Published online Mar 7, 2012. doi: 10.3748/wjg.v18.i9.938
Revised: November 16, 2011
Accepted: December 31, 2011
Published online: March 7, 2012
AIM: To evaluate the results of duodenal stenting for palliation of gastroduodenal malignant obstruction by using a gastric outlet obstruction score (GOOS).
METHODS: A prospective, non-randomized study was performed at a tertiary center between August 2005 and April 2010. Patients were eligible if they had malignant gastric outlet obstruction (GOO) and were not candidates for surgical treatment. Medical history and patient demographics were collected at baseline. Scheduled interviews were made on the day of the procedure and 15, 30, 90 and 180 d later or unscheduled as necessary.
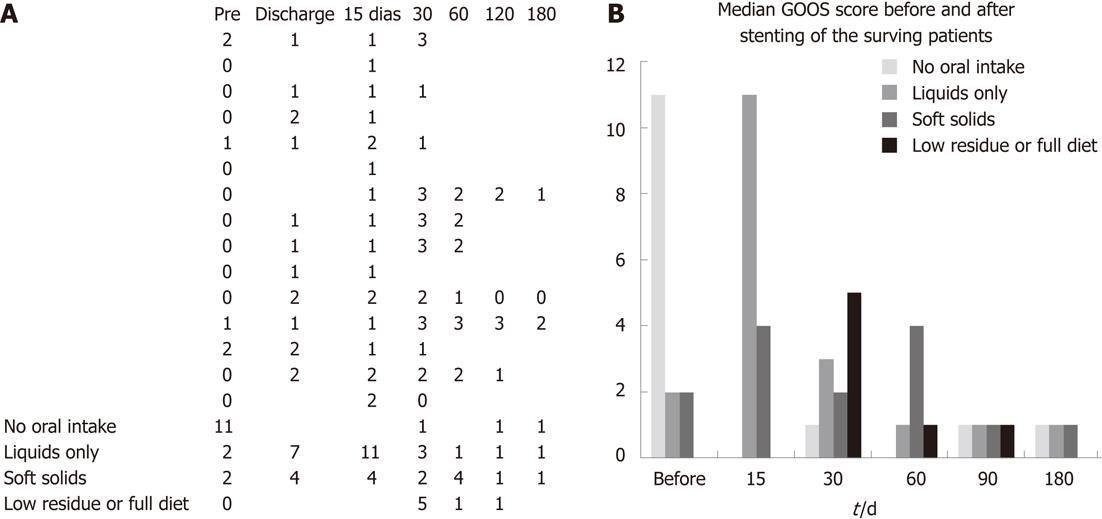
RESULTS: Fifteen patients (6 male, 9 female; median age 61 years) with GOO who had undergone duodenal stenting were evaluated. Ten patients had metastasis at baseline (66.6%) and 14 were unable to accept oral intake (93.33%), including 7 patients who were using a feeding tube. Laboratory data showed biliary obstruction in eight cases (53.33%); all were submitted to biliary drainage. Two patients developed obstructive symptoms due to tumor ingrowth after 30 d and another due to tumor overgrowth after 180 d. Two cases of stent migration occurred. A good response to treatment was observed, with a mean time of approximately 1 d (19 h) until toleration of a liquid diet and slightly more than 2 d for both soft solids (51 h) and a solid food/normal diet (55 h). The mean time to first failure to maintain liquid intake (GOOS ≥ 1) was 93 d. During follow-up, the mean time to first failure to maintain the previously achieved GOOS of 2-3 (solid/semi-solid food), considered technical failure, was 71 d. On the basis of oral intake a GOOS is defined: 0 for no oral intake; 1 for liquids only; 2 for soft solids only; 3 for low-residue or full diet.
CONCLUSION: Enteral stenting to alleviate gastroduodenal malignant obstruction improves quality of life in patients with limited life expectancy, which can be evaluated by using a GOO scoring system.
- Citation: Moura EGH, Ferreira FC, Cheng S, Moura DTH, Sakai P, Zilberstain B. Duodenal stenting for malignant gastric outlet obstruction: Prospective study. World J Gastroenterol 2012; 18(9): 938-943
- URL: https://www.wjgnet.com/1007-9327/full/v18/i9/938.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i9.938
Gastroduodenal strictures may be caused by malignant diseases of the stomach, pancreas and duodenum or by the mass effect of lymphonodal metastasis. Curative resections may not be possible in about 40% of such gastric lesions and in up to 80%-95% of pancreatic lesions, which makes clear the need to develop alternative means to achieve palliation and thus a better quality of life[1-3].
The use of self-expandable metal stents (SEMS) for treatment of gastroduodenal malignancy as a surgical alternative for palliation in patients with high morbidity and limited life expectancy is a feasible, safe and effective method[4-7].
In this study, we aimed to analyze the usefulness of a gastric outlet obstruction score to assess results of duodenal stenting for palliation of gastroduodenal malignant obstruction.
A prospective, non-randomized study was performed at a tertiary care center between August 2005 and April 2010. Patients over 18 years of age were eligible if they had gastric outlet obstruction (GOO) and were not candidates for surgical treatment due to high morbidity of the procedure, refusal or poor nutritional status. The main exclusion criteria were multiple lesions with enteral stenosis, suspected intestinal ischemia, impossibility of passing a guide-wire, gastric cancer presenting as linitis plastica, and contraindication to gastrointestinal endoscopy. All participants signed an informed consent approved by a review board.
Medical history and patient demographics were collected at baseline, and scheduled interviews were made on the day of the procedure and 15 d, 30 d, 90 d and 180 d
later or unscheduled as necessary (Table 1). Patients were withdrawn from the study at the end of 180 d or if death occurred before that time.
| Characteristic | Data (average) |
| Age (yr) | 61.33 |
| Gender | Male (6)/female (9) |
| Weight (kg) | 55.93 |
| Height (m) | 1.61 |
| Weight loss (kg)/(mo) | 14.53/5.40 |
| Previous chemotherapy/radiation therapy | Yes (5)/no (10) |
| Previous biliary drainage | Yes (8)/no (7) |
| Only local cancer | Yes (5)/no (10) |
| Malignancy | |
| Pancreatic adenocarcinoma | 9 |
| Gastric adenocarcinoma | 3 |
| Cholangiocarcinoma | 1 |
| Metastatic disease | 2 |
| Location of the obstruction | |
| Stomach | 3 |
| Bulb | 7 |
| Second and third portion | 5 |
| Tumor extension (cm) | 4.93 |
| Biliary stenosis | Bismuth I (2)/bismuth II and III (6) |
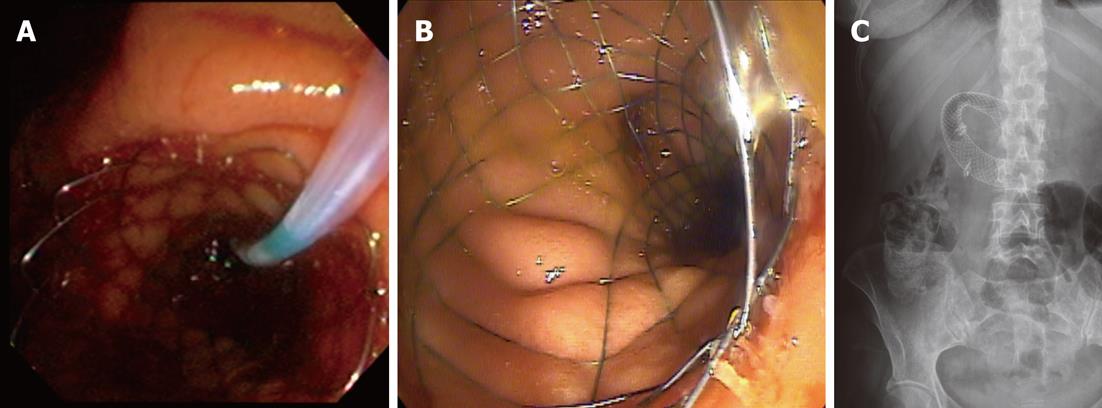
The stent used was an uncovered SEMS with a 27 mm diameter (22 mm at the mid-body) and length of 60, 90 or 120 mm preloaded in a 10 Fr delivery system (Wallflex, Boston Scientific Corporation, MA, United States) (Figure 1). All of the procedures were conducted under sedation or general anesthesia, under radiologic guidance with iodine contrast.
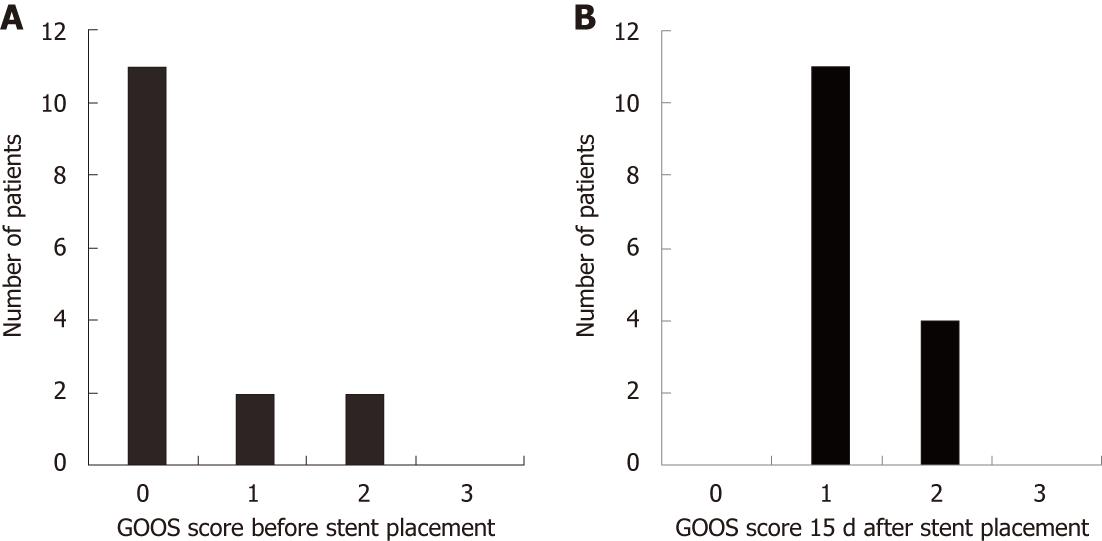
Fifteen patients (6 male, 9 female; median age 61 years) were submitted to duodenal stenting. The procedure was carried out under sedation in six cases, and under general anesthesia in nine patients due to poor clinical status. Most patients had metastasis at baseline (66.60%) and no acceptance of oral intake (93.33%), including seven patients who were using a feeding tube. Three patients had a gastric outlet obstruction score (GOOS) ≥ 1 (Figure 2).
Laboratory data showed biliary obstruction in 8 cases (53.33%), all of whom were submitted to biliary drainage (50.00% endoscopic and 50.00% surgical).
One patient developed obstructive symptoms after 1 mo of stent placement due to tumor ingrowth, which was treated by placing another stent inside the original one, with no recurrence of obstruction. Another patient developed obstructive symptoms after 6 mo due to tumor overgrowth, but it was not possible either to remove the stent or to bridge it because of bleeding and friability. This patient died 9 mo after stent placement.
There were two cases of stent migration. In the first case the patient had a duodenal metastasis from colonic cancer and presented obstructive symptoms after 8 d of stent placement. Two new stents were placed inside the original one, but the patient died 9 d later. The second patient was 53 years old with gastric cancer, liver metastasis and poor nutritional status who developed signs of obstruction within 3 mo after the procedure. Surgical removal of the migrated stent was done without complications and a surgical bypass was performed owing to better nutritional status at that time. One patient was submitted to removal of a foreign body, without complications.
Regarding tolerance to oral diet, a good response to the treatment was observed, with a mean time of approximately 1 d (19 h) till toleration of a liquid diet and slightly more than 2 d for soft solids (51 h) and solid food/normal diet (55 h). These data differ from those of other studies, which showed a faster acceptance of solid food after the procedure, with return to solid food on the same day of the procedure in up to 52% of the cases[8]. During the present study, the prescriptions were made by the patients’ physicians, mostly surgeons who prescribed liquids on the first day and, if they had good acceptance, prescribed solid food on the second day. The first failure to maintain liquid intake (GOOS ≥ 1) occurred at a mean of 3.1 mo. During follow-up, the first failure to maintain the previously achieved GOOS of 2-3 (solid/semi-solid food), considered technical failure, occurred at a mean of 2.35 mo (Figure 3).
Patients with gastroduodenal malignancies usually have limited prognosis with low life expectancy and also a poor quality of life due to inability to swallow at least semi-solid food. Consequently, there is a high incidence of poor nutritional status and dehydration, which reduce the resources that can be used to palliate this scenario[9,10]. Surgical treatment has better results on long-term follow-up but it cannot be offered, initially, to patients with poor clinical status because of increased morbidity and mortality. Based on the fact that less than 40% of patients who require palliative care are fit to undergo a surgical procedure, the need to achieve this objective with a less invasive, safer and effective method has been made clear[1,8,11]. The application of stents to the gastrointestinal tract has addressed this need, with attendant advantages of the surgical procedure although with a lesser long-term patency than desirable, mostly because of growth of tumoral tissue through the mesh (tumor ingrowth) or over the stent (tumor overgrowth) leading to recurrence of obstructive symptoms[7,12,13].
In studies of malignancies it is important to assess each patient’s quality of life and performance status, and several scales are used for this purpose such as the World Health Organization performance status (WHO status), the standard Short Form-36 questionnaire and the European Organization for Research and Treatment of Cancer scale. In GOO malignancies, however, the ability to ingest food seems to be the most important factor analyzed in terms of quality of life status, with the GOOS system being used most frequently for assessment[14]. A retrospective multicenter study enrolling 62 patients and using GOOS to evaluate the clinical success of enteral stenting, stated that all patients had resumed oral intake, although in 14.5% (n = 9) there was no improvement in the score. Some patients had a maximum score prior to stenting, and in all of them relief of symptoms was observed[15].
In a recent prospective study of 101 patients with incurable malignancies of the gastric outlet, three independent predictors of survival were identified: the ability to maintain self-care (WHO status), pain score, and the use of morphinomimetics. A 30-d survival rate of below 10% was found for patients who had all three prognostic indicators (WHO status of 3-4, pain score over 83 at baseline, the use of morphinomimetics stronger than tramadol), suggesting that a less invasive treatment should be considered for this group[1,15].
Analysis of each individual’s clinical status, associated diseases, and independent predictors of survival may provide objective data in helping to decide between surgical or endoscopic palliation. Patients with better prognosis and greater life expectancy should obtain more benefit from surgical treatment due to higher long-term patency rates, and patients with shorter life expectancy might benefit more from endoscopic treatment, enjoying a better quality of life, a quick return to oral diet, and less morbidity[16-18].
The cost of gastrojejunostomy (GJJ) vs stent placement in GOO was compared in a recent randomized trial that considered both direct and indirect costs of the 2 treatments. The study concluded that, although GJJ had a higher total cost, largely due to longer hospital stays, the difference was small and of lesser importance when deciding on the kind of treatment[17].
The choice of stent is also very important for achieving lower complication rates and higher patency leading to better quality of life. Plastic stents are associated with higher migration (self-expandable plastic stents) and perforation (non-expandable plastic stents) than SEMS, which are used more often[19-21]. Metallic stents may be covered by a membrane made of various plastic materials (covered SEMS) which provide greater resistance to tumor ingrowth but they may lose functionality because of higher migration rates in the colon. Uncovered SEMS have lower migration rates because they are anchored by the tumor, but are associated with higher recurrence of symptoms due to tumor ingrowth; nevertheless, they are used more often than covered metallic stents in colon and gastroduodenal malignant obstruction because overall they bring better results[18,21-25].
A recent randomized prospective study comparing the use of covered SEMS vs uncovered SEMS and enrolling 40 patients with gastric cancer in each group, demonstrated a higher stent migration rate within 8 weeks of stent placement in the covered SEMS group (25.8%) than in the uncovered SEMS group (2.8%). At the same time, the restenosis rate related to tumor ingrowth was higher in the uncovered SEMS group (25.0%) than in the covered SEMS (0.0%). In that study a routine endoscopy was performed independent of obstruction symptoms, which could explain the higher migration rates[25].
The evaluation of obstructive biliary signs is crucial in patients with gastroduodenal malignant obstruction because there is an association between them in over 61% of cases[22,25]. When jaundice is present it is important to perform imaging exams to determine its cause and help in its characterization, such as excluding other causes like liver failure due to metastasis. Obstructive jaundice may be successfully treated by endoscopic procedures, interventional radiology or surgery with comparable results but with higher morbidity and longer hospitalization periods in the case of surgery. Treatment decision should be made based on the clinical status and tumor staging. Biliary endoscopic drainage can be accomplished with the use of plastic or metallic stents, the latter having lower rates of colangitis and occlusion and shorter hospitalization stays but being more expensive. Both stents are equally effective in maintaining patency during the first 3 mo. If the patient has a short life expectancy, then plastic stents can be used safely and effectively at a lower cost, but they must be changed every 3 to 6 mo or in suspected cases of colangitis[22,25-27].
In conclusion, gastroduodenal malignancies are associated with gastric outlet obstruction symptoms and consequently with poor quality of life due to incapacity to swallow solid food, intractable nausea and vomiting, post-prandial epigastric tenderness and pain, and usually presenting with poor nutritional and clinical status that limit the options for treatment[18-20]. It has been shown that the use of SEMS for gastroduodenal malignancies is a feasible, safe and effective method, especially in those patients with limited life expectancy or in more critical conditions, allowing improvement not only in nutritional status but also in quality of life. SEMS placement may serve as a bridge to definitive surgical treatment in high-risk patients[28,29], as was conducted in one patient in the present study. We observed a quick return to an oral diet in our cohort after the procedure, and patency was estimated by the clinical efficacy to maintain an oral diet of solid/semi-solid food (GOOS ≥ 1), with a mean time to recurrence of obstructive symptoms of 3.1 mo.
The complications regarding the recurrence of symptoms observed in this study in two cases of stent migration, one case of tumor ingrowth and one of tumor overgrowth are similar to those reported in other publications and can be treated with a high success rate in most cases.
The association of GOO symptoms in gastroduodenal malignancies with biliary obstruction was shown in several published studies; therefore, we performed biliary stenting in eight of our patients (53.33%) prior to duodenal stenting. During the follow-up, three biliary stents were changed but there was no need to implant a new stent. In cases of biliary obstruction after duodenal stenting, biliary stents can be placed through the mesh of the duodenal SEMS to successfully palliate this condition.
Most published studies regarding endoscopic treatment of gastroduodenal malignancies have included only a limited number of patients, thus highlighting the need to perform more comparative studies between this method and surgery and to assess the costs involved.
Use of self-expandable metal stents in gastroduodenal malignancy as a surgical alternative for palliation in patients with high morbidity and limited life expectancy is a feasible, safe and effective method, allowing a quick return to oral intake and low morbidity.
Cost analyses comparing treatment of gastric outlet obstruction (GOO) malignancies by surgery or endoscopic procedures shows that any difference, considering direct and indirect costs, is small and should not have influence on patient’s
treatment.
A prospective, non-randomized study was performed at a tertiary center between August 2005 and April 2010. Patients were eligible if they had GOO and were not candidates for surgical treatment. Medical history and patient demographics were collected at baseline. Scheduled interviews were made on the day of the procedure and 15, 30, 90 and 180 d later or unscheduled as necessary.
Assessment of patient’s quality of life and performance status is crucial to provide more accurate information on whether the treatment being offered to the patient is satisfactory. Gastric outlet obstruction score (GOOS) system is an important tool to achieve this goal when the ability to ingest food seems to be an important factor to patient’s quality of life in GOO malignancies.
This is a good descriptive study in which authors analyze the results of duodenal stenting, by using a GOOS, for palliation of gastroduodenal malignant obstruction.
Peer reviewer: Fausto Catena, MD, PhD, Department of General, Emergency and Transplant Surgery, St Orsola-Malpighi University Hospital, Via Massarenti 9, Bologna 40139, Italy
S- Editor Gou SX L- Editor Logan S E- Editor Xiong L
| 1. | Pinto IT. Malignant gastric and duodenal stenosis: palliation by peroral implantation of a self-expanding metallic stent. Cardiovasc Intervent Radiol. 1997;20:431-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Dormann A, Meisner S, Verin N, Wenk Lang A. Self-expanding metal stents for gastroduodenal malignancies: systematic review of their clinical effectiveness. Endoscopy. 2004;36:543-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 336] [Cited by in F6Publishing: 355] [Article Influence: 17.8] [Reference Citation Analysis (1)] |
| 3. | Lindsay JO, Andreyev HJ, Vlavianos P, Westaby D. Self-expanding metal stents for the palliation of malignant gastroduodenal obstruction in patients unsuitable for surgical bypass. Aliment Pharmacol Ther. 2004;19:901-905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Jeurnink SM, van Eijck CH, Steyerberg EW, Kuipers EJ, Siersema PD. Stent versus gastrojejunostomy for the palliation of gastric outlet obstruction: a systematic review. BMC Gastroenterol. 2007;7:18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 279] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 5. | Moon JH, Choi HJ, Ko BM, Koo HC, Hong SJ, Cheon YK, Cho YD, Lee MS, Shim CS. Combined endoscopic stent-in-stent placement for malignant biliary and duodenal obstruction by using a new duodenal metal stent (with videos). Gastrointest Endosc. 2009;70:772-777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Keränen I, Udd M, Lepistö A, Halttunen J, Kylänpää L. Outcome for self-expandable metal stents in malignant gastroduodenal obstruction: single-center experience with 104 patients. Surg Endosc. 2009;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Larssen L, Medhus AW, Hauge T. Treatment of malignant gastric outlet obstruction with stents: an evaluation of the reported variables for clinical outcome. BMC Gastroenterol. 2009;9:45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Piesman M, Kozarek RA, Brandabur JJ, Pleskow DK, Chuttani R, Eysselein VE, Silverman WB, Vargo JJ, Waxman I, Catalano MF. Improved oral intake after palliative duodenal stenting for malignant obstruction: a prospective multicenter clinical trial. Am J Gastroenterol. 2009;104:2404-2411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Baron TH, Harewood GC. Enteral self-expandable stents. Gastrointest Endosc. 2003;58:421-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Tang T, Allison M, Dunkley I, Roberts P, Dickinson R. Enteral stenting in 21 patients with malignant gastroduodenal obstruction. J R Soc Med. 2003;96:494-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Aslanian H, Jamidar P. The duodenal stent-in-stent: a stent at the crossroads. Gastrointest Endosc. 2009;70:778-779. [Cited in This Article: ] |
| 12. | Pua U. Strut perforation of the duodenum by a WallFlex duodenal stent: detection using multi-detector CT. Gastrointest Endosc. 2010;71:220-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Kim JH, Song HY, Shin JH, Choi E, Kim TW, Lee SK, Kim BS. Stent collapse as a delayed complication of placement of a covered gastroduodenal stent. AJR Am J Roentgenol. 2007;188:1495-1499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | van Hooft J, Mutignani M, Repici A, Messmann H, Neuhaus H, Fockens P. First data on the palliative treatment of patients with malignant gastric outlet obstruction using the WallFlex enteral stent: a retrospective multicenter study. Endoscopy. 2007;39:434-439. [PubMed] [Cited in This Article: ] |
| 15. | van Hooft JE, Dijkgraaf MG, Timmer R, Siersema PD, Fockens P. Independent predictors of survival in patients with incurable malignant gastric outlet obstruction: a multicenter prospective observational study. Scand J Gastroenterol. 2010;45:1217-1222. [PubMed] [Cited in This Article: ] |
| 16. | Das A, Sivak MV. Endoscopic palliation for inoperable pancreatic cancer. Cancer Control. 2000;7:452-457. [PubMed] [Cited in This Article: ] |
| 17. | Jeurnink SM, Polinder S, Steyerberg EW, Kuipers EJ, Siersema PD. Cost comparison of gastrojejunostomy versus duodenal stent placement for malignant gastric outlet obstruction. J Gastroenterol. 2010;45:537-543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Tierney W, Chuttani R, Croffie J, DiSario J, Liu J, Mishkin DS, Shah R, Somogyi L, Petersen BT, ASGE . Enteral stents. Technology status evaluation report. Gastrointest Endosc. 2006;63:920-926. [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Lee DW, Chan AC, Ng EK, Wong SK, Lau JY, Chung SC. Through-the-scope stent for malignant gastric outlet obstruction. Hong Kong Med J. 2003;9:48-50. [PubMed] [Cited in This Article: ] |
| 20. | Simmons DT, Baron TH. Technology insight: Enteral stenting and new technology. Nat Clin Pract Gastroenterol Hepatol. 2005;2:365-74; quiz 1 p following 374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Dek IM, van den Elzen BD, Fockens P, Rauws EA. Biliary drainage of the common bile duct with an enteral metal stent. World J Gastroenterol. 2009;15:2423-2424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Kaw M, Singh S, Gagneja H. Clinical outcome of simultaneous self-expandable metal stents for palliation of malignant biliary and duodenal obstruction. Surg Endosc. 2003;17:457-461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 23. | Iwamuro M, Kawamoto H, Harada R, Kato H, Hirao K, Mizuno O, Ishida E, Ogawa T, Okada H, Yamamoto K. Combined duodenal stent placement and endoscopic ultrasonography-guided biliary drainage for malignant duodenal obstruction with biliary stricture. Dig Endosc. 2010;22:236-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Song GA, Kang DH, Kim TO, Heo J, Kim GH, Cho M, Heo JH, Kim JY, Lee JS, Jeoung YJ. Endoscopic stenting in patients with recurrent malignant obstruction after gastric surgery: uncovered versus simultaneously deployed uncovered and covered (double) self-expandable metal stents. Gastrointest Endosc. 2007;65:782-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Kim CG, Choi IJ, Lee JY, Cho SJ, Park SR, Lee JH, Ryu KW, Kim YW, Park YI. Covered versus uncovered self-expandable metallic stents for palliation of malignant pyloric obstruction in gastric cancer patients: a randomized, prospective study. Gastrointest Endosc. 2010;72:25-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Lee BH, Choe DH, Lee JH, Kim KH, Chin SY. Metallic stents in malignant biliary obstruction: prospective long-term clinical results. AJR Am J Roentgenol. 1997;168:741-745. [PubMed] [Cited in This Article: ] |
| 27. | Baron TH. Minimizing endoscopic complications: endoluminal stents. Gastrointest Endosc Clin N Am. 2007;17:83-104, vii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Wassef W, Rullan R. Interventional endoscopy. Curr Opin Gastroenterol. 2005;21:644-652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 29. | Kaw M, Singh S, Gagneja H, Azad P. Role of self-expandable metal stents in the palliation of malignant duodenal obstruction. Surg Endosc. 2003;17:646-650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |