Published online Jun 7, 2012. doi: 10.3748/wjg.v18.i21.2640
Revised: February 1, 2012
Accepted: March 9, 2012
Published online: June 7, 2012
AIM: To evaluate the effect of nigericin on colorectal cancer and to explore its possible mechanism.
METHODS: The human colorectal cancer (CRC) cell lines HT29 and SW480 were treated with nigericin or oxaliplatin under the conditions specified. Cell viability assay and invasion and metastasis assay were performed to evaluate the effect of nigericin on CRC cells. Sphere-forming assay and soft agar colony-forming assay were implemented to assess the action of nigericin on the cancer stem cell properties of CRC cells undergone epithelial-mesenchymal transition (EMT).
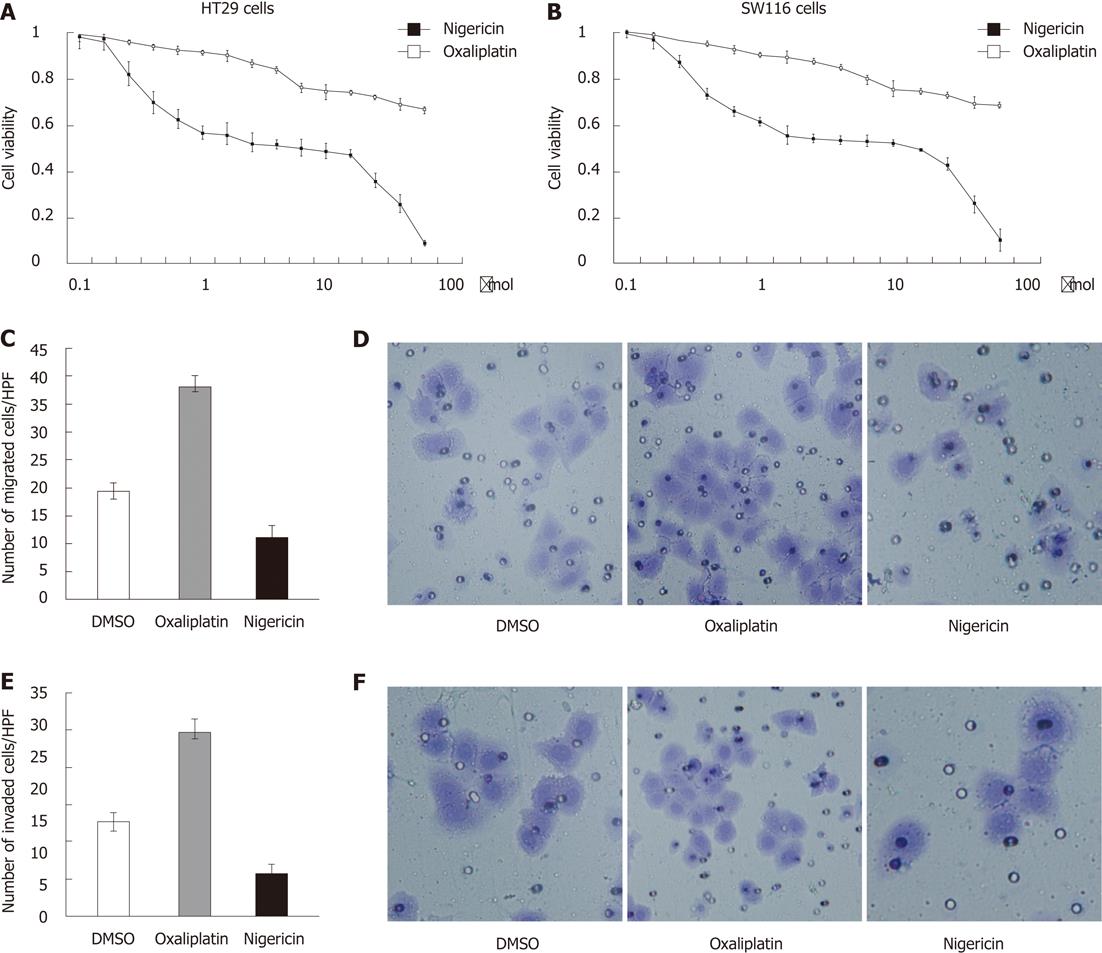
RESULTS: Compared with oxaliplatin, nigericin showed more toxicity for the HT29 cell line (IC50, 12.92 ± 0.25 μmol vs 37.68 ± 0.34 μmol). A similar result was also obtained with the SW116 cell line (IC50, 15.86 ± 0.18 μmol vs 41.02 ± 0.23 μmol). A Boyden chamber assay indicated that a significant decrease in the number of HT29 cells migrating through polyvinylidene fluoride membrane was observed in the nigericin-treated group, relative to the vehicle-treated group [11 ± 2 cells per high-power field (HPF) vs 19.33 ± 1.52 cells per HPF, P < 0.05]. Compared to the control group, the numbers of HT29 cells invading through the Matrigel-coated membrane also decreased in the nigericin-treated group (6.66 ± 1.52 cells per HPF vs 14.66 ± 1.52 cells per HPF, P < 0.05). Nigericin also reduced the proportion of CD133+ cells from 83.57% to 63.93%, relative to the control group (P < 0.05). Nigericin decreased the number of spheres relative to the control group (0.14 ± 0.01 vs 0.35 ± 0.01, P < 0.05), while oxaliplatin increased the number of spheres relative to the control group (0.75 ± 0.02 vs 0.35 ± 0.01; P < 0.05). Nigericin also showed a decreased ability to form colonies under anchorage-independent conditions in a standard soft agar assay after 14 d in culture, relative to the control group (1.66 ± 0.57 vs 7 ± 1.15, P < 0.05), whereas the colony numbers were higher in the oxaliplatin group relative to the vehicle-treated controls (14.33 ± 0.57 vs 7 ± 1.15, P < 0.05). We further detected the expression of E-cadherin and vimentin in cells treated with nigericin and oxaliplatin. The results showed that HT29 cells treated with nigericin induced an increase in E-cadherin expression and a decrease in the vimentin expression relative to vehicle controls. In contrast, oxaliplatin downregulated the expression of E-cadherin and upregulated the expression of vimentin in HT29 cells relative to vehicle controls.
CONCLUSION: This study demonstrated that nigericin could partly reverse the EMT process during cell invasion and metastasis.
- Citation: Zhou HM, Dong TT, Wang LL, Feng B, Zhao HC, Fan XK, Zheng MH. Suppression of colorectal cancer metastasis by nigericin through inhibition of epithelial-mesenchymal transition. World J Gastroenterol 2012; 18(21): 2640-2648
- URL: https://www.wjgnet.com/1007-9327/full/v18/i21/2640.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i21.2640
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in men and the second most commonly diagnosed cancer in women[1], with a 5-year survival rate < 10% for patients with metastatic disease[2]. Despite the use of active targeted drugs for treatment of metastatic CRC, the cure rate has remained low in the past decade. Activating invasion and metastasis is the hallmark of cancer[3,4], during which malignant cells spread from the primary tumor to distant organs.
The pathogenesis of metastasis involves a series of steps, often termed the invasion and metastasis cascade, which includes the following: local invasion of the host stroma by tumor cells; detachment and embolization of tumor cell aggregates; extravasation of the tumor embolus; survival of tumor cells that are transported through the circulation and stop in the capillary bed; extravasation of the tumor embolus; proliferation of the tumor cells within the organ parenchyma, resulting in a metastatic focus; and reinitiation of these processes for the development of metastases. The first and decisive step of this process is the local invasion through the epithelial basement membrane, because it requires alteration in cell-cell and cell-matrix interactions, reconstruction of the extracellular matrix, remodeling of the cytoskeleton, and enhancement of cell modulation. Great progress has been made on the capacity for invasion and metastasis over the past decade with powerful novel research tools and refined experimental models becoming available. On the other hand, many critical regulatory genes have been identified.
Epithelial-mesenchymal transition (EMT), a transdifferentiation characterized by decreased epithelial markers such as E-cadherin and increased mesenchymal markers such as fibronectin, has become prominently implicated as a means by which transformed epithelial tumor cells acquire the ability to invade, resist apoptosis, and propagate[5-9]. More importantly, EMT has been shown to result in cancer cells with stem-cell-like characteristics that have a propensity to invade surrounding tissue and display resistance to chemotherapeutic interventions[6,10,11]. Nigericin is a potassium ionophore, which has been reported to be toxic to breast stem cells passing through EMT[12]. Lu et al[13] have reported that nigericin, like salinomycin, selectively inhibits Wnt1-mediated signaling in HEK293 cells at nanomolar concentrations.
In this study, we aimed to ascertain the specific activities of nigericin on human CRC cell lines. We selected CD133 as the marker of stem cells of CRC.
Human CRC cell lines, HT29 and SW116 were used. HT29 cells were cultured in McCoy’s 5A medium (Gibco, United States) with 10% fetal bovine serum (FBS). SW116 cells were cultured in RPMI 1640 medium with 10% FBS. The cells were cultured at 37 °C in a humidified atmosphere containing 5% CO2.
Oxaliplatin and nigericin were both purchased from Sigma-Aldrich (St. Louis, MO, United States). Antibodies used for immunofluorescence staining and Western blotting were as follows: mouse anti-E-cadherin (Abcam Inc., Cambridge, MA, United States), mouse anti-vimentin (Abcam), mouse anti-CD133 (Abcam; used for Western blotting and immunocytochemistry), allophycocyanin-conjugated CD133 antibody (Miltenyi Biotec, Auburn, CA, United States) used for fluorescence-activated cell sorting (FACS), mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Abcam).
For assessment of cell viability in HT29 and SW116 cell lines under different treatments, cells growing at the exponential stage were plated in triplicate, in 96-well plates at a density of 2000 cells/well in a final volume of 100 μL. After incubation for 24 h, oxaliplatin, nigericin and dimethylsulfoxide (DMSO) control were added to each well of the plates. Cell viability was detected after 24 h using Cell Counting Kit 8 (DOJINDO, Japan). Absorbance for each well was read at 570 nm using a microplate reader. Growth inhibition was calculated as a percentage of the untreated controls. Experiments were done three or more times, often in triplicate, for each cell line, and IC50 was determined using the four-parameter logistic model.
Analysis of cell migration was performed using Boyden chambers according to the manufacturer’s protocol (Becton Dickinson Labware, Bedford, MA, United States). For cell invasion study, the inserts of the chamber were prepared by coating the upper surfaces with Matrigel (BD Matrigel Matrix, Phenol Red-free). HT29 cells (3 × 103) treated with DMSO control, oxaliplatin and nigericin in McCoy’s 5A medium without FBS were plated to the upper chamber. McCoy’s 5A with 20% FBS as chemoattractants were plated in the lower chamber of the 24-well pates. After 24 h, nonmigrating or noninvading cells were removed mechanically from the upper chamber using a cotton swab. Cells that migrated or invaded to the lower surface of the Transwell membrane were fixed in methanol for 30 min at 37 °C and stained with 0.05% crystal violet for 1 h. Cells were quantified by counting the number of stained nuclei in five individual fields by fluorescence microscopy, in triplicate.
Cell lysates were subjected to sodium dodecylsulfate polyacrylamide gel electrophoresis, and the separated proteins were electrophoretically transferred to hydrophobic polyvinylidene fluoride (PVDF) membrane. After blocking in 5% skimmed milk solution for 2 h, the membranes were incubated with the primary antibodies diluted with anti-CD133, anti-E-cadherin, and anti-vimentin. Primary antibodies were detected with mouse secondary antibodies directed against human IgG and visualized with Odyssey Infrared Imaging System.
mRNA expression was determined by real-time polymerase chain reaction. RNA was extracted by using the TRIzol reagent (Invitrogen, Carlsbad, CA, United States) and reverse transcription was performed using Superscript II (Invitrogen) according to the manufacturer’s instructions. TaqMan reactions were done utilizing an ABI 7500 real-time quantitative polymerase chain reaction (PCR) system. For data analysis, raw counts were normalized to housekeeping gene average for the same time point and condition (ΔCt). The following primers were used in this study: CD133 forward CATCCACAGATGCTCCTAAGGC and reverse GCTTTATGGGAGTCTTGGTC; E-cadherin forward CGAGAGCTACACGTTCACGG and reverse GTGTCG AGGGAAAAATAGGCTG; vimentin forward CTCCTCCCCCTGTCACATAC and reverse TGATTGGCATCAGGACCGTTG. GAPDH was used as an internal control. Analysis was performed with the ΔΔCt method.
