Published online Nov 21, 2012. doi: 10.3748/wjg.v18.i43.6302
Revised: September 10, 2012
Accepted: September 12, 2012
Published online: November 21, 2012
AIM: To investigate whether the addition of probiotics can improve the eradication effect of triple therapy for Helicobacter pylori (H. pylori) infection.
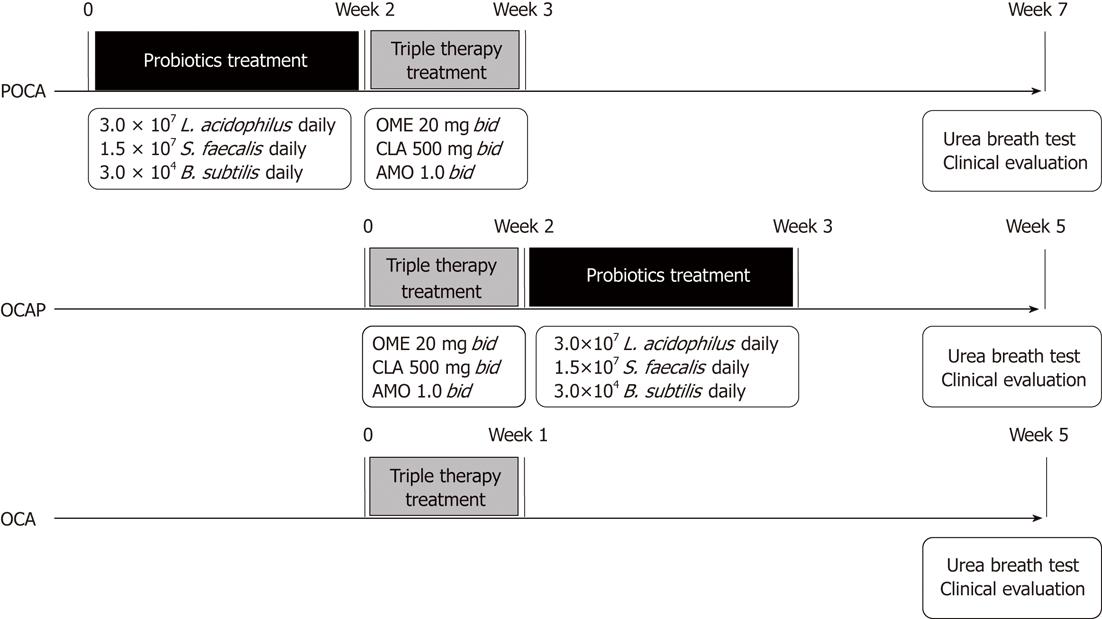
METHODS: This open randomized trial recruited 234 H. pylori positive gastritis patients from seven local centers. The patients were randomized to one-week standard triple therapy (omeprazole 20 mg bid, clarithromycin 500 mg bid, and amoxicillin 1000 mg bid; OCA group, n = 79); two weeks of pre-treatment with probiotics, containing 3 × 107Lactobacillus acidophilus per day, prior to one week of triple therapy (POCA group, n = 78); or one week of triple therapy followed by two weeks of the same probiotics (OCAP group, n = 77). Successful eradication was defined as a negative C13 or C14 urease breath test four weeks after triple therapy. Patients were asked to report associated symptoms at baseline and during follow-up, and side effects related to therapy were recorded. Data were analyzed by both intention-to-treat (ITT) and per-protocol (PP) methods.
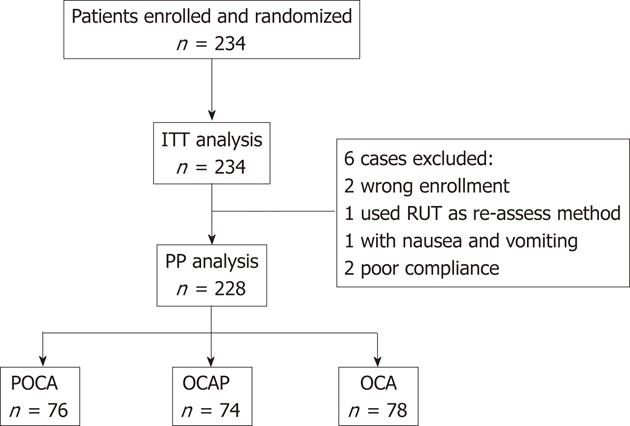
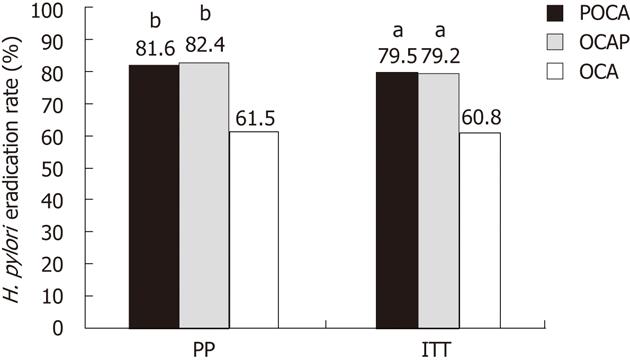
RESULTS: PP analysis involved 228 patients, 78 in the OCA, 76 in the POCA and 74 in the OCAP group. Successful eradication was observed in 171 patients; by PP analysis, the eradication rates were significantly higher (P = 0.007 each) in the POCA (62/76; 81.6%, 95% CI 72.8%-90.4%) and OCAP (61/74; 82.4%, 95% CI 73.6%-91.2%) groups than in the OCA group (48/78; 61.5%, 95% CI 50.6%-72.4%). ITT analysis also showed that eradication rates were significantly higher in the POCA (62/78; 79.5%, 95% CI 70.4%-88.6%) and OCAP (61/77; 79.2%, 95% CI 70%-88.4%) groups than in the OCA group (48/79; 60.8%, 95% CI 49.9%-71.7%), (P = 0.014 and P = 0.015). The symptom relieving rates in the POCA, OCAP and OCA groups were 85.5%, 89.2% and 87.2%, respectively. Only one of the 228 patients experienced an adverse reaction.
CONCLUSION: Administration of probiotics before or after standard triple therapy may improve H. pylori eradication rates.
-
Citation: Du YQ, Su T, Fan JG, Lu YX, Zheng P, Li XH, Guo CY, Xu P, Gong YF, Li ZS. Adjuvant probiotics improve the eradication effect of triple therapy for
Helicobacter pylori infection. World J Gastroenterol 2012; 18(43): 6302-6307 - URL: https://www.wjgnet.com/1007-9327/full/v18/i43/6302.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i43.6302
Eradication therapy has been widely used since Helicobacter pylori (H. pylori) was recognized as a major cause of peptic ulcers, gastric atrophy and stomach neoplasms[1]. However, the classic one-week triple therapy, consisting of a proton pump inhibitor (PPI), clarithromycin and amoxicillin, has become less effective, with eradication rates as low as 50%-70%[2,3], especially in areas with a high prevalence of clarithromycin resistance[4]. For example, in Shanghai from 2000 to 2009, the resistance rates of H. pylori to clarithromycin and levofloxacin increased from 8.6% to 20.7% and from 10.3% to 32.5%, respectively, whereas the resistance rates to metronidazole remained stable at 40%-50%[5]. In addition, the high rates of antibiotic-associated side-effects may result in poor patient compliance[6]. Administration of probiotics to adults[7,8] and children[9,10] has been reported to improve H. pylori eradication rates and to reduce the side-effects of PPI-based eradicating therapies. However, the timing of probiotic administration relative to the triple therapy has not been well characterized. In most studies, probiotics were started immediately after the start of triple therapy and were administered for one[11] to four[12] weeks. Bacteria of the Lactobacillus family were shown to inhibit H. pylori colonization of the stomach[13,14] and the binding of H. pylori to its glycolipid receptors[15]. To better investigate the effects of probiotics, in the present work we examined whether probiotics administered before or after eradication therapy to H. pylori-infected individuals could better enhance the eradication rate.
Lactobacillus acidophilus (L. acidophilus) is a common bacterium that resides in the human gastrointestinal tract and is added to food and milk. Compared with other Lactobacillus species, L. acidophilus has shown promising effects in the treatment of H. pylori[16,17]. We therefore used a compound probiotic, with L. acidophilus as the predominant bacterium. In this multicenter, open and randomized trial, we assessed whether probiotics, administered before or after H. pylori eradication therapy, could enhance eradication rates. We found that both approaches improved eradication rates, although the rate was somewhat higher in patients administered probiotics after than before triple therapy.
The patient population consisted of individuals, aged 18-65 years, newly diagnosed with gastritis or dyspepsia and infected with H. pylori. H. pylori infection was determined by rapid urease tests (RUT) during endoscopy; by pathologic examination of antrum specimens; or by C13 or C14 urea breath tests (UBT)[18]. The method used by each participating center is shown in Table 1. Candidates for inclusion were screened from November 2008 to July 2009. Exclusion criteria were: (1) neoplasm or peptic ulcer with or without complications, or gastroesophageal reflux disease; (2) previous failure of H. pylori eradication or a history of gastric surgery; (3) consumption of acid-inhibitors, bismuth compounds, antibiotics, or probiotics during the previous 4 wk; and (4) known allergy to antibiotics or probiotics.
| POCA (n = 76) | OCAP (n = 74) | OCA (n = 78) | P value | |
| Age, yr | 44.9 ± 13.8 | 48.2 ± 12.2 | 48.0 ± 13.3 | 0.2271 |
| Sex (male %) | 32 (42.1) | 26 (35.1) | 33 (42.3) | 0.5942 |
| H. pylori test (pre-therapy) | 0.9492 | |||
| 13C-UBT | 13 (17.1) | 10 (13.5) | 14 (17.9) | |
| 14C-UBT | 23 (30.3) | 24 (32.4) | 21 (26.9) | |
| RUT | 34 (44.7) | 34 (45.9) | 34 (43.6) | |
| HE | 6 (7.9) | 6 (8.1) | 9 (11.5) | |
| H. pylori test (post-therapy) | 0.8792 | |||
| 13C-UBT | 38 (50.0) | 34 (45.9) | 38 (48.7) | |
| 14C-UBT | 38 (50.0) | 40 (54.1) | 40 (51.3) | |
| Mean follow-up time, d | 58.2 ± 15.5 | 44.9 ± 12.3 | 43.2 ± 11.0 | < 0.0011 |
The enrolled patients were randomized 1:1:1 into three groups (Figure 1). One group received standard triple therapy, consisting of omeprazole 20 mg bid, clarithromycin 500 mg bid, and amoxicillin 1000 mg bid for 7 d (OCA group). The second group received two weeks of pre-treatment with probiotics, containing 3 × 107L. acidophilus per day, prior to one week of triple therapy (POCA group), and the third group received one week of triple therapy followed by two weeks of the same probiotics (OCAP group).
Due to uncertainties about the effects of the addition of probiotics, we estimated sample size by the non-inferiority method. Assuming eradication rates of 90% and 85% in probiotic-combined and OCA groups (α = 0.05 and β = 0.2), respectively, at least 70 individuals per group would be required. We calculated a final sample size of 240, including 30 patients at each center and 60 at the leading institute. The randomization number was produced by SPSS 18.0 software with a block of three and assigned to each center.
Each probiotic tablet (Yi Jun Kang®, He Li Pharm. Co. Ltd. China), weighing 0.5 g, contained 5 × 106L. acidophilus, 2.5 × 106Streptococcus faecalis (S. faecalis) and 5 × 103Bacillus subtilis (B. subtilis). Patients were instructed to take two of these tablets 30 min after meal, three times a day. Boxes containing a sufficient number of these tablets for the study period were provided to each patient by the probiotics producer.
Patient compliance was evaluated by counting the number of tablets returned, with an error rate lower than 5% considered acceptable. Four weeks after the completion of H. pylori eradication therapy, H. pylori status was assessed using a C13 or C14 based UBT (Table 2), with complete eradication defined as < 4.0 dpm and < 100 dpm, respectively.
| Center | UBT | n | H. pylori eradication rate (%) | |||
| POCA | OCAP | OCA | Total | |||
| 01 | C13 | 52 | 83.3 (15/18) | 80.0 (12/15) | 52.6 (10/19) | 71.2 |
| 02 | C13 | 29 | 80.0 (8/10) | 90.0 (9/10) | 88.9 (8/9) | 86.2 |
| 03 | C14 | 30 | 70.0 (7/10) | 50.0 (5/10) | 30.0 (3/10) | 50.0 |
| 04 | C13 | 29 | 80.0 (8/10) | 88.9 (8/9) | 70.0 (7/10) | 79.3 |
| 05 | C14 | 30 | 100 (10/10) | 70.0 (7/10) | 60.0 (6/10) | 76.7 |
| 06 | C14 | 29 | 88.9 (8/9) | 100 (10/10) | 40.0 (4/10) | 75.9 |
| 07 | C14 | 29 | 66.7 (6/9) | 100 (10/10) | 100 (10/10) | 89.7 |
The study was performed in accordance with good clinical practice and the guidelines of the Declaration of Helsinki. All patients provided a written informed consent and the study protocol was approved by the Ethics Committee of Changhai Hospital (CHEC2008-041).
All patients were asked to report associated symptoms at baseline and during follow-up, including abdominal pain, acid regurgitation, heartburn, nausea, vomiting, abdominal distension and diarrhea. Any side effect related to therapy was recorded and analyzed.
Data were computerized and analyzed with SPSS 18.0 software (IBM Corporation, NY, United States). The intention-to-treat (ITT) population consisted of all randomized patients, whereas the per-protocol (PP) population consisted of subjects who completed the entire study without any major protocol violations. The baseline demographic and clinical characteristics of the ITT and PP populations, and of the three groups of randomized patients in each population, were compared using Student’s t tests and Fisher’s exact tests, as warranted. Eradication rates in the three groups were compared by Fisher’s exact tests. The eradication rate and 95% confidence intervals in each group were calculated for both the PP and ITT populations. All statistical tests were two-sided, with a 5% level of significance.
We enrolled 234 H. pylori positive subjects. Of them, 228 were included in the PP population, with 76, 74 and 78 subjects in the POCA, OCAP and OCA groups, respectively (Figure 2). Two patients were randomized in error, including one patient aged 81 years randomized to OCAP and one patient with a bleeding duodenal ulcer randomized to POCA. One subject in the OCAP group was reassessed by RUT. Two patients showed a poor compliance with treatment, one each in the OCAP and OCA groups. One patient in the POCA group complained of nausea and vomiting on the fourth day of triple therapy and discontinued the study. The baseline demographic and clinical characteristics of the 228 enrolled patients are shown in Table 1. There were no significant differences in mean age, sex distribution, and distribution of H. pylori detecting methods. H. pylori was initially assessed by RUT in 102 (44.7%) patients, by UBT in 105 (46.1%) and by pathology in 21 (9.2%). C13 UBT was used for the second assessment of H. pylori in about 50% of patients, and C14 UBT in the other 50%. The mean follow-up time was significantly longer in the POCA (58.2 d) than in the OCAP and OCA (44 d) groups, due to the study design and treatment protocol (Figure 1).
Four weeks after the completion of triple therapy, H. pylori test on C13 or C14 UBT was negative in 171 (75%) of the 228 patients. There were significant differences among the groups, however. PP analysis showed that the eradication rates were significantly higher in the POCA (81.6%, 95% CI: 72.8%-90.4%) and OCAP (82.4%, 95% CI: 73.6%-91.2%) groups than in the OCA (61.5%, 95% CI: 50.6%-72.4%) group (P = 0.007 each, Figure 3). ITT analysis showed that, compared with the OCA group (48/79; 60.8%, 95% CI: 49.9%-71.7%), success rates were significantly higher in the POCA (62/78; 79.5%, 95% CI: 70.4%-88.6%, P = 0.014) and OCAP (61/77; 79.2%, 95% CI: 70%-88.4%, P = 0.015) groups. Further analysis showed that probiotic supplementation was associated with a higher eradication rate in all centers except for Centers 2 and 7 (Table 2).
The symptom relieving rates in the POCA, OCAP and OCA groups were 85.5% (65/76), 89.2% (66/74) and 87.2% (68/78), respectively. Only one of the 228 (0.4%) patients experienced an adverse effect. This patient, randomized to the POCA group, experienced severe vomiting on the fourth day of antibiotic treatment. Side-effect rates did not differ significantly among the three groups.
To our knowledge, this study is the first to show that administration of probiotics, either before or after traditional triple therapy, enhances H. pylori eradication rates. We found, in particular, that probiotic treatment after triple therapy significantly increased the eradication rate. Other than the studies, in which probiotics were started at the same time as PPI-based therapy, only one study has found that four weeks of pre-treatment with Lactobacillus- and Bifidobacterium-containing yogurt resulted in a higher H. pylori eradication rate than quadruple therapy alone (85% vs 71%, P < 0.05)[19]. That study, however, assessed second-line therapy for H. pylori, whereas we evaluated first-line treatment. We found that pre-treatment with L. acidophilus, S. faecalis and B. subtilis for two weeks prior to triple therapy improved the eradication rate, from 60.8% to 79.5%. Pretreatment with probiotics may decrease H. pylori load despite antimicrobial resistance, thus improving the efficacy of eradication therapy[19].
Pretreatment with probiotics has been found to significantly reduce the H. pylori colonization rate in mice, from 100% to 50% (P = 0.02)[20] and to reduce inflammation in the gastric antrum. In humans, four weeks of treatment with Lactobacillus reuteri ATCC 55730 reduced H. pylori load and decreased the occurrence of dyspeptic symptoms[21]. Although our results confirm previous findings[22], that pretreatment with suitable probiotics could benefit H. pylori infected patients, the optimal treatment period (two or four weeks) and optimal dose of probiotics have not been determined.
It is not clear whether simultaneously administered probiotics will be destroyed by anti-H. pylori drugs. Although pre-treatment with probiotics may constitute a solution, it results in a prolonged course of therapy, two weeks longer than the routine method. It is also unclear whether pre-treatment with probiotics could reduce the rates of antibiotic-related side effects to those observed with combined therapy[23-26]. Large clinical trials are required to compare the side-effect reduction rates of patients administered probiotics before and during eradication therapy.
Another possible regimen is to treat patients with probiotics after the completion of eradication therapy. Interestingly, we found that this regimen significantly increased the H. pylori eradication rate compared with eradication therapy alone, from 60.8% to 79.2%. To compare the outcome, we performed repeat UBT in our OCAP group four weeks after triple therapy (two weeks after probiotic therapy). Probiotics may inhibit residual H. pylori and mask UBT tests as ‘false negatives’, similar to the effects of PPIs. Further long-term studies are needed to determine if H. pylori eradication is true and permanent.
The recommended species and number of bacteria in probiotics have not been determined[27,28]. An 833-fold higher dose of L. acidophilus (2.5 × 1010/d) than ours, when added to triple therapy containing esomeprazole, amoxicillin, and clarithromycin, failed to increase the eradication rate observed with triple therapy alone (an eradication rate, 83.9% vs 80.6%, P = 0.74)[29]. This may have been due to the relatively small number of subjects, the short duration of probiotic administration (8 d) and the use of a single species of bacteria. Probiotic mixtures appear to be effective against a wide range of end points, including treatment of H. pylori infection, with multi-strain probiotics showing greater efficacy than single strains, including strains that are components of the mixtures themselves[30]. H. pylori may be less sensitive to single than to multiple probiotics, similar to resistance to antibiotics.
The probiotic mixture we tested is easily acquired in China and has been used widely to treat diarrhea and inflammatory bowel disease. If our findings are confirmed, Chinese H. pylori infected patients may benefit from probiotic treatment.
Due to the low incidence of side effects we observed, we could not evaluate whether probiotics reduce the adverse effects of antibiotics. However, safety evaluation was not a primary end point of this study. Other limitations include the lack of blinding; the absence of a placebo group; the lack of standardization of the H. pylori assay method; the short follow-up period; and the lack of confirmation of H. pylori load by biopsy or culture. Nevertheless, this study is the first to provide information on the timing of probiotics relative to routine eradication therapy in H. pylori infected patients. We found that both pre- and post-treatment with probiotics increased the eradication rates of triple therapy, with post-treatment being more effective than pre-treatment.
In conclusion, our results suggest that either pre- or post-administration of probiotics may improve the H. pylori eradication effect of standard triple therapy.
Eradication therapy has been widely used since Helicobacter pylori (H. pylori) was first recognized as a major cause of gastric diseases. Classic triple therapy, including a proton pump inhibitor (PPI) and the antibiotics clarithromycin and amoxicillin, has become less effective over time. Addition of probiotics may improve the eradication rates of triple therapy, but the optimal timing of probiotics remains unclear.
Pretreatment with probiotics has been shown to reduce H. pylori colonization in animals and humans.
This is the first study to show that both pre- and, particularly, post-treatment with probiotics increased the eradication rates of triple therapy. These results suggest that mixed probiotics may improve the H. pylori eradication effect of standard triple therapy with fewer adverse effects.
These results provide a guide for better use of probiotics, in combination with triple therapy, for the clinical treatment of H. pylori-infected patients. This new regimen may be particularly applicable to H. pylori eradication in areas of high resistance to antibiotics.
This study was designed to determine whether the administration of probiotics, before or after standard triple therapy, could improve H. pylori eradication rates. Interestingly, both regimens were effective in improving the efficacy of standard triple therapy. The study is well designed and the results are interesting.
Peer reviewer: Francesco Franceschi, MD, PhD, Assistant Professor, Internal Medicine, Catholic University of Rome, Gemelli Hospital, Largo A. Gemelli, 8, 00168 Rome, Italy
S- Editor Lv S L- Editor Ma JY E- Editor Xiong L
| 1. | Vergara M, Vallve M, Gisbert JP, Calvet X. Meta-analysis: comparative efficacy of different proton-pump inhibitors in triple therapy for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2003;18:647-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Ables AZ, Simon I, Melton ER. Update on Helicobacter pylori treatment. Am Fam Physician. 2007;75:351-358. [PubMed] [Cited in This Article: ] |
| 3. | Higuchi K, Maekawa T, Nakagawa K, Chouno S, Hayakumo T, Tomono N, Orino A, Tanimura H, Asahina K, Matsuura N. Efficacy and safety of Helicobacter pylori eradication therapy with omeprazole, amoxicillin and high- and low-dose clarithromycin in Japanese patients: a randomised, double-blind, multicentre study. Clin Drug Investig. 2006;26:403-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Gao W, Cheng H, Hu F, Li J, Wang L, Yang G, Xu L, Zheng X. The evolution of Helicobacter pylori antibiotics resistance over 10 years in Beijing, China. Helicobacter. 2010;15:460-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Sun QJ, Liang X, Zheng Q, Gu WQ, Liu WZ, Xiao SD, Lu H. Resistance of Helicobacter pylori to antibiotics from 2000 to 2009 in Shanghai. World J Gastroenterol. 2010;16:5118-5121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 52] [Cited by in F6Publishing: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Archimandritis A, Souyioultzis S, Katsorida M, Tzivras M. Clostridium difficile colitis associated with a ‘triple’ regimen, containing clarithromycin and metronidazole, to eradicate Helicobacter pylori. J Intern Med. 1998;243:251-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Bekar O, Yilmaz Y, Gulten M. Kefir improves the efficacy and tolerability of triple therapy in eradicating Helicobacter pylori. J Med Food. 2011;14:344-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Yoon H, Kim N, Kim JY, Park SY, Park JH, Jung HC, Song IS. Effects of multistrain probiotic-containing yogurt on second-line triple therapy for Helicobacter pylori infection. J Gastroenterol Hepatol. 2011;26:44-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Gotteland M, Poliak L, Cruchet S, Brunser O. Effect of regular ingestion of Saccharomyces boulardii plus inulin or Lactobacillus acidophilus LB in children colonized by Helicobacter pylori. Acta Paediatr. 2005;94:1747-1751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Gotteland M, Andrews M, Toledo M, Muñoz L, Caceres P, Anziani A, Wittig E, Speisky H, Salazar G. Modulation of Helicobacter pylori colonization with cranberry juice and Lactobacillus johnsonii La1 in children. Nutrition. 2008;24:421-426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | de Bortoli N, Leonardi G, Ciancia E, Merlo A, Bellini M, Costa F, Mumolo MG, Ricchiuti A, Cristiani F, Santi S. Helicobacter pylori eradication: a randomized prospective study of triple therapy versus triple therapy plus lactoferrin and probiotics. Am J Gastroenterol. 2007;102:951-956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Song MJ, Park DI, Park JH, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI. The effect of probiotics and mucoprotective agents on PPI-based triple therapy for eradication of Helicobacter pylori. Helicobacter. 2010;15:206-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Nam H, Ha M, Bae O, Lee Y. Effect of Weissella confusa strain PL9001 on the adherence and growth of Helicobacter pylori. Appl Environ Microbiol. 2002;68:4642-4645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Gotteland M, Cruchet S. Suppressive effect of frequent ingestion of Lactobacillus johnsonii La1 on Helicobacter pylori colonization in asymptomatic volunteers. J Antimicrob Chemother. 2003;51:1317-1319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Mukai T, Asasaka T, Sato E, Mori K, Matsumoto M, Ohori H. Inhibition of binding of Helicobacter pylori to the glycolipid receptors by probiotic Lactobacillus reuteri. FEMS Immunol Med Microbiol. 2002;32:105-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 178] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 16. | Canducci F, Armuzzi A, Cremonini F, Cammarota G, Bartolozzi F, Pola P, Gasbarrini G, Gasbarrini A. A lyophilized and inactivated culture of Lactobacillus acidophilus increases Helicobacter pylori eradication rates. Aliment Pharmacol Ther. 2000;14:1625-1629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 142] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | De Francesco V, Stoppino V, Sgarro C, Faleo D. Lactobacillus acidophilus administration added to omeprazole/amoxycillin-based double therapy in Helicobacter pylori eradication. Dig Liver Dis. 2000;32:746-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Malfertheiner P, Megraud F, O’Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772-781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1348] [Cited by in F6Publishing: 1286] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 19. | Sheu BS, Cheng HC, Kao AW, Wang ST, Yang YJ, Yang HB, Wu JJ. Pretreatment with Lactobacillus- and Bifidobacterium-containing yogurt can improve the efficacy of quadruple therapy in eradicating residual Helicobacter pylori infection after failed triple therapy. Am J Clin Nutr. 2006;83:864-869. [PubMed] [Cited in This Article: ] |
| 20. | Johnson-Henry KC, Mitchell DJ, Avitzur Y, Galindo-Mata E, Jones NL, Sherman PM. Probiotics reduce bacterial colonization and gastric inflammation in H. pylori-infected mice. Dig Dis Sci. 2004;49:1095-1102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 21. | Francavilla R, Lionetti E, Castellaneta SP, Magistà AM, Maurogiovanni G, Bucci N, De Canio A, Indrio F, Cavallo L, Ierardi E. Inhibition of Helicobacter pylori infection in humans by Lactobacillus reuteri ATCC 55730 and effect on eradication therapy: a pilot study. Helicobacter. 2008;13:127-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Gotteland M, Brunser O, Cruchet S. Systematic review: are probiotics useful in controlling gastric colonization by Helicobacter pylori? Aliment Pharmacol Ther. 2006;23:1077-1086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Myllyluoma E, Veijola L, Ahlroos T, Tynkkynen S, Kankuri E, Vapaatalo H, Rautelin H, Korpela R. Probiotic supplementation improves tolerance to Helicobacter pylori eradication therapy--a placebo-controlled, double-blind randomized pilot study. Aliment Pharmacol Ther. 2005;21:1263-1272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Sheu BS, Wu JJ, Lo CY, Wu HW, Chen JH, Lin YS, Lin MD. Impact of supplement with Lactobacillus- and Bifidobacterium-containing yogurt on triple therapy for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2002;16:1669-1675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 142] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Cremonini F, Di Caro S, Covino M, Armuzzi A, Gabrielli M, Santarelli L, Nista EC, Cammarota G, Gasbarrini G, Gasbarrini A. Effect of different probiotic preparations on anti-helicobacter pylori therapy-related side effects: a parallel group, triple blind, placebo-controlled study. Am J Gastroenterol. 2002;97:2744-2749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 244] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 26. | Duman DG, Bor S, Ozütemiz O, Sahin T, Oğuz D, Iştan F, Vural T, Sandkci M, Işksal F, Simşek I. Efficacy and safety of Saccharomyces boulardii in prevention of antibiotic-associated diarrhoea due to Helicobacterpylori eradication. Eur J Gastroenterol Hepatol. 2005;17:1357-1361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Lionetti E, Indrio F, Pavone L, Borrelli G, Cavallo L, Francavilla R. Role of probiotics in pediatric patients with Helicobacter pylori infection: a comprehensive review of the literature. Helicobacter. 2010;15:79-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Wang KY, Li SN, Liu CS, Perng DS, Su YC, Wu DC, Jan CM, Lai CH, Wang TN, Wang WM. Effects of ingesting Lactobacillus- and Bifidobacterium-containing yogurt in subjects with colonized Helicobacter pylori. Am J Clin Nutr. 2004;80:737-741. [PubMed] [Cited in This Article: ] |
| 29. | Medeiros JA, Gonçalves TM, Boyanova L, Pereira MI, de Carvalho JN, Pereira AM, Cabrita AM. Evaluation of Helicobacter pylori eradication by triple therapy plus Lactobacillus acidophilus compared to triple therapy alone. Eur J Clin Microbiol Infect Dis. 2011;30:555-559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Chapman CM, Gibson GR, Rowland I. Health benefits of probiotics: are mixtures more effective than single strains? Eur J Nutr. 2011;50:1-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 274] [Article Influence: 21.1] [Reference Citation Analysis (0)] |