Published online Mar 14, 2013. doi: 10.3748/wjg.v19.i10.1661
Revised: November 26, 2012
Accepted: December 22, 2012
Published online: March 14, 2013
We present the case of a 29-year-old patient with a history of abdominal pain and vomiting. Based on wireless video capsule findings he was previously diagnosed with ileal Crohn’s disease at a different institution, although the clinical and radiological picture was not typical and the response to corticosteroids was poor. We performed a single-balloon enteroscopy showing a short, ulcerous stenosis 50 cm proximal from Bauhin’s valve. The endoscopic and clinical histopathological findings were compatible with cryptogenic multifocal ulcerous stenosing enteritis (CMUSE). High dose corticosteroids were again started, without effect. The monoclonal tumor necrosis factor-α (TNF-α) antibody infliximab was added to the medical therapy. After induction therapy, both clinical and endoscopic amelioration was obtained. Larger case studies are needed to confirm the efficacy of TNF-α inhibition in steroid refractory CMUSE.
- Citation: De Schepper H, Macken E, Van Marck V, Spinhoven M, Pelckmans P, Moreels T. Infliximab induces remission in cryptogenic multifocal ulcerous stenosing enteritis: First case. World J Gastroenterol 2013; 19(10): 1661-1664
- URL: https://www.wjgnet.com/1007-9327/full/v19/i10/1661.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i10.1661
Ulceration of the small intestine poses a rather limited but difficult differential diagnosis. The most common causes are Crohn’s disease, nonsteroidal anti-inflammatory drug (NSAID) associated enteritis, lymphoma, tuberculous enteritis and (mainly in immunocompromised patients) cytomegalovirus (CMV) enteritis. Less frequent etiologies should be kept in mind however. A recently described differential diagnosis is cryptogenic multifocal ulcerous stenosing enteritis (CMUSE), which may be difficult to treat.
A 29-year-old male patient presented at our department in April 2011 for a second opinion. He suffered from aspecific abdominal complaints since 2003. Initially, a diagnosis of irritable bowel syndrome was put forth. In 2008, an ileocolonoscopy was performed in a different institution which showed several small ulcerations in the terminal ileum in the presence of a normal colon. Gastroduodenoscopy was normal. A wireless video capsule examination was performed showing multiple small ulcerations in the ileum. A tentative diagnosis of Crohn’s disease was made. The patient was started on corticosteroids in January 2009 (prednisolone 40 mg/d), azathioprine was associated as a maintenance therapy but not tolerated due to intractable nausea. The steroids were slowly tapered over 8 mo, after which the correctness of the diagnosis of Crohn’s disease was questioned for unspecified reasons and all treatment stopped. The patient consulted our department for a second opinion.
When we first met this patient in April 2011, diffuse abdominal cramping pain was still the chief complaint. The pain was worse postprandially leading to sitophobia. There was frequent vomiting and a 15 kg weight loss over the past 5 years was noted. Stools tended to be loose with the occasional presence of bloody slimes, the frequency was approximately once daily. There was no fever. His medical history was unremarkable apart from the presumed inflammatory bowel disease. There was no familial history of digestive disease. He smoked 20 cigarettes per week and used marihuana on a daily basis for medicinal purposes. There was no other substance (ab) use mentioned nor detected upon repeated urine toxicology. The use of NSAIDs was systematically denied. Blood work showed normal hemoglobin and red blood cell count and no leucocytosis or C-reactive protein elevation. Antinuclear antibodies and anti-neutrophil cytoplasmic antibodies titers were not elevated.
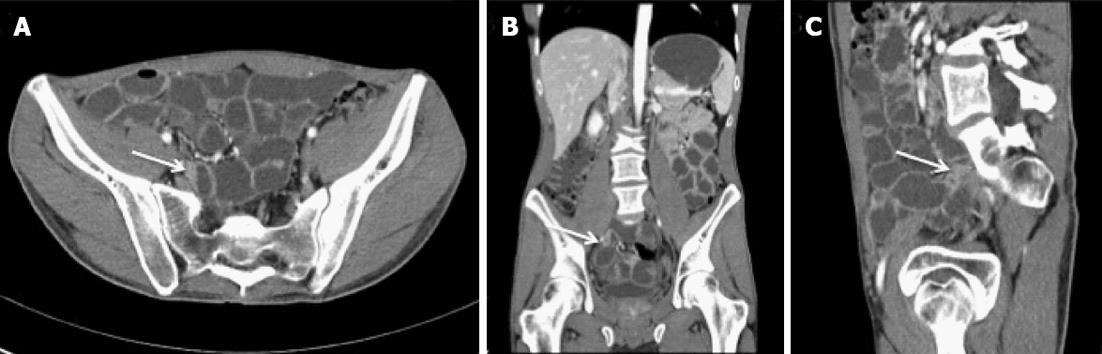
Since all previous examinations only mentioned small intestinal involvement, a computed tomography (CT) enterography was performed to guide subsequent endoscopy but failed to show intestinal inflammation or sequelae of inflammation such as strictures. We subsequently performed a retrograde single-balloon enteroscopy in May 2011 showing the presence of multiple circular but short ileal ulcerations over a distance of 20 cm, starting at the ileocaecal valve. Based upon these findings the CT enterography was consequently reviewed with the radiologist. After a careful second reading, a very short segment in the preterminal ileum was identified which could represent a small intestinal stricture although differentiation with a segmental contraction could not be made (Figure 1). Reconsidering the possibility of a mild form of Crohn’s disease, the patient was again started on corticosteroids (budesonide tapered over 3 mo).
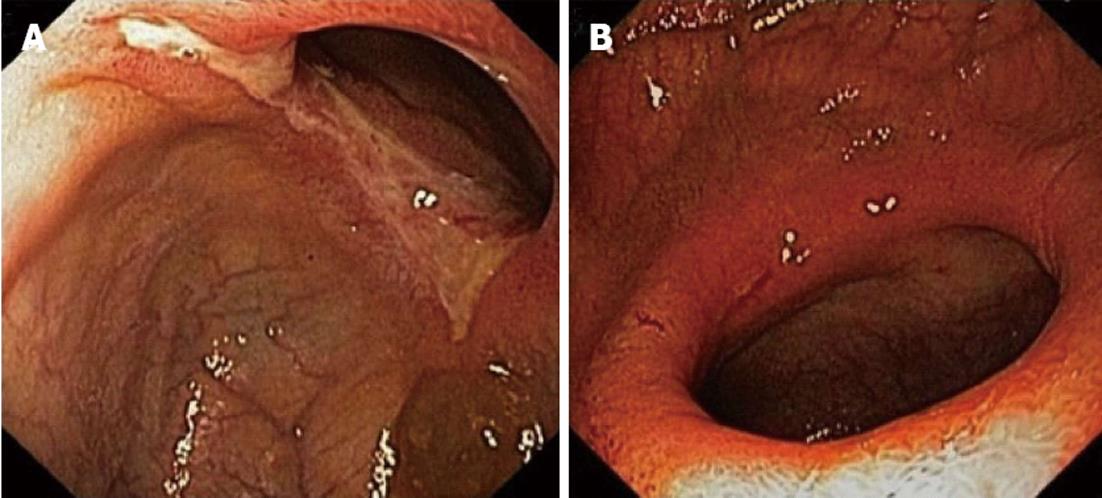
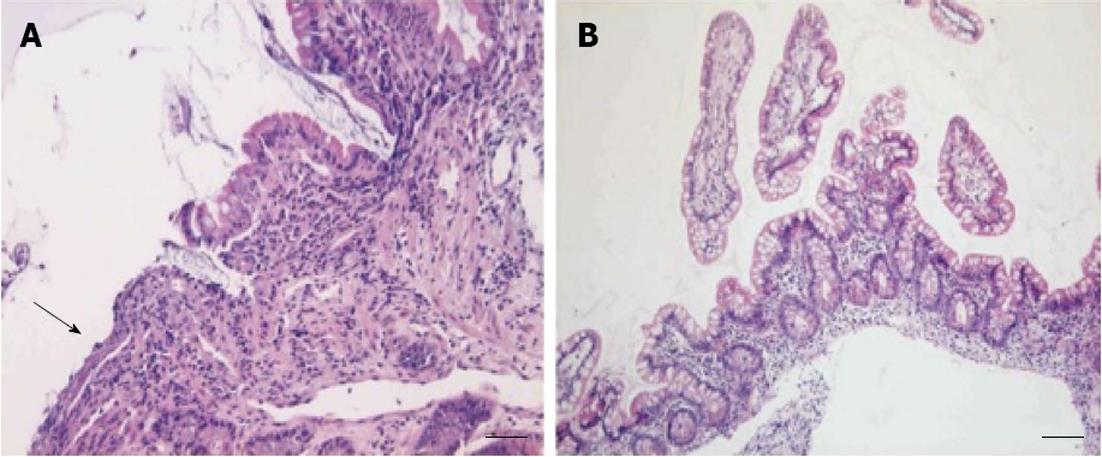
In November 2011 a repeat enteroscopy was performed, which showed a small ulceration 20 cm proximally from the ileocaecal valve and a short but circular and stenosing ulceration 30 cm more proximally (Figure 2). The microscopic examination showed superficial ulceration in the ileal mucosa, without associated signs of Crohn’s disease in the surrounding, preserved mucosa (Figure 3A).
Based upon the clinical course and the characteristic endoscopic image, CMUSE was diagnosed. Considering the lack of endoscopic or clinical response to treatment with budesonide so far, the patient was switched to high dose prednisolone (40 mg daily) in December 2011. After two months, no clinical improvement was noticed, in contrast, the patient almost completely stopped oral food intake because of postprandial cramping and was started on continuous nasogastric tube feeding. In addition, a third enteroscopy confirmed that the endoscopic image was unaltered. To avoid surgery, the ethical committee of our institution was consulted to consider our patient for a compassionate use treatment with the monoclonal tumor necrosis factor-α (TNF-α) antibody infliximab. A standard induction scheme was started in March 2012 (intravenous infliximab at a dose of 5 mg/kg on weeks 0, 2 and 6), followed by maintenance treatment (intravenous infliximab at a dose of 5 mg/kg every 8 wk). After administration of the induction scheme, significant clinical improvement occured with a reduction in abdominal pain and significant amelioration of sitophobia, leading to cessation of nasogastric tube feeding and prednisolone treatment. A fourth enteroscopy was performed, showing disappearance of the ileal circular ulceration (Figure 2A). A nonsignificant and probably irreversible stenosis remained. Histological examination showed only minor, non-specific inflammatory changes and a restoration of the villus architecture in the ileal biopsies (Figure 3B).
Infliximab treatment is currently still ongoing, clinical remission is maintained up till 6 mo after starting treatment.
Small intestinal ulceration is a less frequent cause of abdominal pain. Its differential diagnosis includes lymphoma, Crohn’s disease, tuberculous enteritis, CMV enteritis, NSAID related enteritis, oral potassium chloride toxicity, severe celiac disease (ulcerative jejunoileitis), systemic vasculitis and CMUSE. Small intestinal strictures are seen in chronic NSAID, ischemic enteritis, abdominal irradiation, Crohn’s disease and CMUSE[1].
CMUSE is an independent entity showing characteristics of inflammatory bowel disease and ischemic enteritis. The etiology is unclear but angiography studies suggest that in at least a subset of patients, CMUSE may involve vasculitis and even represent a visceral variant of polyarteritis nodosa. It seems likely that CMUSE incorporates chronic nonspecific ulcers of the small intestine (CNSU), a disease reported solely in the japanese population[2]. CNSU does not respond to corticosteroid administration, and can therefore be seen as steroid-refractory CMUSE.
CMUSE patients always report intestinal symptoms such as abdominal pain, diarrhea and vomiting related to intestinal (sub)obstruction. In 70% of cases, extraintestinal complaints are mentioned: 50% of patients lose weight, 20% develop fever and 10% complains of joint aches. The relatively frequent presence of extraintestinal signs such as oral aphtae, Raynaud’s phenomenon, sicca syndrome and pulmonary disease suggests that CMUSE may be part of a more systemic disorder.
Diagnostic criteria for CMUSE were proposed by Perlemuter et al[3,4], who published the 2 largest collections of patients (totaling 28 patients): unexplained small intestinal strictures found in adolescents and in middle-aged subjects, superficial ulceration restricted to the mucosa and submucosa, a chronic or relapsing clinical course (even after surgery), no biological signs of systemic inflammatory reaction and a typically (initially) beneficial effect produced by steroids. These criteria can be distilled from the clinical history and (balloon assisted) enteroscopy, the latter providing the histological material necessary to confirm the diagnosis. Histopathology shows an inflammatory response and ulcerative damage restricted to the mucosa and submucosa, different from the transmural involvement typical from Crohn’s disease. Blood analysis is needed to detect anemia, to exclude systemic inflammation and may show hypoproteinemia due to protein-loosing enteropathy. Medical imaging has its own role in diagnosing small intestinal ulceration/stenosis. A simple contrast follow-through fluoroscopy can dynamically show the presence of intestinal stenosis, but provides no specificity. As mesenteric vascular changes only occur in a subset of patients, performing a diagnostic angiography will not per se guide the diagnosis nor the therapy, and should be reserved for selected cases. Our case shows that CT enterography may lack the sensitivity to capture the often short and circular stenoses that are characteristic for CMUSE, in contrast to the often longer and more complex strictures seen in Crohn’s disease.
Differential diagnosis was mentioned earlier and should at least and most importantly include Crohn’s disease and NSAID-related enteropathy[5].
Current medical therapy consists of corticosteroid induction and maintenance with slow tapering of daily dosage, although no randomized clinical trials or guidelines are available to defend this strategy. Corticosteroids may prevent surgical intervention but often lead to steroid-dependence. Steroid-refractory CMUSE has recently been reported in one patient[6]. The use of other immune suppressive medication such as azathioprine or anti TNF-α inhibition is not reported in current literature. The mainstay of treatment of CMUSE stenosis is surgery, although recurrence is frequent (symptoms and strictures recur in 50% of patients). Endoscopic balloon dilatation can be used as a bridge to surgery, in order to prevent extensive resections of the small intestine and the threat of a short bowel syndrome.
Our patient fits the Perlemuter criteria for CMUSE (although we have no proof that the inflammation does not extend to the submucosa, as the latter can only be confirmed on a resection specimen). He evidently was refractory to steroid treatment, confirming an earlier anecdotal report[6]. To avoid surgical resection and the high risk of disease recurrence it implies, we opted to treat our patient with the standard of care treatment in inflammatory bowel disease, i.e., the monoclonal TNF-α antibody infliximab. In a small series of patients, this immunosuppressive drug was able to induce disease remission for symptomatic enteric Crohn’s strictures[7], providing a rationale for our treatment trial. Moreover, infliximab has shown its worth in treating rheumatoid arthritis and several forms of vasculitis (although the data for the latter are less extensive)[8].
After the infliximab induction regimen, clinical remission was immediately noted. Enteroscopy showed near complete endoscopic remission as well. To our knowledge, this is the first report to describe the beneficial effect of TNF-α blockade in steroid-refractory CMUSE. Evidently, larger series are necessary to confirm this observation.
P- Reviewers Bourke B, M’Koma A S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Chang DK, Kim JJ, Choi H, Eun CS, Han DS, Byeon JS, Kim JO. Double balloon endoscopy in small intestinal Crohn’s disease and other inflammatory diseases such as cryptogenic multifocal ulcerous stenosing enteritis (CMUSE). Gastrointest Endosc. 2007;66:S96-S98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Matsumoto T, Iida M, Matsui T, Yao T. Chronic nonspecific multiple ulcers of the small intestine: a proposal of the entity from Japanese gastroenterologists to Western enteroscopists. Gastrointest Endosc. 2007;66:S99-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Perlemuter G, Guillevin L, Legman P, Weiss L, Couturier D, Chaussade S. Cryptogenetic multifocal ulcerous stenosing enteritis: an atypical type of vasculitis or a disease mimicking vasculitis. Gut. 2001;48:333-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Perlemuter G, Chaussade S, Soubrane O, Degoy A, Louvel A, Barbet P, Legman P, Kahan A, Weiss L, Couturier D. Multifocal stenosing ulcerations of the small intestine revealing vasculitis associated with C2 deficiency. Gastroenterology. 1996;110:1628-1632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Chung SH, Jo Y, Ryu SR, Ahn SB, Son BK, Kim SH, Park YS, Hong YO. Diaphragm disease compared with cryptogenic multifocal ulcerous stenosing enteritis. World J Gastroenterol. 2011;17:2873-2876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 6. | Kim CW, Yu CS, Yoon YS, Yoon SN, Lim SB, Kim JC. Steroid-refractory cryptogenic multifocal ulcerous stenosing enteritis. Am J Surg. 2011;202:e48-e51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Pelletier AL, Kalisazan B, Wienckiewicz J, Bouarioua N, Soulé JC. Infliximab treatment for symptomatic Crohn’s disease strictures. Aliment Pharmacol Ther. 2009;29:279-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Atzeni F, Doria A, Carrabba M, Turiel M, Sarzi-Puttini P. Potential target of infliximab in autoimmune and inflammatory diseases. Autoimmun Rev. 2007;6:529-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |