Published online Sep 7, 2012. doi: 10.3748/wjg.v18.i33.4570
Revised: March 2, 2012
Accepted: March 20, 2012
Published online: September 7, 2012
AIM: To investigate whether the combined methods of unilateral thyroparathyroidectomy (TPX) and subdiaphragmatic vagotomy (VAX) can be adapted for rats and used as a reliable method to produce a rat model of long-term reduction of gastrointestinal (GI) motor function.
METHODS: Male Sprague-Dawley rats were randomly divided into 3 groups, normal, sham-operated and unilateral TPX plus VAX. The TPX plus VAX rats received VAX 7 d after application of TPX, and dietary intake and fecal output were then measured daily for 1 wk. After completion of the experiments, gastric emptying and small bowel transit were measured in vivo, and the contractile responses of colonic strips to excitatory and inhibitory neurotransmitters were estimated using isometric force transducers in vitro.
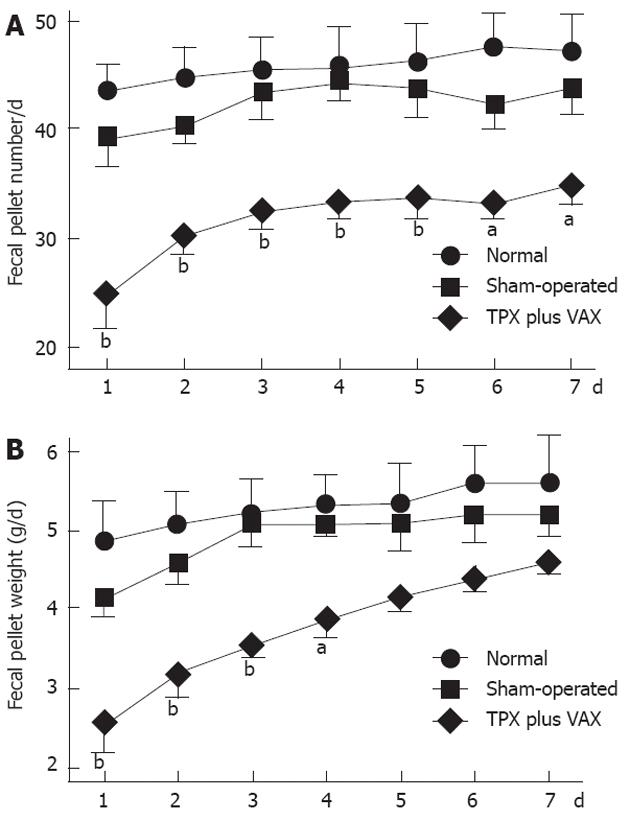
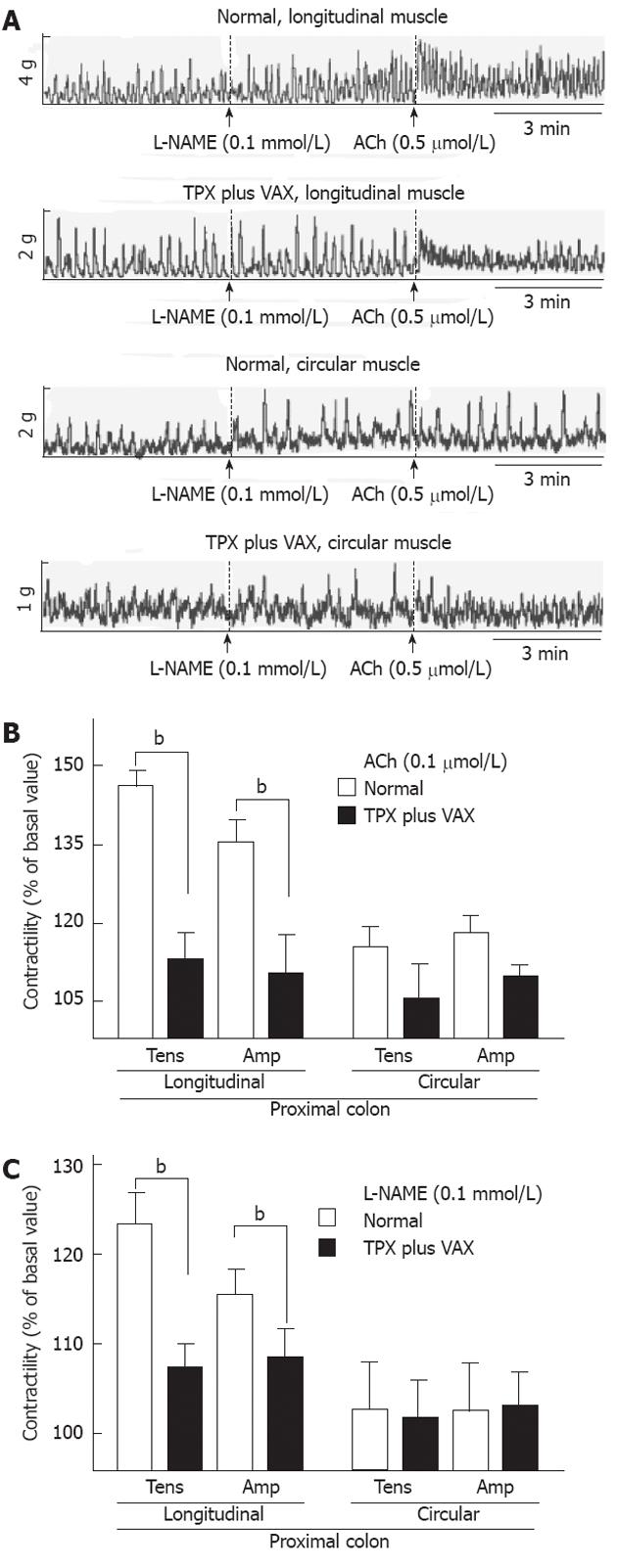
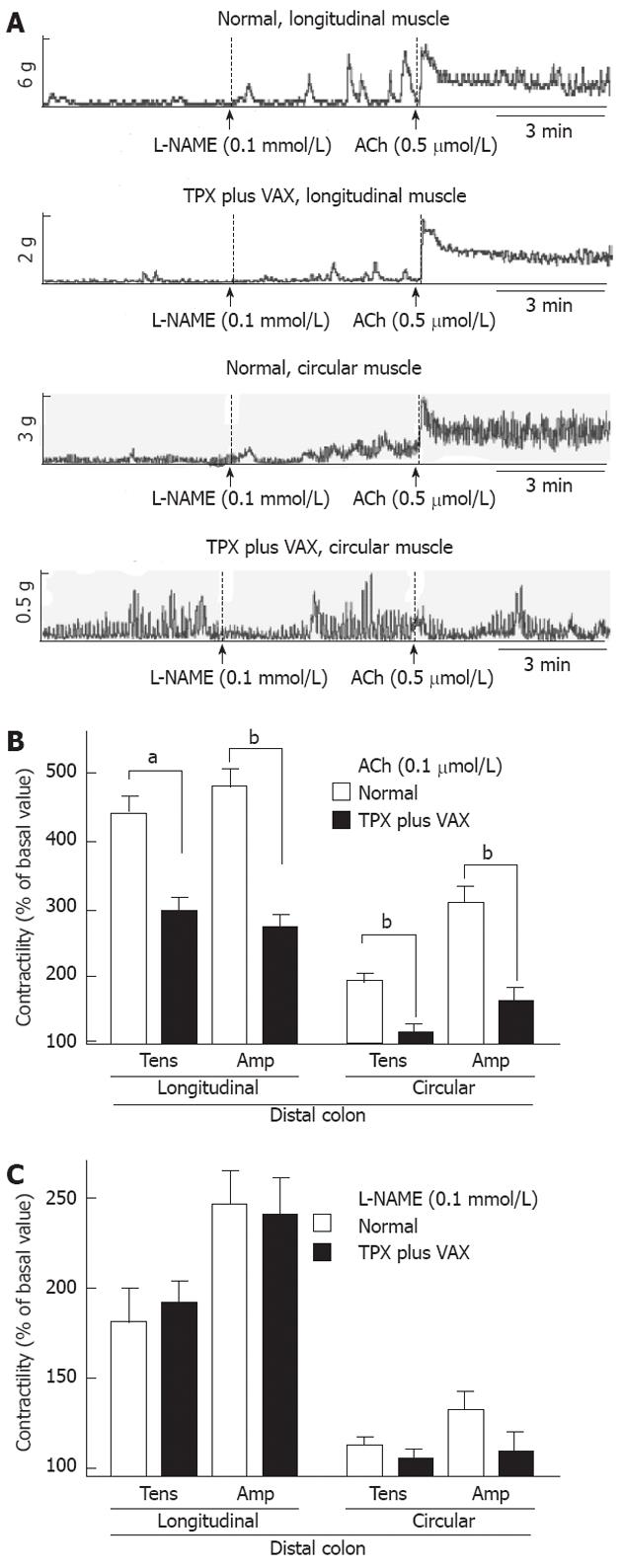
RESULTS: In comparison with normal and sham-operated rats, rats which received unilateral TPX plus VAX showed a significant decrease in body weight and in fecal pellet number and weight throughout the entire week. Application of TPX plus VAX to rats markedly delayed gastric emptying and small bowel transit. In TPX plus VAX rats, the longitudinal muscles of the proximal colon showed a significant reduction in contractile responses to acetylcholine (5 × 10-6 mol/L), and a dramatic attenuation of contractile responses was also observed in both the longitudinal and circular muscles of the distal colon. However, the spontaneous contractility of the colonic strips from TPX plus VAX rats was not significantly affected by treatment with N-nitro-L-arginine-methyl ester (0.1 mol/L).
CONCLUSION: The results indicate that unilateral TPX plus VAX reduced the motor function of the GI tract in rats, and the reduced gut motility is likely mediated, at least in part, by inhibition of the excitatory neurotransmitter system.
- Citation: Lee JH, Kwon OD, Ahn SH, Choi KH, Park JH, Lee S, Choi BK, Jung KY. Reduction of gastrointestinal motility by unilateral thyroparathyroidectomy plus subdiaphragmatic vagotomy in rats. World J Gastroenterol 2012; 18(33): 4570-4577
- URL: https://www.wjgnet.com/1007-9327/full/v18/i33/4570.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i33.4570
Vagal nerves and thyroid hormones regulate the physiological functions of the gastrointestinal (GI) tract. The subdiaphragmatic vagal nerve and its branches innervate the GI tract from the lower esophageal sphincter to the upper GI tract in humans[1,2], and the extensive distribution of vagal nerves to the gut plays an important role in the regulation of many digestive functions including food intake, gastric accommodation and GI motility[1,3]. Therefore, abnormalities in the subdiaphragmatic vagal nerves pathologically mediates the development of several GI diseases including dyspepsia and constipation[1,3,4]. In addition, hyper- and hypo-thyroidism also gives rise to a variety of GI symptoms including abnormal gastric secretion and GI motility[5,6]. The effects of hypothyroidism on the GI tract seem to be multi-factorial with alterations in neuroregulation, and a reduction in peristaltic activity being the most frequent GI complaint in patients with hypothyroidism[7].
It has been suggested that the vagal nerves predominantly act on the stomach and small intestine rather than the lower GI tract, and that subdiaphragmatic vagotomy (VAX) results in a transient slowing of the rate of gastric emptying and transit of the upper small intestine[8-10]. In our preliminary studies, rats which received subdiaphragmatic VAX transiently showed delayed gastric emptying and small bowel transit after surgery. Additionally, it has been reported that a complete depletion of thyroid hormones by bilateral thyroparathyroidectomy (TPX) in rats can induce a severe tetany which has a high mortality rate[11]. We also observed a similar phenomenon in preliminary studies, where bilateral TPX caused an extremely high fatality rate ≥ 90%) of rats within 48 h after surgical application. Taken together, we consider that bilateral TPX or subdiaphragmatic VAX alone is not a reliable and reproducible method to induce a long-term reduction in upper GI motility.
Therefore, we hypothesized that in comparison with bilateral TPX or subdiaphragmatic VAX, a combined method (TPX plus VAX) of unilateral TPX and subdiaphragmatic VAX might be a better strategy to provide a long-term reduction in spontaneous motility of the entire GI tract, but with reduced postoperative mortality. To examine this hypothesis, this study measured the effects of unilateral TPX plus VAX on the alteration of GI motility using rats, which are not only a popular laboratory animal for biomedical research, but also are a good model for studying the pharmacological properties of medicines, and for investigating the pathophysiological characteristics of human GI dysfunction[12,13].
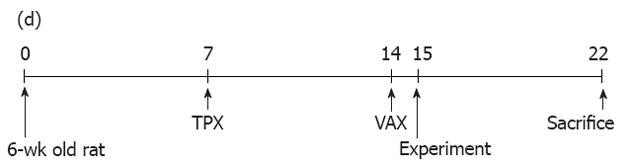
Male Sprague-Dawley rats (6-wk old) were obtained from Samtako BioKorea (Kyungki, South Korea) and acclimatized for 1 wk with free access to a solid normal rodent diet (Samyang Co., Kyungki, South Korea) and drinking water under a temperature of 23 °C ± 2 °C, a relative humidity of 50%-60% and a 12-h/12-h dark-light cycle. After acclimatization, rats were randomly divided into 3 groups: normal, sham-operated and unilateral TPX plus VAX groups. Each group comprised 7-9 rats. The experimental procedures employed in this study are illustrated in Figure 1. All experiments were conducted in accordance with the institutional guidelines established by the Wonkwang University Commolittee for the Care and Use of Laboratory Animals.
Unilateral TPX was carried out according to the previous method with a slight modification[14]. In brief, rats (7-wk old) were anesthetized using chloropent (5 mg/kg ip) for deep anesthesia, and thyroparathyroid glands on the right side were exposed by a mid-line incision of the neck under aseptic conditions and excised under stereomicroscopic observation. For sham-operated rats, the operation was performed using the same procedures except with no dissection of the thyroparathyroid glands. After surgery, animals were individually housed for 1 wk in cages with bedding of wood shavings.
At 7 d after unilateral TPX, subdiaphragmatic VAX was carried out according to a previous report with a slight modification[15]. Briefly, rats (8-wk old) were anesthetized using chloropent (5 mg/kg ip), and the abdomen around the stomach was opened under aseptic conditions. The ventral and dorsal branches of the subdiaphragmatic vagal nerves were cut (0.5 cm above the gastroesophageal junction). In sham-operated rats, the vagal trunks were similarly exposed, but without cutting of the vagal nerves. At the end of surgery, the completeness of VAX was verified by gastric responses to electrical stimulation, and inspection of the vagal nerve endings using a stereomicroscope as described in previous reports[8,15]. After dissecting the vagal nerves, animals were individually acclimatized for 1 d in wood-bedded cages and then housed in stainless-steel metabolic cages throughout the entire experimental period. Body weight, dietary intake, and fecal pellet number and weight were measured daily (9:00 am) for 7 d.
Gastric emptying was measured according to the previous method with a slight modification[16]. At 8 d after subdiaphragmatic VAX, rats were starved overnight but with drinking water available ad libitum. Each animal was sacrificed by cervical dislocation 10 min after oral administration of a test meal (1 mL) containing 0.05% (w/v) phenol red in aqueous carboxymethyl cellulose (4.5%, w/v), and the stomach was immediately removed, followed by clamping of the gastro-esophageal and gastro-duodenal junctions. The stomach was rinsed with phosphate-buffered saline (PBS, pH 7.4) and dissected in 20 mL 0.1 mol/L NaOH. The stomach, containing phenol red solution, was shaken for 30 min and centrifuged at 3000 g for 10 min. To precipitate proteins, 0.5 mL of supernatant was added to 20% trichloroacetic acid (50 mL) and mixed vigorously. The samples were centrifuged at 10 000 g for 30 min, and 0.3 mL supernatant was mixed with 0.5 mol/L NaOH (0.4 mL) to develop the color. The absorbance of samples at 558 nm wavelength was measure with a spectrophotometer (U-2000; Hitachi, Tokyo, Japan). Gastric emptying was estimated from the following formulation: (amount of residual phenol red/phenol red present in stomach immediately after administration) × 100.
To examine the spontaneous motility of the small intestine, a small bowel transit test was measured an established method[17]. Briefly, rats at 8 d after application of subdiaphragmatic VAX were starved overnight but with drinking water available ad libitum. Each animal was orally administered 1 mL of a charcoal meal, 10% (w/v) activated charcoal in aqueous Arabic gum (5%, w/v), and sacrificed by cervical dislocation 20 min later. The gastro-duodenal and ileo-cecal junctions were tied, and the entire small intestine was then quickly removed to avoid stretching. After rinsing the intestine with PBS, small bowel transit was estimated by comparing the distance travelled by the charcoal meal from the pyloric sphincter to the total length of the small intestine from the pyloric sphincter to the ileo-cecal junction. The distance travelled by the charcoal meal was expressed as a percentage of the total length of the small intestine.
At 8 d after subdiaphragmatic VAX, rats were starved overnight but with drinking water available ad libitum and sacrificed by CO2 asphyxiation and cervical dislocation. The entire proximal and distal colons were removed, and the luminal contents were flushed out using Krebs-Ringer bicarbonate buffer (composition 118 mmol NaCl, 4.7 mmol KCl, 1.2 mmol KH2PO4, 1.2 mmol MgSO4, 2.6 mmol CaCl2, 25 mmol NaHCO3 and 11.5 mmol D-glucose, pH 7.4). Strips (1 cm length) of proximal colon at a distance of 1 cm from the ileo-cecal junction and distal colon and at a distance of 1 cm from the anus were dissected. The intact colonic strips were longitudinally or circularly mounted in an organ bath (10 mL) containing Krebs-Ringer bicarbonate buffer bubbled with 5% CO2/95% O2 and maintained at 37 °C. One end of each colonic strip was tied with suture silk and fixed to the bottom of the organ bath, and the other end was connected to an isometric force transducer (Grass Technologies, West Warwick, RI).
Colonic contractility was measured as described in our previous report[18]. Briefly, after the colonic strips were allowed to equilibrate for 30-60 min with washout every 10 min, 1 g of tension was slowly applied to the tissues before starting the experiments. The strips were treated with 0.5 mol of acetylcholine (ACh) or 0.1 mmol of N-nitro-L-arginine methyl ester (L-NAME) for 10 min, and the contractile responses of the colonic strips prepared from the unilateral TPX plus VAX rats to ACh and L-NAME were estimated and compared with the colonic contractility in strips of normal animals. Isometric contractile responses from the force transducer were measured by a biological recording system equipped with an amplifier (PowerLab 4/25, AD Instruments, Colorado Springs, CO, United States). The mean tensions and amplitudes of colonic contraction produced under resting and drug-treated conditions were measured over a period of 10 min.
To estimate the change of serum thyroxine (T4) level caused by unilateral TPX, total serum T4 level at 15 d after application of unilateral TPX were measured using the T4 enzyme-linked immunosorbent assay kit (GenWay Biotech; San Diego, CA, United States). Blood samples were collected from the abdominal vein at the time of sacrifice, and serum was obtained by centrifugation at 3000 g for 10 min. Serum samples were stored at -70 °C until T4 analysis.
All results are presented as mean ± SE. One-way analysis of variance was performed to compare the statistical significance of multi-groups, followed by post hoc analysis by the Student-Newman-Keuls test for comparison of 2 groups. P < 0.05 was considered statistically significant.
No deaths occurred in the rats which received unilateral TPX plus VAX. In these rats, an obvious dilatation of the stomach and significant residual luminal content in the GI tract were found, but marked morphological changes of the gut were not observed. There were no significant differences in visible features and behaviors among the normal (4.74 ± 0.85 g/dL), sham-operated (4.97 ± 1.14 g/dL) and unilateral TPX plus VAX rats (2.86 ± 0.98 g/dL). Serum T4 levels in the unilateral TPX plus VAX rats were not significantly lower than those in the normal and sham-operated animals.
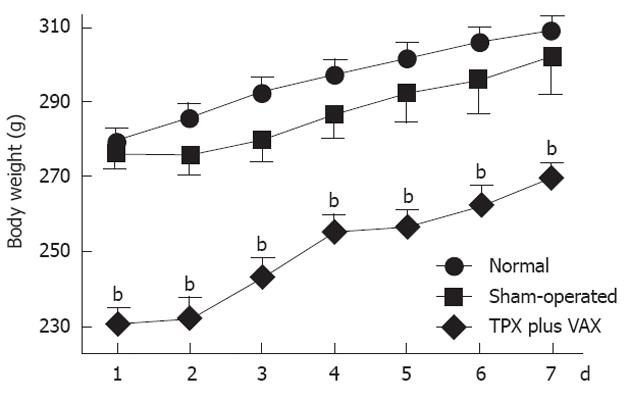
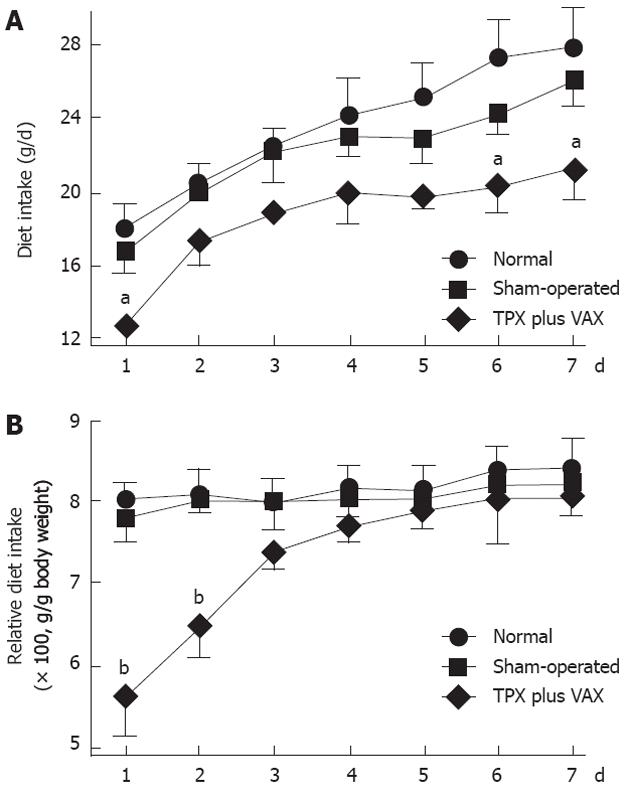
As shown in Figure 2, body weights of unilateral TPX plus VAX rats were significantly lower than those of normal and sham-operated animals throughout the entire experimental period. However, there was no significant difference in body weights between normal and sham-operated rats. Daily dietary intake of the unilateral TPX plus VAX rats was not significantly lower than in normal and sham-operated animals throughout the entire experimental period (Figure 3A). The relative daily dietary intake in unilateral TPX plus VAX rats transiently decreased, but almost completely returned to normal levels after 4 d (Figure 3B).
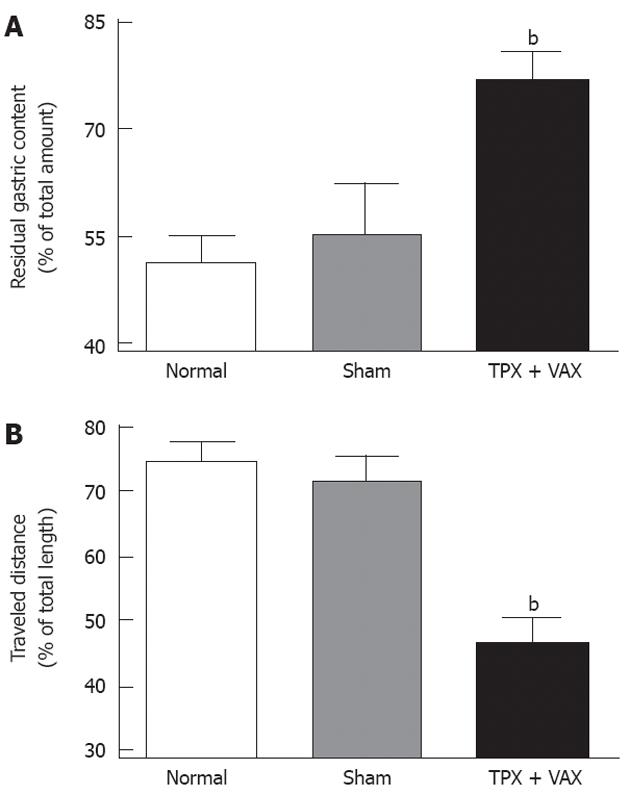
The motor functions of upper GI tracts were estimated by the gastric emptying test and small bowel transit in vivo, and the obtained results are shown in Figure 4. In comparison with normal and sham-operated rats, the residual gastric contents of unilateral TPX plus VAX rats were significantly elevated (Figure 4A), and the traveled distances of the luminal content in the small intestines of unilateral TPX plus VAX rats were markedly shortened (Figure 4B).
Defecatory function was determined by measuring fecal pellet numbers and weights, and the obtained results are shown in Figure 5. In comparison with normal and sham-operated rats, daily fecal pellet numbers of unilateral TPX plus VAX rats were significantly reduced throughout the entire experimental periods (Figure 5A), and their daily fecal weights were markedly reduced for the first 4 d of the experimental period (Figure 5B).
To examine changes in the spontaneous contractility of colonic smooth muscles, the contractile responses of intact colonic strips to ACh (0.5 mol) and L-NAME (0.5 mmol) were measured. The representative tracings obtained for proximal and distal colons are shown in the Figures 6A and 7A, respectively. In comparison with normal rats, the contractile responses to ACh (0.5 mol), both tension and amplitude, of the proximal colon longitudinal muscles prepared from the unilateral TPX plus VAX rats were significantly attenuated, whereas no significant changes were observed in the circular muscles (Figure 6B). L-NAME-induced contractility of the proximal colon did not significantly differ between the normal and unilateral TPX plus VAX rats (Figure 6C). Additionally, ACh-induced contractions of the distal colonic longitudinal and circular muscles prepared from the unilateral TPX plus VAX rats were significantly reduced compared with those of normal animals (Figure 7B), whereas the contractile responses of colonic strips to L-NAME did not significantly differ between normal and unilateral TPX plus VAX rats (Figure 7C).
To produce a rat model of a long-term reduction of the physiological motility of the entire GI tract, a partial depletion of thyroparathyroid hormones and denervation of subdiaphragmatic vagal nerves were sequentially applied to rats. In the rats receiving unilateral TPX plus VAX, an obvious dilatation of the stomach and significant residual luminal contents of GI tracts were found without marked morphological changes of the gut, and gastric emptying and small bowel transit were significantly delayed. The rats also showed a significant retardation of defecatory function. Additionally, we observed a significant reduction in distal colonic contractile responses to ACh, but not to L-NAME. These findings possibly indicate that a partial depletion of thyroparathyroid hormones and denervation of subdiaphragmatic vagal nerves decrease the spontaneous motility of the entire GI tracts through a reduction in the physiological activity of excitatory neurotransmitter systems. These dysfunctions are likely the pathological characteristics of the GI diseases related to reduced GI motility in humans.
Although a variety of animal models has been developed to examine the pathological mechanisms involved in the reduction of GI motility and to evaluate the pharmacological properties of drugs[19-22], it is unfortunately difficult to translate the findings of these animal models to human GI diseases. Although pathological characteristics of the bilateral TPX- or subdiaphragmatic VAX-induced animal models have been intensively investigated[1,3,6], the precise values for the combined method of unilateral TPX and subdiaphragmatic VAX are entirely, to our knowledge, lacking. Moreover, in the preliminary studies, we observed several scientific and methodological limitations of bilateral TPX or subdiaphragmatic VAX alone. A complete depletion of thyroparathyroid hormones by bilateral TPX caused an extremely high fatality rate (≥ 90%) within 48 h after surgery, and subdiaphragmatic VAX transiently (less than 3 d after surgery) reduced only gastric emptying and small bowel transit, but not colonic transit. These results led us to consider that bilateral TPX or subdiaphragmatic VAX may not be a reliable and reproducible method to produce animal models of GI diseases induced by long-term functional reduction of GI motility in humans.
It has been reported that subdiaphragmatic VAX in guinea pigs increased daily food intake and weight gain, whereas the reverse occurred in rats[23,24], and a pathological depletion of thyroid hormones causes a reduction in normal weight gain in rats[14]. These findings are partially in accord with our findings, indicating that unilateral TPX plus VAX significantly decreases body weight gain, but not daily dietary intake. We carefully consider that these may be related to the experimental methods used, which may differently regulate the physiological functions of gut including gastric secretion and bowel motility, resulting in different magnitudes of food intake and weight gain. This is supported by previous reports[1,25], suggesting that alteration of food intake and weight gain following bilateral TPX and subdiaphragmatic VAX is absolutely dependent on the ability of the GI tract to digest food.
The delayed gastric emptying in some patients with hypothyroidism is likely to result from reduced muscle contractility of the antrum[26], and vagal nerves are involved in the regulation of contractile patterns during gastric phase III[27]. In rats, chronic hypothyroidism or hyperthyroidism alters the expression patterns of cholinergic muscarinic receptors distributed in the ileal muscles[28], and administration of carbachol obviously increases ileal transit decline by vagal denervation[8]. These suggestions are well in accord with our results, indicating that unilateral TPX plus VAX markedly delays gastric emptying and small bowel transit. Although this study did not evaluate the cellular mechanisms implicated in the unilateral TPX plus VAX-induced reduction of upper GI motility, the results found in this study likely suggest that unilateral TPX plus VAX may be a reliable and reproducible method to produce a rat model characterized by a long-term reduction in gastric emptying and small bowel transit.
A previous report[8] suggested that colonic transit was delayed soon after subdiaphragmatic VAX, but returned to essentially normal levels at a later time. We also observed a similar effect of subdiaphragmatic VAX on defecatory function. Importantly, a reduction in defecatory function was maintained for 7 d when unilateral TPX and subdiaphragmatic VAX were sequentially applied to rats. These results clearly indicated that unilateral TPX plus VAX obviously reduces the colonic motor activity which propels the luminal contents, and the reduced propulsive activity of the upper and lower GI tract is maintained for long time periods compared with subdiaphragmatic VAX alone. Moreover, it is considered that a significant decrease in the daily numbers of fecal pellets is possibly caused by a reduction in the defecatory function of the colon, and this likely reflects the reduction in motor function of colonic smooth muscle observed in patients with constipation and defecatory disorders[25].
It has been well known that ACh[29] is the major excitatory neurotransmitter, and nitric oxide[30] is the major non-adrenergic, non-cholinergic inhibitory neurotransmitter in the regulation of colonic motility. Therefore, to assess the alteration in excitatory and inhibitory neurotransmitter systems by unilateral TPX plus VAX, we examined the contractile responses of colonic strips to ACh and L-NAME. In comparison with colonic strips of normal rats, the colonic strips prepared from unilateral TPX plus VAX rats exhibited a markedly impaired contractile response to ACh, but the contractile response to L-NAME was not significantly altered. We also found that unilateral TPX plus VAX did not significantly affect the contractile properties of adrenergic and purinergic inhibitory mechanisms (data not shown). These results are possibly suggestive of cholinergic neuronal remodeling during a partial depletion of thyroparathyroid hormones and disruption of subdiaphragmatic vagal nerves, resulting in functional reduction in colonic motility leading to pathological conditions such as constipation. Findings comparable with our results have previously been reported, suggesting that depletion of thyroparathyroid hormones reduces GI motility through a reduction in excitatory neurotransmitter activity[6]. Therefore, the results found in this study suggest that, in addition to dysfunction of vagal nerves regulating GI motility, the reduced colonic contractility may result from enteric excitatory neuronal loss associated with a decrease in thyroparathyroid hormones. However, further studies are needed to investigate the detailed cellular mechanisms involved in the unilateral TPX plus VAX-induced reduction of GI motility.
Taken together, sequential application of unilateral TPX and subdiaphragmatic VAX to rats may be a reliable method to produce a model of delayed gastric emptying and defecatory disorders in rats. In comparison with bilateral TPX or subdiaphragmatic VAX alone, TPX plus VAX showed valuable advantages, including no animal deaths and long-term persistence of reduced GI motility. Rats sequentially undergoing unilateral TPX and subdiaphragmatic VAX may be taken into consideration as a putative animal model for disorders of GI motility such as dyspepsia and constipation.
The prevalence of dyspepsia and constipation in humans continues to increase throughout the world. Although attenuation of gastric and colonic motility is widely considered as the primary causal factors for the occurrence of dyspepsia and constipation, respectively, there are still many questions to be answered, and a useful animal model to evaluate the pathophysiological mechanisms responsible for the reduction of gut motility remains to be developed.
Thyroid diseases affect gastrointestinal (GI) functions including disturbance of gut motility, and the most common GI complaints in patients with hypothyroidism are nausea, vomiting, constipation and abdominal pain. The vagal nerves are extensively distributed in the digestive tracts from the upper esophageal sphincter muscles to the transverse colon in humans, and the extensive distribution of vagal nerves to the gut provides a key bi-directional link with the brain, which regulates GI behavior such as food intake, gastric accommodation and muscle contractility.
It has been demonstrated in our study using rats that complete depletion of thyroparathyroid hormones by thyroparathyroidectomy caused an extremely high fatality rate, and the disturbance of vagal activity by thyroparathyroidectomy produced a transient, less than 3 d, reduction of gastrointestinal motility. However, in comparison with thyroparathyroidectomy or vagotomy alone, application of both unilateral thyroparathyroidectomy and subdiaphragmatic vagotomy to rats had several advantages, including no deaths of the animals and a long-term, at least 7 d, reduction in gut motility. In the present study, the authors suggest for the first time that both unilateral thyroparathyroidectomy and vagotomy may be reliably applied to produce a rat model of GI disorders characterized by long-term reduction in GI motility.
Rats subject to both unilateral thyroparathyroidectomy and subdiaphragmatic thyroparathyroidectomy may provide an animal model of dyspepsia and constipation, and may be usefully used in studies of new drugs to reverse the reduced motility of the GI tract.
Dyspepsia: A condition of impaired gastric digestive function associated with gastritis and gastroesophageal reflux. The common symptoms of dyspepsia are upper abdominal fullness, bloating, nausea, heartburn or feeling full earlier than expected.
The authors have attempted to develop a novel animal model for reducing gastrointestinal motility in aid of studies examining dyspepsia and constipation. This is a straightforward study that addresses an important issue.
Peer reviewer: Daniela M Sartor, Clinical Pharmacology and Therapeutics Unit, Department of Medicine, Austin Health, Heidelberg, Victoria 3084, Australia
S- Editor Gou SX L- Editor Cant MR E- Editor Li JY
| 1. | Andrews PL, Sanger GJ. Abdominal vagal afferent neurones: an important target for the treatment of gastrointestinal dysfunction. Curr Opin Pharmacol. 2002;2:650-656. [PubMed] [Cited in This Article: ] |
| 2. | Berthoud HR, Patterson LM, Neumann F, Neuhuber WL. Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat Embryol (Berl). 1997;195:183-191. [PubMed] [Cited in This Article: ] |
| 3. | Takahashi T, Owyang C. Characterization of vagal pathways mediating gastric accommodation reflex in rats. J Physiol. 1997;504:479-488. [PubMed] [Cited in This Article: ] |
| 4. | Neya T, Takaki M, Nakayama S. Effects of vagotomy on feeding and defecation in guinea pigs. Acta Med Okayama. 1979;33:371-377. [PubMed] [Cited in This Article: ] |
| 5. | Miller LJ, Gorman CA, Go VL. Gut-thyroid interrelationships. Gastroenterology. 1978;75:901-911. [PubMed] [Cited in This Article: ] |
| 6. | Portela-Gomes GM, Albuquerque JP, Ferra MA. Serotonin and gastrin cells in rat gastrointestinal tract after thyroparathyroidectomy and induced hyperthyroidism. Dig Dis Sci. 2000;45:730-735. [PubMed] [Cited in This Article: ] |
| 7. | Daher R, Yazbeck T, Jaoude JB, Abboud B. Consequences of dysthyroidism on the digestive tract and viscera. World J Gastroenterol. 2009;15:2834-2838. [PubMed] [Cited in This Article: ] |
| 8. | Gutierrez LV, Kocak N, Cox AG. Alimentary transit and supersensitivity after vagotomy in the rat. Gut. 1971;12:625-628. [PubMed] [Cited in This Article: ] |
| 9. | Shafer RB, Prentiss RA, Bond JH. Gastrointestinal transit in thyroid disease. Gastroenterology. 1984;86:852-855. [PubMed] [Cited in This Article: ] |
| 10. | Yang M, Fang DC, Li QW, Sun NX, Long QL, Sui JF, Gan L. Effects of gastric pacing on gastric emptying and plasma motilin. World J Gastroenterol. 2004;10:419-423. [PubMed] [Cited in This Article: ] |
| 11. | Johansson H. Gastrointestinal motility function related to thyroid activity. An experimental study in the rat. Acta Chir Scand Suppl. 1966;359:1-88. [PubMed] [Cited in This Article: ] |
| 12. | La JH, Kim TW, Sung TS, Kim HJ, Kim JY, Yang IS. Increase in neurokinin-1 receptor-mediated colonic motor response in a rat model of irritable bowel syndrome. World J Gastroenterol. 2005;11:237-241. [PubMed] [Cited in This Article: ] |
| 13. | McDougal JN, Miller MS, Burks TF, Kreulen DL. Age-related changes in colonic function in rats. Am J Physiol. 1984;247:G542-G546. [PubMed] [Cited in This Article: ] |
| 14. | Goto S, Billmire DF, Grosfeld JL. Hypothyroidism impairs colonic motility and function. An experimental study in the rat. Eur J Pediatr Surg. 1992;2:16-21. [PubMed] [Cited in This Article: ] |
| 15. | Ghia JE, Blennerhassett P, Collins SM. Vagus nerve integrity and experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G560-G567. [PubMed] [Cited in This Article: ] |
| 16. | Tsubouchi T, Saito T, Mizutani F, Yamauchi T, Iwanaga Y. Stimulatory action of itopride hydrochloride on colonic motor activity in vitro and in vivo. J Pharmacol Exp Ther. 2003;306:787-793. [PubMed] [Cited in This Article: ] |
| 17. | Jeong SI, Lee S, Kim KJ, Keum KS, Choo YK, Choi BK, Jung KY. Methylisogermabullone isolated from radish roots stimulates small bowel motility via activation of acetylcholinergic receptors. J Pharm Pharmacol. 2005;57:1653-1659. [PubMed] [Cited in This Article: ] |
| 18. | Jeong SI, Kim YS, Lee MY, Kang JK, Lee S, Choi BK, Jung KY. Regulation of contractile activity by magnolol in the rat isolated gastrointestinal tracts. Pharmacol Res. 2009;59:183-188. [PubMed] [Cited in This Article: ] |
| 19. | Meshkinpour H, Harmon D, Thompson R, Yu J. Effects of thoracic spinal cord transection on colonic motor activity in rats. Paraplegia. 1985;23:272-276. [PubMed] [Cited in This Article: ] |
| 20. | Ro S, Hwang SJ, Muto M, Jewett WK, Spencer NJ. Anatomic modifications in the enteric nervous system of piebald mice and physiological consequences to colonic motor activity. Am J Physiol Gastrointest Liver Physiol. 2006;290:G710-G718. [PubMed] [Cited in This Article: ] |
| 21. | Serio R, Bonvissuto F, Mulè F. Altered electrical activity in colonic smooth muscle cells from dystrophic (mdx) mice. Neurogastroenterol Motil. 2001;13:169-175. [PubMed] [Cited in This Article: ] |
| 22. | Sivarao DV, Mashimo H, Goyal RK. Pyloric sphincter dysfunction in nNOS-/- and W/Wv mutant mice: animal models of gastroparesis and duodenogastric reflux. Gastroenterology. 2008;135:1258-1266. [PubMed] [Cited in This Article: ] |
| 23. | Kraly FS, Jerome C, Smith GP. Specific postoperative syndromes after total and selective vagotomies in the rat. Appetite. 1986;7:1-17. [PubMed] [Cited in This Article: ] |
| 24. | Kanno H, Kiyama T, Fujita I, Kato S, Uchida E, Tajiri T. Vagotomy suppresses body weight gain in a rat model of gastric banding. Dig Surg. 2010;27:302-306. [PubMed] [Cited in This Article: ] |
| 25. | Ebert EC. The thyroid and the gut. J Clin Gastroenterol. 2010;44:402-406. [PubMed] [Cited in This Article: ] |
| 26. | Kahraman H, Kaya N, Demirçali A, Bernay I, Tanyeri F. Gastric emptying time in patients with primary hypothyroidism. Eur J Gastroenterol Hepatol. 1997;9:901-904. [PubMed] [Cited in This Article: ] |
| 27. | Tanaka T, VanKlompenberg LH, Sarr MG. Selective role of vagal and nonvagal innervation in initiation and coordination of gastric and small bowel patterns of interdigestive and postprandial motility. J Gastrointest Surg. 2001;5:418-433. [PubMed] [Cited in This Article: ] |
| 28. | Gaginella TS, Wietecha M, Hecht RM, Kerzner B. Thyroid status and muscarinic receptor density and affinity in rat intestinal smooth muscle. Arch Int Pharmacodyn Ther. 1981;253:200-209. [PubMed] [Cited in This Article: ] |
| 29. | Tobin G, Giglio D, Lundgren O. Muscarinic receptor subtypes in the alimentary tract. J Physiol Pharmacol. 2009;60:3-21. [PubMed] [Cited in This Article: ] |
| 30. | Van Crombruggen K, Lefebvre RA. Nitrergic-purinergic interactions in rat distal colon motility. Neurogastroenterol Motil. 2004;16:81-98. [PubMed] [Cited in This Article: ] |