, Tatematsu M, Yang HK, Ushijima T. Insufficient role of cell proliferation in aberrant DNA methylation induction and involvement of specific types of inflammation. Carcinogenesis. 2011;32:35-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |