Published online Jun 28, 2012. doi: 10.3748/wjg.v18.i24.3099
Revised: March 26, 2012
Accepted: May 6, 2012
Published online: June 28, 2012
AIM: To investigate morphological changes of intestinal smooth muscle contractile fibres in small bowel atresia patients.
METHODS: Resected small bowel specimens from small bowel atresia patients (n = 12) were divided into three sections (proximal, atretic and distal). Standard histology hematoxylin-eosin staining and enzyme immunohistochemistry was performed to visualize smooth muscle contractile markers α-smooth muscle actin (SMA) and desmin using conventional paraffin sections of the proximal and distal bowel. Small bowel from age-matched patients (n = 2) undergoing Meckel’s diverticulum resection served as controls.
RESULTS: The smooth muscle coat in the proximal bowel of small bowel atresia patients was thickened compared with control tissue, but the distal bowel was unchanged. Expression of smooth muscle contractile fibres SMA and desmin within the proximal bowel was slightly reduced compared with the distal bowel and control tissue. There were no major differences in the architecture of the smooth muscle within the proximal bowel and the distal bowel. The proximal and distal bowel in small bowel atresia patients revealed only minimal differences regarding smooth muscle morphology and the presence of smooth muscle contractile filament markers.
CONCLUSION: Changes in smooth muscle contractile filaments do not appear to play a major role in postoperative motility disorders in small bowel atresia.
- Citation: Gfroerer S, Fiegel H, Ramachandran P, Rolle U, Metzger R. Changes of smooth muscle contractile filaments in small bowel atresia. World J Gastroenterol 2012; 18(24): 3099-3104
- URL: https://www.wjgnet.com/1007-9327/full/v18/i24/3099.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i24.3099
Small bowel atresia is a congenital disorder of unknown pathogenesis, which carries significant morbidity[1-8]. Because of the severity of the dilatation of the proximal bowel and the hypoplasia of the distal bowel, various postoperative gastrointestinal motility problems might occur. The postoperative course can be complicated by a prolonged adynamic ileus (11%) and need for total parenteral nutrition (30%-70%)[9]. Although the underlying cause of this postoperative intestinal motility disorder is unclear, it has been clearly shown that normal gastrointestinal motility depends on the coordinated function of the enteric nervous system (ENS), the intestinal smooth muscle and the interstitial cells of Cajal (ICCs)[10]. Consequently, previous publications have examined these factors for changes in small bowel atresia patients, especially within the atretic and adjacent proximal and distal bowel. Hypertrophy of the small bowel muscle proximal to the atresia has been found in clinical and experimental studies on small bowel atresia. Furthermore, various changes have been reported within the ENS in small bowel atresia[11]. We have recently shown differential changes of the ENS and the ICCs within the proximal bowel in small bowel atresia[12]. However, the smooth muscle of small bowel atresia patients has not been studied in detail. Therefore, the aim of this study was to investigate specific contractile filaments of the smooth muscle cells in resected specimens of small bowel atresia.
Resected small bowel specimens (ileum) from term newborn small bowel atresia patients (n = 12) were included in the investigation after obtaining parental consent. The resected ileal specimens were divided into three segments (proximal, atretic and distal). Bowel specimens of two age-matched patients (who underwent surgery for Meckel’s diverticulum) served as control tissue.
The specimens were fixed in 4% paraformaldehyde, embedded in paraffin blocks and sectioned at 2-4 μm (Leica SM 2000 R) followed by drying overnight at 37 °C. Before immunohistochemical staining, the paraffin sections were dewaxed for 10 min in xylene, 10 min in acetone and 10 min in acetone/Tris-buffered saline (TBS: 1:1). After treatment, the slides were washed in TBS.
When antigen retrieval by heat was required, dewaxed paraffin sections were placed in microwave-proof tubes containing target retrieval solution (Dako). The slides were treated in the tubes for 5 min at 600 W in a microwave (SS 566H; Bosch, Munich, Germany). The evaporated volume was replaced with distilled water, and the procedure was repeated twice. After microwave treatment, the slides were cooled and washed in TBS.
Standard HE histology was performed. For immunohistochemistry, an alkaline phosphatase-anti-alkaline phosphatase (APAAP) staining kit (Dako Real™ Detection System, APAAP, Mouse) using anti-smooth muscle actin (SMA, polyclonal, Dako, 1:500) and anti-desmin (Desmin, polyclonal, Dako, 1:25) antibodies was used. A nonsense mAb (clone: MR 12/53) served as the negative control.
Immunohistochemical analysis focused on the proximal and distal parts of the resected ileum. The sections were evaluated by two independent investigators using light microscopy (magnification: 40 ×). HE staining was used to visualize the overall histology of the specimens. The distribution and density of immunoreactive SMA-positive and desmin-positive muscle filaments were studied in each part of the resected bowel (proximal, atretic and distal). Because immunohistochemical staining cannot be quantified, semi-quantitative scoring was performed as follows: - no expression, + low expression, ++ moderate expression, +++ high expression.
The study included resected ileal segments from 12 term newborn small bowel atresia patients (gestational age: 38-40 wk). Eleven patients presented with type III a ileal atresia and one patient presented with multiple ileal atresia. All patients were operated during the first or second day of life.
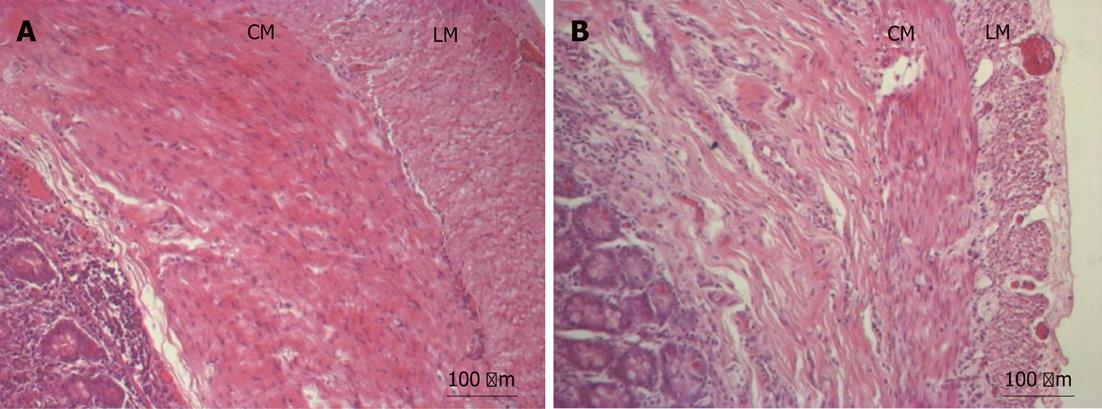
Standard HE staining revealed normal muscle components within the bowel wall. The muscle layers of the proximal bowel appeared to be slightly thicker. However, these findings were not consistent in all specimens (Figure 1).
The gross histology of the affected (proximal) bowel remained unchanged. There was a variable increase in muscle layer thickness.
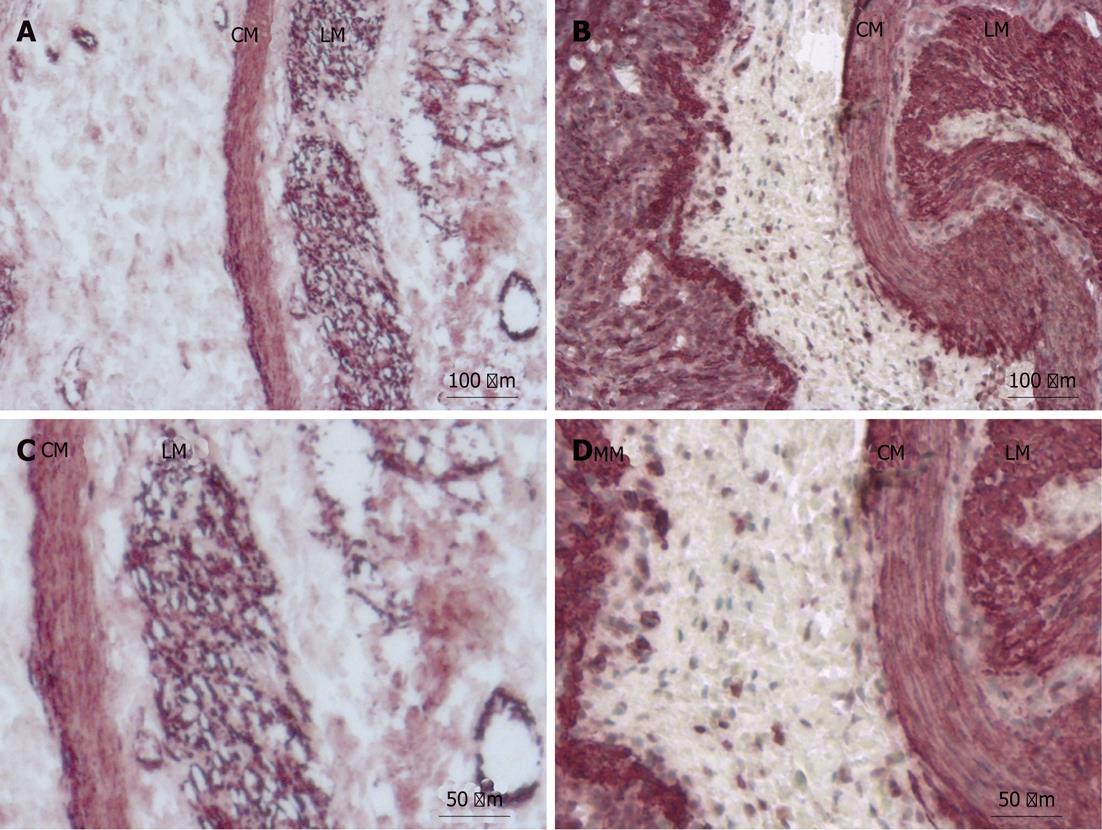
SMA expression in filaments was moderately decreased within the proximal bowel (moderate expression: ++) of small bowel atresia patients compared with control tissues (high expression: +++) (Figure 2A and C).
SMA positive filaments were uniformly less expressed in the tunica muscularis mucosa compared with the tunica muscularis propria (circular muscle, longitudinal muscle).
The distal bowel had normal expression of SMA-positive filaments (high expression: +++) within the smooth muscle of the tunica muscularis propria and the tunica muscularis mucosa compared to controls (high expression: +++) (Figure 2B and D).
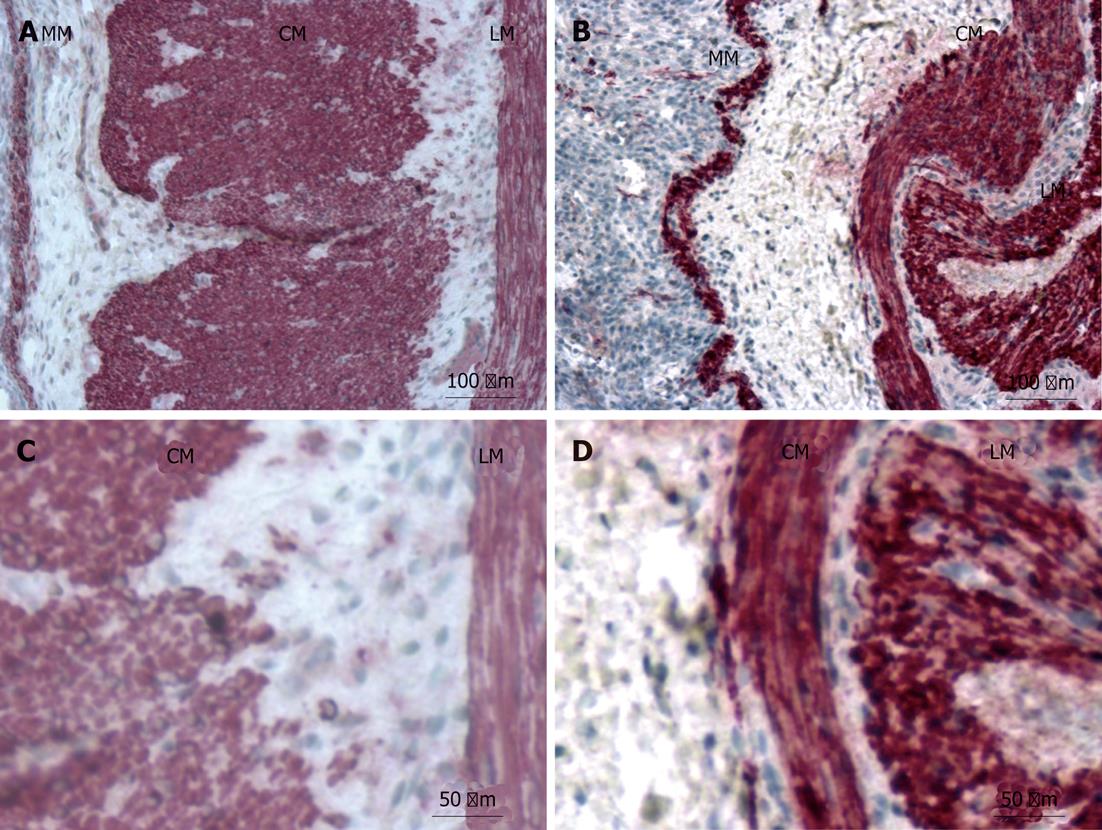
The gross histology of the proximal and distal small bowel was unchanged, as observed by desmin immunohistochemistry. The expression of desmin-positive filaments was moderately reduced within all muscle layers of the affected proximal bowel (moderate expression: ++) compared with controls (high expression: +++). There were no differences in desmin expression between the tunica muscularis propria (circular and longitudinal muscle) and the tunica muscularis mucosa within the proximal bowel (Figure 3A and C).
The distal bowel showed normal expression of desmin-positive filaments (high expression: +++) within the smooth muscle of the tunica muscularis propria and the tunica muscularis mucosa compared to controls (high expression: +++) (Figure 3B and D).
The extent of damage to the smooth muscle in small bowel atresia has not been well characterized. Previous studies of histological and ultrastructural changes of the affected bowel have investigated the ENS and the ICCs[13-20]. In these studies, differential changes of the ENS and ICCs were especially found in the proximal dilated bowel in small bowel atresia. Our present study revealed only moderate changes in the morphology of the of the smooth muscle and contractile filaments of the resected bowel proximal to the small bowel atresia. Furthermore, varying degrees of smooth muscle layer thickening was evident. The correlation between the degree of smooth muscle layer thickening and the duration of small bowel obstruction could not be evaluated since no data on the prenatal onset of the true obstruction were available.
A previous animal study creating a partial obstruction in dog ileum tissue revealed that the ganglion cells increased in size and the smooth muscle of the dilated bowel became thicker[21].
Another study used a murine partial small bowel obstruction model and revealed that 2 wk following the onset of a partial obstruction, the bowel increased in diameter, and hypertrophy of the tunica muscularis occurred oral to the obstruction site[22]. ICC networks were disrupted orally to the obstruction, and this disruption was accompanied by the loss of electrical slow waves and responses to enteric nerve stimulation. These defects were not observed aboral to the obstruction. Furthermore, it was shown that the removal of the obstruction led to the redevelopment of ICC networks and the recovery of slow wave activity within 30 d. Neural responses were partially restored in 30 d[22]. Similar repair mechanisms may occur after surgical correction of small bowel atresia. It seems obvious that decreases in ICCs in small bowel atresia and their restoration after removal of the obstruction contributes to the regulation of gastrointestinal motility.
Masumoto et al[11] showed that the circular muscle of the proximal small bowel is hypertrophied and expresses less SMA. This study showed that the ultrastructure and distribution pattern of the SMA-positive smooth muscle cells remained similar in the affected bowel compared with controls.
In another study, Masumoto et al[23] showed muscular alterations, such as abnormal smooth muscle bundles within the proximal segment of small bowel atresia. We could not confirm the existence of these abnormal smooth muscle bundles in our investigations. A case report recently revealed long lasting chronological changes within the ENS, muscle components and ICCs in small bowel atresia[24]. Interestingly, in this case, SMA-positive areas were found in both the circular and longitudinal musculature, and an increase in the proximal segment was observed at two different time points (newborn and 6 mo of age) compared with controls. The expression of SMA was similar in the distal segments compared with controls. Again, no clear changes were seen using SMA in the proximal bowel compared with the distal bowel and controls.
Ozguner et al[25] reported that the proximal segment of the atretic intestine showed structural deficits. Abnormal ganglia cells and defects in the intestinal musculature were prominent, but the intestinal mucosa remained intact. They found that abnormalities on both the antimesenteric and mesenteric sides, and their interpretation supported a vascular accident as a causative factor. Nevertheless, our study was not able to show muscular disruptions within the proximal bowel.
Previous studies have clearly shown that the ENS and ICCs are altered in the proximal and dilated bowel in small bowel atresia[12]. The innervation pattern of the proximal bowel resembles intestinal neuronal dysplasia[12]. These changes might be the result of a long-lasting bowel obstruction and bowel content stasis. Surprisingly, the smooth muscle appears hypertrophied, but substantial changes within its ultrastructure were not observed. Therefore, we believe that the moderate histological changes within the smooth muscle do not contribute to the pathogenesis of small bowel atresia. Furthermore, the moderately altered smooth muscle does not seem to play a major role in the postoperative gastrointestinal motility of the affected patients.
In conclusion the possible restoration of ICCs and the moderate changes within the contractile filaments of smooth muscle components in small bowel atresia suggest that changes within the ENS are responsible for the postoperative motility problems. Furthermore, we speculate that extensive resection of the dilated proximal bowel is necessary to restore passage in adequate time.
Small bowel atresia is a congenital anomaly of unknown cause. Despite early corrective surgery, patients carry a substantial morbidity because of postoperative gastrointestinal motility problems. Normal gastrointestinal motility is generated by the complex interaction of the enteric nervous system (ENS), the intestinal smooth muscle and the interstitial cells of Cajal (ICCs). Alterations in the ENS and ICCs may contribute to the motility problems in patients with small bowel atresia after surgery. It has not yet been investigated whether changes in the smooth muscle occur in small bowel atresia and whether these possible changes influence the postoperative course.
The relationship between the macroscopic and histological changes of the affected bowel and the postoperative motility disorder are still under investigation. Furthermore, the role of the smooth muscle and its contractile filaments in small bowel atresia needs to be further elucidated.
This study showed that the smooth muscle contractile filaments are only moderately altered in the proximal and dilated bowel in small bowel atresia. These results suggest that extensive resections of dilated proximal is not necessary in affected patients.
Previously shown changes within the ENS and the ICCs may influence postoperative gastrointestinal motility in affected patients. The moderate variations of smooth muscle contractile filaments in small bowel atresia do not seem to play a role in the postoperative course.
Bowel atresia is a congenital defect in the continuity of the bowel. The incidence of small bowel atresia is higher than that of large bowel atresia and varies between 1:300 and 1:3000.
The manuscript is well written and addresses an important concept of a clinical problem.
Peer reviewer: Kareem M Abu-Elmagd, Professor, Department of Surgery, University of Pittsburgh Medical Center, 3459 Fifth Avenue, MUH 7 South, Pittsburgh, PA 15213, United States
S- Editor Shi ZF L- Editor A E- Editor Zheng XM
| 1. | Tandler J. Zur entwicklungsgeschichte des menschlichen duodenum in fruhen embryonalstadien. Morphol Jahrb. 1900;29:187-216. [Cited in This Article: ] |
| 2. | LOUW JH, BARNARD CN. Congenital intestinal atresia; observations on its origin. Lancet. 1955;269:1065-1067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 389] [Cited by in F6Publishing: 389] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Dalla Vecchia LK, Grosfeld JL, West KW, Rescorla FJ, Scherer LR, Engum SA. Intestinal atresia and stenosis: a 25-year experience with 277 cases. Arch Surg. 1998;133:490-496; discussion 490-496. [PubMed] [Cited in This Article: ] |
| 4. | Walker K, Badawi N, Hamid CH, Vora A, Halliday R, Taylor C, Shi E, Roy GT, Simpson E, Holland AJ. A population-based study of the outcome after small bowel atresia/stenosis in New South Wales and the Australian Capital Territory, Australia, 1992-2003. J Pediatr Surg. 2008;43:484-488. [PubMed] [Cited in This Article: ] |
| 5. | Burjonrappa SC, Crete E, Bouchard S. Prognostic factors in jejuno-ileal atresia. Pediatr Surg Int. 2009;25:795-798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Stollman TH, de Blaauw I, Wijnen MH, van der Staak FH, Rieu PN, Draaisma JM, Wijnen RM. Decreased mortality but increased morbidity in neonates with jejunoileal atresia; a study of 114 cases over a 34-year period. J Pediatr Surg. 2009;44:217-221. [PubMed] [Cited in This Article: ] |
| 7. | Waldhausen JH, Sawin RS. Improved long-term outcome for patients with jejunoileal apple peel atresia. J Pediatr Surg. 1997;32:1307-1309. [PubMed] [Cited in This Article: ] |
| 8. | Festen S, Brevoord JC, Goldhoorn GA, Festen C, Hazebroek FW, van Heurn LW, de Langen ZJ, van Der Zee DC, Aronson DC. Excellent long-term outcome for survivors of apple peel atresia. J Pediatr Surg. 2002;37:61-65. [PubMed] [Cited in This Article: ] |
| 9. | Kumaran N, Shankar KR, Lloyd DA, Losty PD. Trends in the management and outcome of jejuno-ileal atresia. Eur J Pediatr Surg. 2002;12:163-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Wallace AS, Burns AJ. Development of the enteric nervous system, smooth muscle and interstitial cells of Cajal in the human gastrointestinal tract. Cell Tissue Res. 2005;319:367-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 176] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | Masumoto K, Suita S, Nada O, Taguchi T, Guo R. Abnormalities of enteric neurons, intestinal pacemaker cells, and smooth muscle in human intestinal atresia. J Pediatr Surg. 1999;34:1463-1468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Gfroerer S, Metzger R, Fiegel H, Ramachandran P, Rolle U. Differential changes in intrinsic innervation and interstitial cells of Cajal in small bowel atresia in newborns. World J Gastroenterol. 2010;16:5716-5721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Di Nardo G, Stanghellini V, Cucchiara S, Barbara G, Pasquinelli G, Santini D, Felicani C, Grazi G, Pinna AD, Cogliandro R. Enteric neuropathology of congenital intestinal obstruction: A case report. World J Gastroenterol. 2006;12:5229-5233. [PubMed] [Cited in This Article: ] |
| 14. | Watanabe Y, Ando H, Seo T, Katsuno S, Marui Y, Horisawa M. Two-dimensional alterations of myenteric plexus in jejunoileal atresia. J Pediatr Surg. 2001;36:474-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Ramachandran P, Vincent P, Ganesh S, Sridharan S. Morphological abnormalities in the innervation of the atretic segment of bowel in neonates with intestinal atresia. Pediatr Surg Int. 2007;23:1183-1186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Tander B, Bicakci U, Sullu Y, Rizalar R, Ariturk E, Bernay F, Kandemir B. Alterations of Cajal cells in patients with small bowel atresia. J Pediatr Surg. 2010;45:724-728. [PubMed] [Cited in This Article: ] |
| 17. | Khen N, Jaubert F, Sauvat F, Fourcade L, Jan D, Martinovic J, Vekemans M, Landais P, Brousse N, Leborgne M. Fetal intestinal obstruction induces alteration of enteric nervous system development in human intestinal atresia. Pediatr Res. 2004;56:975-980. [PubMed] [Cited in This Article: ] |
| 18. | Fiegel HC, Schönberg RA, Roth B, Grasshoff S, Kluth D. Submucosal plexus of dilatated gut disappears after ligation in chicken embryos: preliminary results. Eur J Pediatr Surg. 2006;16:407-410. [PubMed] [Cited in This Article: ] |
| 19. | Schoenberg RA, Kluth D. Experimental small bowel obstruction in chick embryos: Effects on the developing enteric nervous system. J Pediatr Surg. 2002;37:735-740. [PubMed] [Cited in This Article: ] |
| 20. | Parisi Salvi E, Vaccaro R, Baglaj SM, Renda T. Nervous system development in normal and atresic chick embryo intestine: an immunohistochemical study. Anat Embryol (Berl). 2004;209:143-151. [PubMed] [Cited in This Article: ] |
| 21. | Earlam RJ. Ganglion cell changes in experimental stenosis of the gut. Gut. 1971;12:393-398. [PubMed] [Cited in This Article: ] |
| 22. | Chang IY, Glasgow NJ, Takayama I, Horiguchi K, Sanders KM, Ward SM. Loss of interstitial cells of Cajal and development of electrical dysfunction in murine small bowel obstruction. J Physiol. 2001;536:555-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 170] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 23. | Masumoto K, Suita S, Taguchi T. The occurrence of unusual smooth muscle bundles expressing alpha-smooth muscle actin in human intestinal atresia. J Pediatr Surg. 2003;38:161-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Masumoto K, Akiyoshi J, Nagata K, Uesugi T, Taguchi S, Tajiri T, Taguchi T. Chronological change in intramural components in severe proximally dilated jejunal atresia: an immunohistochemical study. J Pediatr Gastroenterol Nutr. 2008;46:602-606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Ozguner IF, Savas C, Ozguner M, Candir O. Intestinal atresia with segmental musculature and neural defect. J Pediatr Surg. 2005;40:1232-1237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |