Published online Aug 21, 2012. doi: 10.3748/wjg.v18.i31.4162
Revised: January 10, 2012
Accepted: May 12, 2012
Published online: August 21, 2012
AIM: To investigate the cytotoxic effects of spray-dried extracts of Phyllanthus niruri in combination with cisplatin on two cancer cell lines.
METHODS: Colorectal carcinoma (HT29) and human hepatocellular carcinoma (HepG2) cells were treated with spray-dried extracts of Phyllanthus niruri (SDEPN) either alone or in combination with cisplatin at different concentrations (0.5 mg/mL and 1 mg/mL) for 4 h and 24 h. To verify and quantify cancer cells treated with these products as well as identify the cell cycle stage and cell viability, we stained the cells with propidium iodide and assessed them by flow cytometry. The percentage of cells in different cell cycle phases was quantified and data were expressed as histograms. Significant differences between groups were determined using analysis of variance and Bonferroni’s test, as indicated. A value of P < 0.05 was considered to be statistically significant.
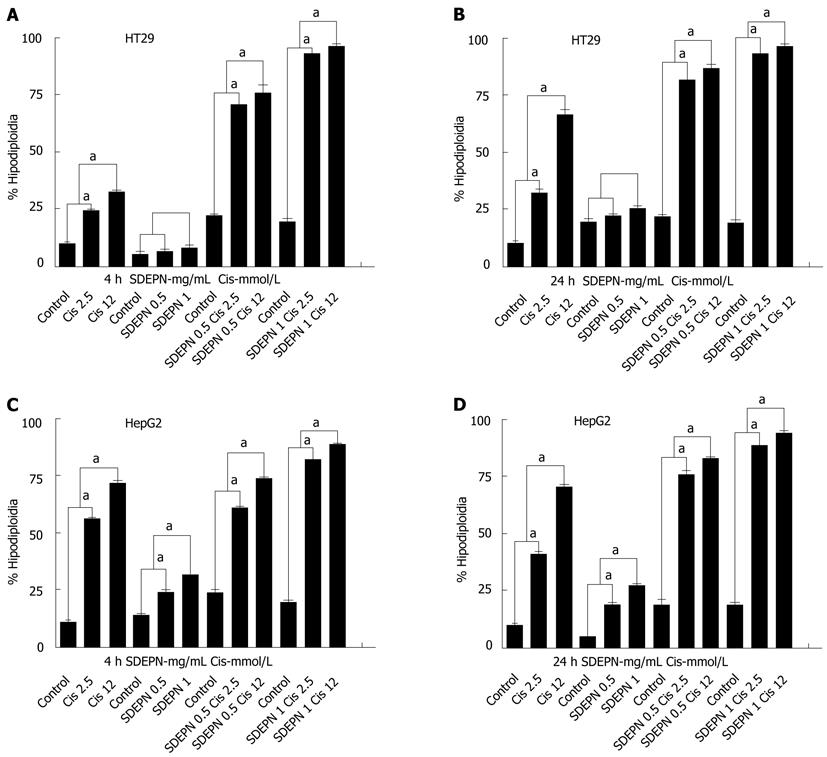
RESULTS: SDEPN had significantly different cytotoxic effects on HT29 (2.81 ± 0.11 vs 3.51 ± 1.13, P > 0.05) and HepG2 (5.07 ± 0.3 vs 15.9 ± 1.04, P < 0.001) cells when compared to control cells for 4 h. SDEPN also had significantly different cytotoxic effects on HT29 (1.91 ± 0.57 vs 4.53 ± 1.22, P > 0.05) and HepG2 (14.56 ± 1.6 vs 35.67 ± 3.94, P < 0.001) cells when compared to control cells for 24 h. Both cell lines were killed by cisplatin in a dose-dependent manner compared to control cells (HepG2 cells for 4 h: 10.78 ± 1.58 vs 53.89 ± 1.53, P < 0.001; 24 h: 8.9 ± 1.43 vs 62.78 ± 1.87, P < 0.001 and HT29 cells for 4 h: 9.52 ± 0.913 vs 49.86 ± 2.89, P < 0.001; 24 h: 11.78 ± 1.05 vs 53.34 ± 2.65, P < 0.001). In HT29 cells, pretreatment with SDEPN and subsequent treatment with cisplatin resulted in a greater number of cells being killed (12.78 ± 1.01 vs 93.76 ± 1.6, P < 0.001). HepG2 cells showed significant cell killing with treatment with SDEPN when combined with cisplatin (12.87 ± 2.78 vs 78.8 ± 3.02, P < 0.001).
CONCLUSION: SDEPN is selectively toxic against two cancer cell lines. Moreover, SDEPN in combination with cisplatin induces a synergistic increase in the cell death of both HT29 and HepG2 cells.
- Citation: Araújo Júnior RF, Soares LAL, da Costa Porto CR, de Aquino RGF, Guedes HG, Petrovick PR, de Souza TP, Araújo AA, Guerra GCB. Growth inhibitory effects of Phyllanthus niruri extracts in combination with cisplatin on cancer cell lines. World J Gastroenterol 2012; 18(31): 4162-4168
- URL: https://www.wjgnet.com/1007-9327/full/v18/i31/4162.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i31.4162
Phyllanthus niruri has many effective traditional uses for a wide variety of diseases. Some of the medicinal uses have been supported in experimental models, suggesting that the plant extracts possess various pharmacological properties. Due to its impressive preclinical therapeutic potential, extracts of species of the genus Phyllanthus have been evaluated to treat hypertension, jaundice, diabetes, hypercalciuria, and urolithiasis[1]. Other studies revealed preclinical pharmacological activity and therapeutic potential of phytochemicals isolated from Phyllanthus niruri. The species has demonstrated an antimutagenic and anticarcinogenic action[2], antitumor[3], antioxidant[4], hepatoprotective[5,6] and antihyperuricemic properties[7], as well as antihyperlipemic activity[8,9].
Phytochemicals exhibit different structural characteristics with various pharmacological actions. For example, lignans have excellent hepatoprotective[10,11] and anti-viral properties[12], whereas terpenes exhibit anti-microbial activities[13]. Flavonoids from Phyllanthus niruri have been shown to have antioxidant[14], antileishmanial[15], and anti-inflammatory properties[16]. Phytochemical studies have shown that extracts of genus Phyllanthus contain a variety of components, including gallic acid[1,17]. Furthermore, studies have demonstrated cytotoxic activity of gallic acid on the human promyelocytic leukemia HL-60 cell lines[18,19]. Gallic acid has also been shown to induce apoptotic cell death in HSC-2 and HL-60 cells[20].
More importantly, no side effects or toxicities have been reported for this herb after many years of research. Although extensive research has been conducted, there is still an abundance of unanswered questions regarding Phyllanthus niruri, particularly towards understanding the mechanism of biological activity of phytochemicals from the herb with emphasis on components that have anti-human immunodeficiency virus (HIV)[21] and anti-hepatitis B[22] properties. Phyllanthus niruri has been found to exhibit a marked inhibitory effect on the hepatitis B virus, as evidenced by its exhaustive utility in cases of chronic jaundice. However, to date, no research has focused on the identification and validation of active pharmacophores of Phyllanthus niruri that are responsible for the reported inhibitory effect of its aqueous extract on HIV[21]. Investigations have examined anti-HIV effects of the alkaloidal extract from Phyllanthus niruri on human cell lines. An alkaloidal extract of Phyllanthus niruri showed suppressive activity on strains of HIV-1 cultured on the huT-4 cell line[21].
Directed studies of botanical extracts may lead to the discovery of new agents with improved and intriguing pharmacological properties. This may be achieved by molecular modeling studies that assess the interactions of bioactive phytochemicals from Phyllanthus niruri with their respective molecular targets. Moreover, upon improvement of binding affinity to the specified target by virtual chemical modification of existing pharmacophores, new small molecules may be identified and synthesized in the laboratory[23].
Other species of the genus Phillantus, such as Phyllanthus polyphyllus and Phyllanthus emblica, have demonstrated growth inhibitory activity with a certain degree of selectivity towards the two cancer cell lines tested. It has been previously shown that cisplatin can inhibit the growth of colorectal carcinoma (HT29) and human hepatocellular carcinoma (HepG2) cells in a dose-dependent manner[20,24,25].
In this study, we investigated the antiproliferative or cell-killing activities of spray-dried extracts of Phyllanthus niruri on the human colorectal carcinoma HT29 and human hepatocellular HepG2 cell lines. In addition, we assessed the capacity of these extracts to potentiate the action of cis-diamminedichloroplatinum II (cisplatin).
A spray-dried extract of Phyllanthus niruri (SDEPN) containing 12.33 mg/g of gallic acid[26] and 94.4 mg/g of total flavonoids[27] was prepared following the manufacturer’s instructions using a Spray Dryer Production Minor (GEA Niro, Denmark).
The human colorectal cancer cell line HT-29 and human hepatocellular carcinoma cell line HepG2 were purchased from the Culture Collection of the Faculty of Medicine, University of São Paulo. The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies, Inc., Grand Island, NY, United States) supplemented with 10% (v/v) heat-inactivated fetal bovine serum.
To verify and quantify cells treated with SDEPN extract and to identify the cell cycle stage and cell viability, we stained cells with propidium iodide (PI). PI emits different fluorescence intensities depending on the phase of the cell cycle, which in turn are captured by the photomultiplier detectors in a flow cytometer (BD Facscanto II). Cancer cells were plated in 6-well plates at a cell density of 5 × 105 cells/well in 2 mL of medium. Cells were treated with SDEPN alone, cisplatin alone, or SDEPN followed by cisplatin. After 24 h, the cells were treated with a series of SDEPN concentrations (0-75 mmol/L) for 4 h or 24 h. After this incubation, the medium containing SDEPN was replaced with medium containing 2.5 or 12 μmol/L cisplatin. The cells were harvested by trypsinization when they reached approximately 80% confluence. The cells were placed in 70% ethanol and centrifuged for 5 min at 300 g. They were then resuspended in 200 μL of a PI solution (20 mL PBS, 20 μL 0.1% Triton X-100, 200 mg/mL RNase, and 20 μg/mL PI), placed in FACS tubes, and incubated for 30 min at room temperature protected from light. The labeled cells were captured with a FACScalibur cytometer (BD Bioscience, Franklin Lakes, NJ, United States) and analyzed with the software CELLQuest™ (BD Biosciences). The percentage of cells in different cell cycle phases was quantified and data were expressed as histograms.
All experiments were performed at least in triplicate. The significance of differences between groups was calculated by applying analysis of variance and Bonferroni’s test, as indicated. A value of P < 0.05 was considered to be statistically significant.
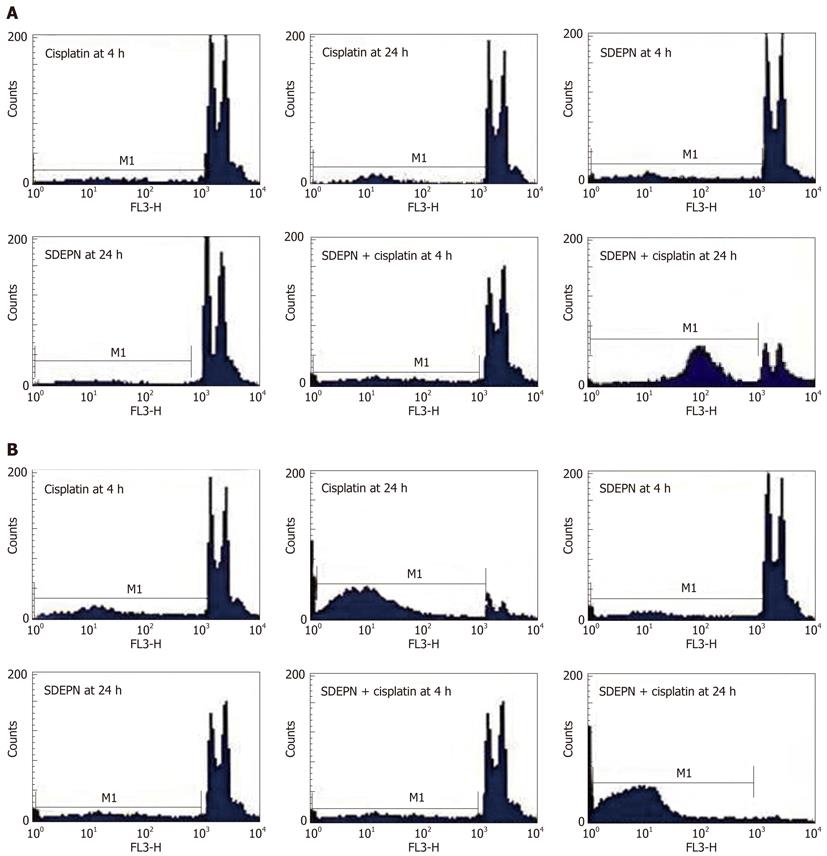
The effects of SDEPN on cell killing in two cancer cell lines were determined by flow cytometry. All cell lines were killed in a dose-dependent manner after exposure to the plant extracts (Figure 1). SDEPN had significantly different cytotoxic effects on HT29 (2.81 ± 0.11 vs 3.51 ± 1.13, P > 0.05) and HepG2 (5.07 ± 0.3 vs 15.9 ± 1.04, P < 0.001) cells when compared to control cells for 4 h. SDEPN also had significantly different cytotoxic effects on HT29 (1.91 ± 0.57 vs 4.53 ± 1.22, P > 0.05) and HepG2 (14.56 ± 1.6 vs 35.67 ± 3.94, P < 0.001) cells when compared to control cells for 24 h. These results indicated that the plant extract was selectively toxic against the cancer cells tested. We therefore hypothesized that the combination of these plant extracts with chemotherapeutic drugs may have a synergistic cytotoxic effect on these cells. Primary flow cytometry analyses are shown in Figure 2.
Using flow cytometry, we assessed the effects of cisplatin on the proliferation of HepG2 and HT29 cells. Both cell lines were killed in a dose-dependent manner compared to control cells (HepG2 cells for 4 h: 10.78 ± 1.58 vs 53.89 ± 1.53, P < 0.001; 24 h: 8.9 ± 1.43 vs 62.78 ± 1.87, P < 0.001 and HT29 cells for 4 h: 9.52 ± 0.913 vs 49.86 ± 2.89, P < 0.001; 24 h: 11.78 ± 1.05 vs 53.34 ± 2.65, P < 0.001; Figure 1). Primary flow cytometry analyses are shown in Figure 2.
HT29 cells did not undergo cell death when SDEPN was used alone (P > 0.05) but HepG2 cells (P < 0.001) showed significant cell killing with treatment with SDEPN alone (Figure 1), but also very significantly (12.87 ± 2.78 vs 78.8 ± 3.02, P < 0.001) when combined with cisplatin (Figure 1). However, HT29 cells underwent cell death (12.78 ± 1.01 vs 93.76 ± 1.6, P < 0.001) when treated with SDEPN for 24 h prior to cisplatin treatment (Figure 1). The primary images of flow cytometry analysis can be seen in Figure 2.
In HT29 cells, pretreatment with SDEPN and subsequent treatment with cisplatin resulted in a greater number of cells being killed compared to treatment with cisplatin or SDEPN alone (Figure 1A and B). This increase in the number of HepG2 cells killed in response to combination treatment was observed at the lower concentration of SDEPN (Figure 1C and D). Therefore, pretreatment with SDEPN allowed the cells (HT29) to be sensitized to killing induced by cisplatin. This suggests that this cell type is more able to adapt to stressful conditions.
In this study, the growth inhibitory activity of SDEPN extract alone and in combination with cisplatin was investigated in HT29 and HepG2 cells. Our results indicated that SDEPN extract significantly inhibited the growth of both cell lines in a dose-dependent manner when combined with cisplatin.
Cisplatin is a well-known cancer therapeutic agent that causes high toxicity to normal tissues during cancer therapy, inducing cell death through interaction of the platinum complex with DNA molecules which induces crosslinking in DNA[28,29]. The platinum compound is used to treat various types of human solid tumors[30]. The major limitation to clinical use of cisplatin is the adverse effects, mainly nephrotoxicity, caused by the compound[31]. In addition to DNA interactions, cisplatin is capable of generating oxidative stress through mitochondrial dysfunction, which results in the increased production of reactive oxygen species (ROS)[32]. Therefore, our data show that HT29 cells are more resistant to SDEPN- or cisplatin-mediated cell death, and this resistance may be related to their ability to adapt to stressful conditions. In addition, HepG2 cells were more sensitive to both treatments, suggesting that these cells may be less adapted to stress. In this study we found that the combination of SDEPN with cisplatin induced growth inhibition and cell death at different dose levels in HT29 and HepG2 cell lines.
The mechanism of action of some plant extracts is still unclear, since they are not a single active ingredient but rather consist of multiple compounds that could potentially induce the observed effects[1,23]. Plant-derived polyphenols, including gallic acid[33,34] and tannins[35], were reported to be the main components of Phyllanthus niruri extracts[1]. Therefore, the effects of SDEPN may induce apoptosis and cell death in the cell lines used in this study through multiple pathways due to the multiple components present in the extract. The active ingredients in Phyllanthus niruri that exert anticancer effects may include polyphenols, such as gallic acid, flavanoids or tannins, which are abundant in the herb. However, the detailed mechanism responsible for the anticancer effect of SDEPN extract and identification of the actual functional components require further elucidation.
It has been previously demonstrated that the effect of gallic acid on cancer cells, particularly lung cancer cells, involves caspase activation and oxidative processes[33]. In addition, gallic acid has the capacity to induce apoptosis and increase the efficacy of cisplatin in LL-2 lung cancer cells[34].
Our study provides corroborative evidence that SDEPN has selective cytotoxic effects against HT29 and HepG2 cells. Moreover, a synergistic effect was seen when the cells were treated with the extract in combination with cisplatin. This finding supports our hypothesis that combinations of plant extracts and chemotherapeutic agents may allow for a reduction in the dosage of the more toxic chemotherapeutic agent while retaining the therapeutic efficacy and minimizing toxicities. The induction of cell death by SDEPN may increase the sensitivity of HT29 cells to cisplatin-mediated cytotoxicity. Other species of the genus Phyllanthus have demonstrated growth inhibitory activity with a certain degree of selectivity towards the two cancer cell lines tested[20].
The findings of this study support our hypothesis that combinations of plant extracts and chemotherapeutic agents allow for a reduction in the dosage of the latter (i.e., cisplatin), while maintaining therapeutic efficacy. Moreover, the induction of cell death by SDEPN may be a strategy for increasing the sensitivity of HT29 cells to cisplatin-mediated cell death.
Phyllanthus niruri is a widespread tropical plant that is commonly found in coastal areas of Mexico, Argentina, and Brazil. In Brazil, some plant extracts have been distributed to patients by the Public Health Programme since 2010 as an adjunctive therapy for various diseases. The extract from aerial sections of Phyllanthus niruri was included in this list due to the long history of use in traditional medicine for the treatment of kidney and bladder diseases, intestinal infections, diabetes, and hepatitis B. Furthermore, this study has demonstrated an in vitro pro-death potential in cancer cells when Phyllanthus niruri is associated with cisplatin. In vivo studies are still needed to confirm our findings.
Currently, a variety of effective phytochemicals have been tested in cancer treatment, which are used alone or in combination with chemotherapeutic agents for treatment. A spray-dried extract from Phyllanthus niruri (SDEPN) was found to be selectively toxic against two cancer cell lines and induced an increase in cell death of HT29 and HepG2 cells when used in combination with cisplatin. HT29 cells did not undergo cell death when SDEPN was used alone. The mechanism of this effect is currently unknown, but may involve apoptosis, since a major component of SDEPN extract is gallic acid, which has been shown to induce apoptotic pathways.
In this report, the authors have demonstrated that combinations of SDEPN with cisplatin in HT29 and HepG2 cell lines have a more potent effect than either agent alone.
Spray-dried extract of SDEPN appears to be cytotoxic against hepatocellular carcinoma and less toxic against colorectal carcinoma. A combination of SDEPN extract with a chemotherapeutic agent may therefore enhance the efficacy of each component against these cancers. Future in vivo studies will be performed by our group to study the anticancer activity of SDEPN alone and in combination with cisplatin as well as the toxicity against normal tissues.
Dried herbal extracts are widely used as therapeutic products with improved pharmaceutical properties, such as stability and content uniformity. Additionally, dried extracts are successfully used to obtain solid dosage forms that contain a high dose of herbal extracts, such as capsules and tablets. Therefore, spray drying is the most commonly used procedure to obtain dried extracts (spray-dried extracts) in the herbal processing industry.
The manuscript is well written and the findings are interesting to some extent. However, some concerns need to be addressed.
Peer reviewer: Chang-Qing Su, Professor, Department of Molecular Oncology, Eastern Hepatobiliary Surgical Hospital, Second Military Medical University, Changhai Rd. 225, Shanghai 200438, China
S- Editor Gou SX L- Editor Kerr C E- Editor Zhang DN
| 1. | Calixto JB, Santos AR, Cechinel Filho V, Yunes RA. A review of the plants of the genus Phyllanthus: their chemistry, pharmacology, and therapeutic potential. Med Res Rev. 1998;18:225-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 2. | Sripanidkulchai B, Tattawasart U, Laupatarakasem P, Vinitketkumneun U, Sripanidkulchai K, Furihata C, Matsushima T. Antimutagenic and anticarcinogenic effects of Phyllanthus amarus. Phytomedicine. 2002;9:26-32. [PubMed] [Cited in This Article: ] |
| 3. | Rajeshkumar NV, Joy KL, Kuttan G, Ramsewak RS, Nair MG, Kuttan R. Antitumour and anticarcinogenic activity of Phyllanthus amarus extract. J Ethnopharmacol. 2002;81:17-22. [PubMed] [Cited in This Article: ] |
| 4. | Bhattacharjee R, Sil PC. The protein fraction of Phyllanthus niruri plays a protective role against acetaminophen induced hepatic disorder via its antioxidant properties. Phytother Res. 2006;20:595-601. [PubMed] [Cited in This Article: ] |
| 5. | Nassar AM, Helaiel EE, Ibrahim KA. Further observations on the ultrastructure of the schistosomal pigment in human liver. J Egypt Soc Parasitol. 1986;16:91-104. [PubMed] [Cited in This Article: ] |
| 6. | Manjrekar AP, Jisha V, Bag PP, Adhikary B, Pai MM, Hegde A, Nandini M. Effect of Phyllanthus niruri Linn. treatment on liver, kidney and testes in CCl4 induced hepatotoxic rats. Indian J Exp Biol. 2008;46:514-520. [PubMed] [Cited in This Article: ] |
| 7. | Murugaiyah V, Chan KL. Mechanisms of antihyperuricemic effect of Phyllanthus niruri and its lignan constituents. J Ethnopharmacol. 2009;124:233-239. [PubMed] [Cited in This Article: ] |
| 8. | Khanna AK, Rizvi F, Chander R. Lipid lowering activity of Phyllanthus niruri in hyperlipemic rats. J Ethnopharmacol. 2002;82:19-22. [PubMed] [Cited in This Article: ] |
| 9. | Latha P, Chaitanya D, Rukkumani R. Protective effect of Phyllanthus niruri on alcohol and heated sunflower oil induced hyperlipidemia in Wistar rats. Toxicol Mech Methods. 2010;20:498-503. [PubMed] [Cited in This Article: ] |
| 10. | Chang CC, Lien YC, Liu KC, Lee SS. Lignans from Phyllanthus urinaria. Phytochemistry. 2003;63:825-833. [PubMed] [Cited in This Article: ] |
| 11. | Yan F, Zhang QY, Jiao L, Han T, Zhang H, Qin LP, Khalid R. Synergistic hepatoprotective effect of Schisandrae lignans with Astragalus polysaccharides on chronic liver injury in rats. Phytomedicine. 2009;16:805-813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Gnabre JN, Itob Y, Mab Y, Huanga RC. Isolation of anti-HIV-l lignans from Larrea tridentata by counter-current chromatography. Journal of Chromatography. 1996;353-364. [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Popova MP, Chinou IB, Marekov IN, Bankova VS. Terpenes with antimicrobial activity from Cretan propolis. Phytochemistry. 2009;70:1262-1271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Hayashi Y, Matsushima M, Nakamura T, Shibasaki M, Hashimoto N, Imaizumi K, Shimokata K, Hasegawa Y, Kawabe T. Quercetin protects against pulmonary oxidant stress via heme oxygenase-1 induction in lung epithelial cells. Biochem Biophys Res Commun. 2012;417:169-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Muzitano MF, Cruz EA, de Almeida AP, Da Silva SA, Kaiser CR, Guette C, Rossi-Bergmann B, Costa SS. Quercitrin: an antileishmanial flavonoid glycoside from Kalanchoe pinnata. Planta Med. 2006;72:81-83. [PubMed] [Cited in This Article: ] |
| 16. | Guardia T, Rotelli AE, Juarez AO, Pelzer LE. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco. 2001;56:683-687. [PubMed] [Cited in This Article: ] |
| 17. | Patel JR, Tripathi P, Sharma V, Chauhan NS, Dixit VK. Phyllanthus amarus: ethnomedicinal uses, phytochemistry and pharmacology: a review. J Ethnopharmacol. 2011;138:286-313. [PubMed] [Cited in This Article: ] |
| 18. | Ishihara M, Sakagami H. Application of semiempirical method to estimate the cytotoxic activity of gallic acid and its related compounds. Anticancer Res. 2003;23:2549-2552. [PubMed] [Cited in This Article: ] |
| 19. | Sakaguchi N, Inoue M, Isuzugawa K, Ogihara Y, Hosaka K. Cell death-inducing activity by gallic acid derivatives. Biol Pharm Bull. 1999;22:471-475. [PubMed] [Cited in This Article: ] |
| 20. | Pinmai K, Chunlaratthanabhorn S, Ngamkitidechakul C, Soonthornchareon N, Hahnvajanawong C. Synergistic growth inhibitory effects of Phyllanthus emblica and Terminalia bellerica extracts with conventional cytotoxic agents: doxorubicin and cisplatin against human hepatocellular carcinoma and lung cancer cells. World J Gastroenterol. 2008;14:1491-1497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 21. | Naik AD, Juvekar AR. Effects of alkaloidal extract of Phyllanthus niruri on HIV replication. Indian J Med Sci. 2003;57:387-393. [PubMed] [Cited in This Article: ] |
| 22. | Sarkar MK, Sil PC. Hepatocytes are protected by herb Phyllanthus niruri protein isolate against thioacetamide toxicity. Pathophysiology. 2007;14:113-120. [PubMed] [Cited in This Article: ] |
| 23. | Bagalkotkar G, Sagineedu SR, Saad MS, Stanslas J. Phytochemicals from Phyllanthus niruri Linn. and their pharmacological properties: a review. J Pharm Pharmacol. 2006;58:1559-1570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 24. | Rajkapoor B, Sankari M, Sumithra M, Anbu J, Harikrishnan N, Gobinath M, Suba V, Balaji R. Antitumor and cytotoxic effects of Phyllanthus polyphyllus on Ehrlich ascites carcinoma and human cancer cell lines. Biosci Biotechnol Biochem. 2007;71:2177-2183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 25. | Krishnaveni M, Mirunalini S. Therapeutic potential of Phyllanthus emblica (amla): the ayurvedic wonder. J Basic Clin Physiol Pharmacol. 2010;21:93-105. [PubMed] [Cited in This Article: ] |
| 26. | De Souza TP, Gómez-Amoza JL, Martínez-Pacheco R, Petrovick PR. Compression behavior of formulations from Phyllanthus niruri spray dried extract. Pharmazie. 2006;61:213-217. [PubMed] [Cited in This Article: ] |
| 27. | Uchiyama T, Kamio M, Kodaka T, Tamori S, Fukuhara S, Amakawa R, Uchino H, Araki K. Leukemic cells from some adult T-cell leukemia patients proliferate in response to interleukin-4. Blood. 1988;72:1182-1186. [PubMed] [Cited in This Article: ] |
| 28. | Yunos NM, Beale P, Yu JQ, Huq F. Synergism from sequenced combinations of curcumin and epigallocatechin-3-gallate with cisplatin in the killing of human ovarian cancer cells. Anticancer Res. 2011;31:1131-1140. [PubMed] [Cited in This Article: ] |
| 29. | Gandara DR, Perez EA, Weibe V, De Gregorio MW. Cisplatin chemoprotection and rescue: pharmacologic modulation of toxicity. Semin Oncol. 1991;18:49-55. [PubMed] [Cited in This Article: ] |
| 30. | Ali BH, Al Moundhri MS. Agents ameliorating or augmenting the nephrotoxicity of cisplatin and other platinum compounds: a review of some recent research. Food Chem Toxicol. 2006;44:1173-1183. [PubMed] [Cited in This Article: ] |
| 31. | Baliga R, Zhang Z, Baliga M, Ueda N, Shah SV. In vitro and in vivo evidence suggesting a role for iron in cisplatin-induced nephrotoxicity. Kidney Int. 1998;53:394-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 32. | Martins NM, Santos NA, Curti C, Bianchi ML, Santos AC. Cisplatin induces mitochondrial oxidative stress with resultant energetic metabolism impairment, membrane rigidification and apoptosis in rat liver. J Appl Toxicol. 2008;28:337-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 33. | Ohno Y, Fukuda K, Takemura G, Toyota M, Watanabe M, Yasuda N, Xinbin Q, Maruyama R, Akao S, Gotou K. Induction of apoptosis by gallic acid in lung cancer cells. Anticancer Drugs. 1999;10:845-851. [PubMed] [Cited in This Article: ] |
| 34. | Kawada M, Ohno Y, Ri Y, Ikoma T, Yuugetu H, Asai T, Watanabe M, Yasuda N, Akao S, Takemura G. Anti-tumor effect of gallic acid on LL-2 lung cancer cells transplanted in mice. Anticancer Drugs. 2001;12:847-852. [PubMed] [Cited in This Article: ] |
| 35. | Markom M, Hasan M, Daud WRW, Singh H, Jahim JM. Extraction of hydrolysable tannins from Phyllanthus niruri Linn. Effects of solvents and extraction methods. Sep Purif Technol. 2007;52:487-496. [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 167] [Article Influence: 9.8] [Reference Citation Analysis (0)] |