HT29 and SW116 cells, after different treatments, were washed with PBS. Single cell suspensions were incubated with allophycocyanin (APC)-conjugated CD133 antibody (Miltenyi Biotec) for 30 min at 4 °C. Mouse IgG1-APC was selected as an isotype control body. 7-Aminoactinomycin was used to eliminate the dead cells. The labeled cells were detected by the BD FACSVantage Systems (Becton Dickinson) according to the manufacturer’s protocols. Gating was implemented on the basis of negative control staining profiles.
McCoy’s 5A with B27 supplement (Invitrogen), 20 μg/mL epidermal growth factor (Invitrogen), 20 μg/mL fibroblast growth factor (Invitrogen), and penicillin-streptomycin served as the stem cell medium (SCM) for this experiment. HT29 cells, after the indicated treatments, were plated at a concentration of 200 cells/100 μL SCM in each of the 20 wells of a 96-well ultralow-attachment plate (Corning Life Sciences, CA, United States). Cells were supplemented with 100 μL SCM after 7 d of incubation and analyzed on day 14, and MTT solution (40 μL) was added to each well, and a colorimetric assessment was done. The average absorbance measurement for each group was used as an index of sphere number.
We mixed 1.2% agar with 2 × McCoy’s 5A medium at a ratio of 1:1 to make a 0.6% agar growth medium solution. We pipetted 2 mL of the 0.6% growth medium mixture into each well of the six-well cell culture cluster (Corning Life Sciences). We avoided bubble formation and spread the mixture evenly by slowly rotating the plate. We allowed the 0.6% agar growth medium layer to harden for 30-40 min at room temperature in a sterile laminar flow hood. We determined the concentration of HT29 cells treated with DMSO control, oxaliplatin and salinomycin, and adjust the suspension to 5 × 103 cells/mL in 0.3% agar diluted with PBS. We transferred 2 mL of the cell suspension to the 0.6% agar growth medium plate and cultured at 37 °C in the presence of 5% CO2 for 14-21 d. We counted the number of colonies using a microscope.
Cells were directly sorted onto a glass slide, fixed with 4% paraformaldehyde, and stained with anti-E-cadherin, anti-CD133 and anti-vimentin monoclonal antibodies. Nuclei were identified by staining with 4’, 6-diamidino-2-phenylindole. Subcellular localizations were determined by using confocal microscopy. The fluorescence intensity of each region was analyzed by different people on three occasions.
All values were shown as mean ± SD. Statistical significance was calculated by t test unless otherwise stated (SPSS 17.0), considering P < 0.05 as statistically significant.
We examined the in vitro effect of nigericin on tumor growth and metastasis. Compared with oxaliplatin, nigericin exhibited more toxicity for the HT29 cell line (IC50, 12.92 ± 0.25 μmol vs 37.68 ± 0.34 μmol) (Figure 1A). We also obtained similar results with the SW116 cell line (IC50, 15.86 ± 0.18 μmol vs 41.02 ± 0.23 μmol) (Figure 1B). We then checked whether nigericin had functional influence on the migratory and invasive capacity of CRC cells. After incubation for 24 h, nigericin induced a conspicuous reduction in the number of cells migrating through the PVDF membrane relative to the vehicle-treated controls [11 ± 2 cells per high-power field (HPF) vs 19.33 ± 1.52 cells per HPF, P < 0.05] (Figure 1C and D). It was surprising that oxaliplatin promoted the migration of CRC cells through PVDF membrane compared with the vehicle-treated controls (38 ± 2 cells per HPF vs 19.33 ± 1.52 cells per HPF, P < 0.05) (Figure 1C and D). Compared to the control group, the numbers of HT29 cells invading through the Matrigel-coated membrane also decreased in the nigericin-treated group (6.66 ± 1.52 cells per HPF vs 14.66 ± 1.52 cells per HPF, P < 0.05) (Figure 1E and F). Correspondingly, oxaliplatin treatment increased the number of HT29 cells invading through the Matrigel-coated membrane (28.66 ± 2.08 cells per HPF vs 14.66 ± 1.52 cells per HPF, P < 0.05) (Figure 1E and F).
In order to complete subsequent experiments logically, we treated the HT29 cells with nigericin, oxaliplatin, and DMSO vehicle control for 3 d, and then replaced the culture medium containing drugs with normal McCoy’s 5A medium with 10% FBS for another 3 d incubation.
The stem cell marker prominin-1 (CD133), a pentaspan membrane protein, may not be the only marker, but it remains the most widely reported marker of cancer stem cells (CSCs) of CRC validated by different groups[14-18].
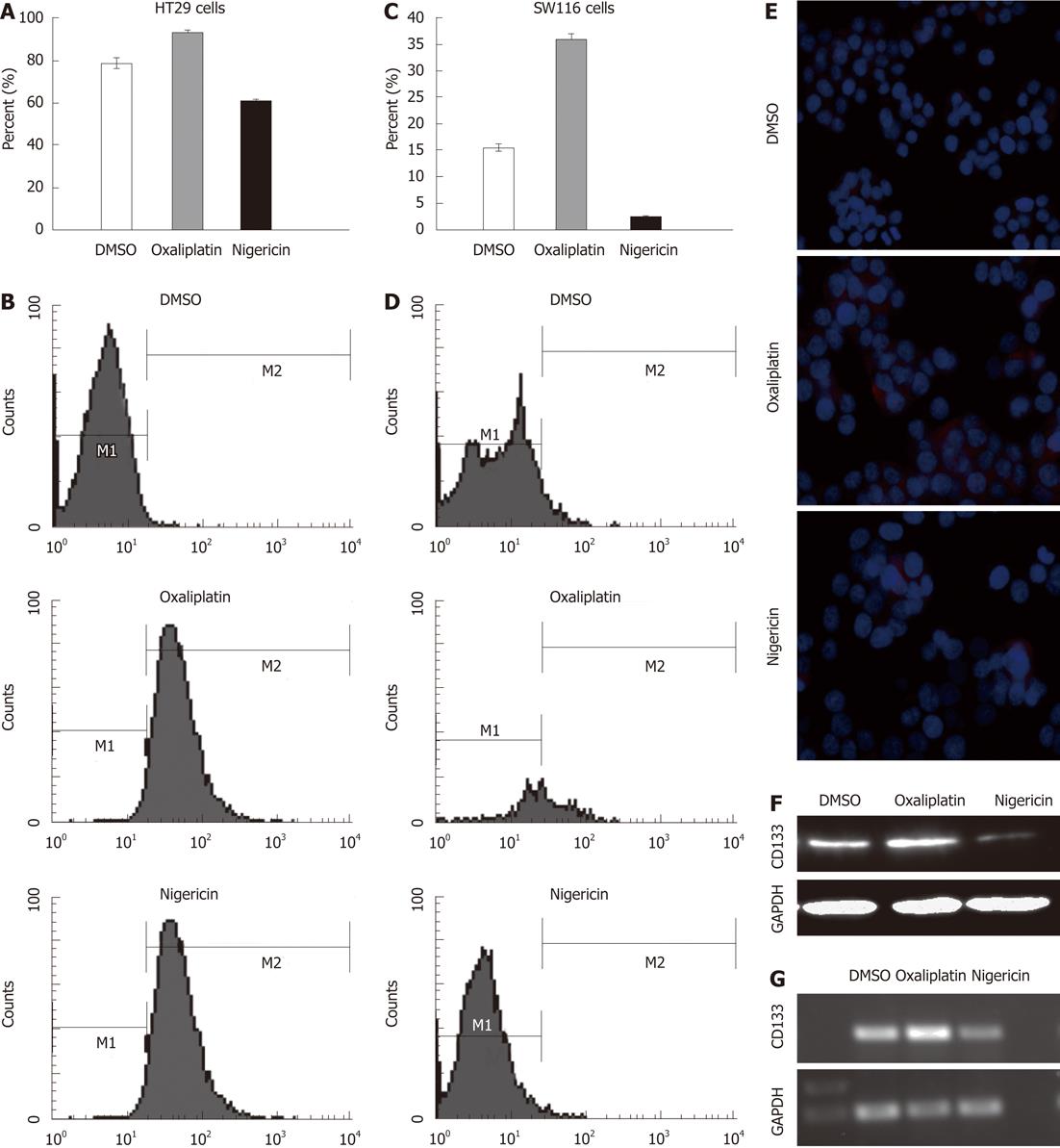
We further assessed the expression of CD133 on HT29 cells after treatment with nigericin and oxaliplatin using flow cytometry. The results demonstrated that nigericin reduced the positive rate of CD133 from 83.57% to 63.93%, relative to the control group (P < 0.05) (Figure 2A and B). In contrast, oxaliplatin treatment increased the expression of CD133 from 79.18% to 97.22%. In order to verify this result, we selected the SW116 cell line to repeat the experiment. Similarly, nigericin decreased the proportion of CD133+ cells from 4.55% to 0.31%; on the contrary, the expression rate of CD133 increased from 4.55% to 36.89% (Figure 2C and D). The data from real-time PCR, Western blotting, and immunocytochemistry indicated analogous results (Figure 2E-G).
To evaluate the ability to form colonies or spheres of HT29 cells treated with nigericin and oxaliplatin in the absence of serum and without attachment to culture plates[19]. We performed the sphere-forming assay and soft agar forming assay under serum-free conditions.
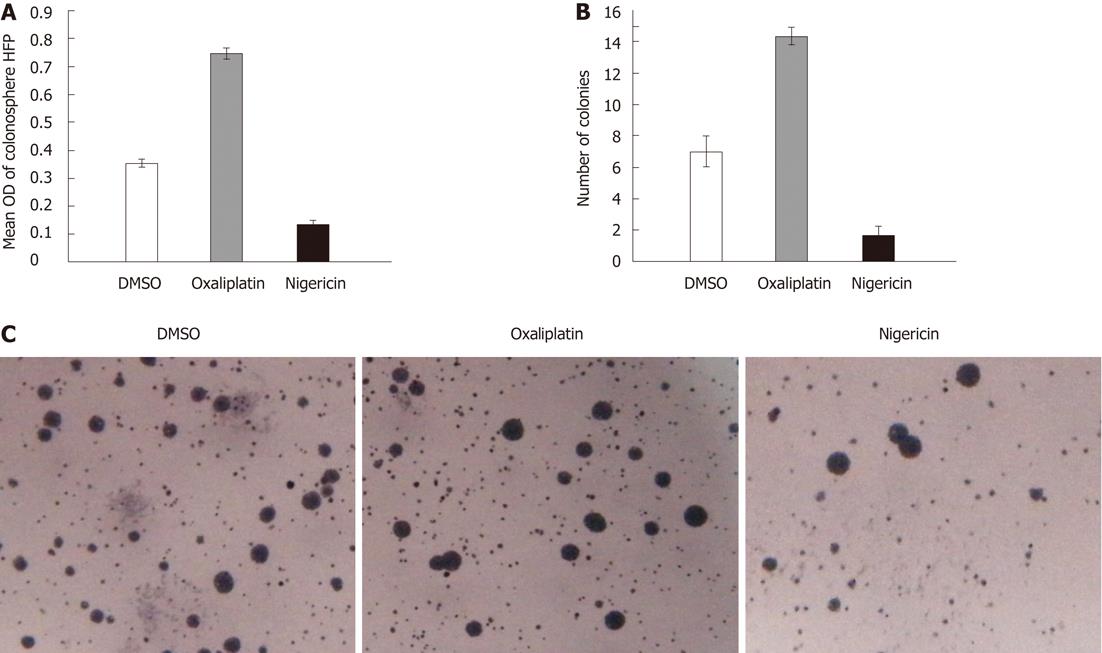
Differences between the nigericin and oxaliplatin groups were quantitated by plating a limited number of cells in each well of a low-attachment 96-well plate and evaluating the ability of HT29 cells to form colonospheres. Nigericin decreased the number of spheres relative to the control group (0.14 ± 0.01 vs 0.35 ± 0.01, P < 0.05), while oxaliplatin increased the number of spheres relative to the control group (0.75 ± 0.02 vs 0.35 ± 0.01, P < 0.05) (Figure 3A). Nigericin also showed a decreased ability to form colonies under anchorage-independent conditions in a standard soft agar assay after 14 d in culture, relative to the control group (1.66 ± 0.57 vs 7 ± 1.15, P < 0.05), whereas the colony numbers were higher in oxaliplatin group relative to the vehicle-treated controls (14.33 ± 0.57 vs 7 ± 1.15, P < 0.05) (Figure 3B and C).
E-cadherin, encoded by the CDH1 gene, has dual functions in epithelial cells: as a cell-cell adhesion molecule and as a negative regulator of the canonical WNT signaling cascade; in particular, of its central mediator β-catenin. E-cadherin downregulation in mammalian cell systems is sufficient to trigger EMT[20]. Gupta et al[12] have reported that nigericin preferentially kills cells that have undergone EMT. In colorectal carcinomas, the embryonic EMT is activated during tumor invasion in disseminating cancer cells[21]. Characteristic of these cells is a loss of E-cadherin expression.
We detected the expression of epithelial marker (E-cadherin) and mesenchymal marker (vimentin) of cells treated with nigericin and oxaliplatin to ascertain the effects of diverse compounds on EMT.
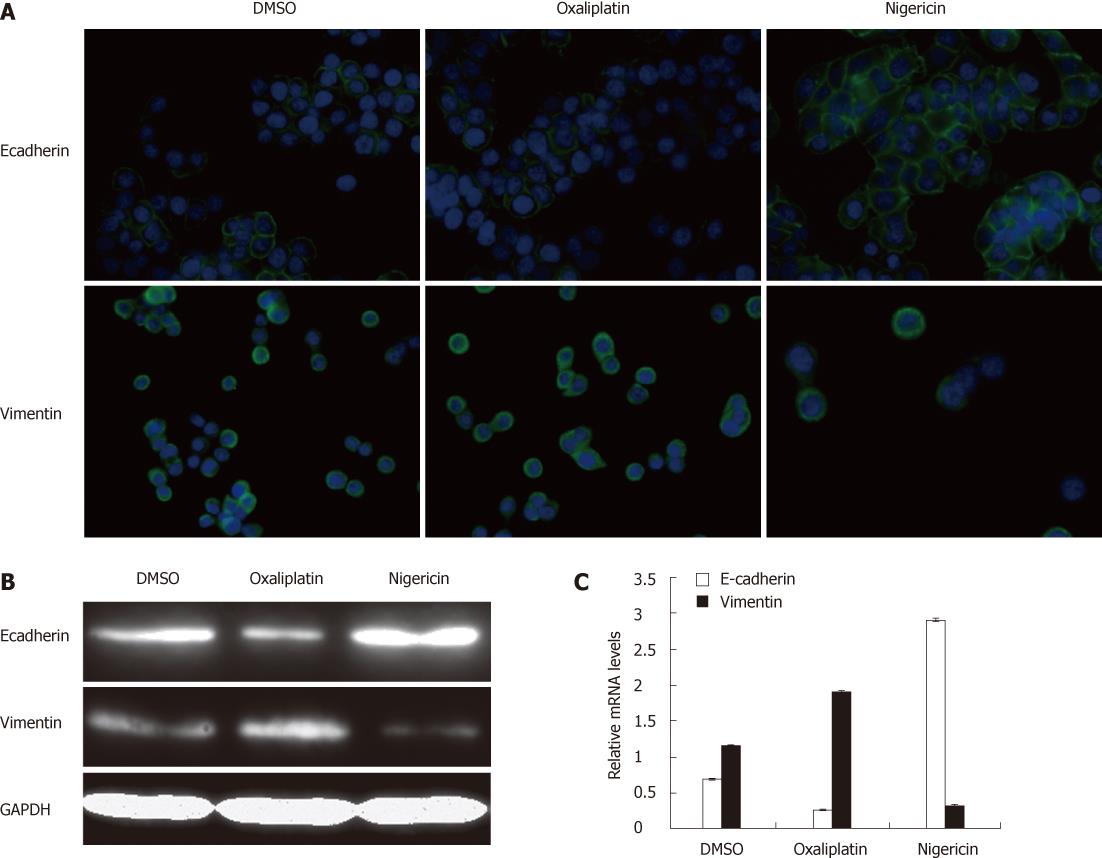
As shown in Figure 4A and B, nigericin induced an increase in expression of E-cadherin and a decrease in expression of vimentin relative to vehicle controls. In contrast, the expression of E-cadherin in the cells treated with oxaliplatin was downregulated in contrast to vehicle controls; correspondingly, oxaliplatin treatment upregulated the expression level of vimentin. The data from real-time PCR showed similar results to immunocytochemistry and Western blotting (Figure 4C).
Significant progress has been made in understanding the molecular pathogenesis, diagnosis (hereditary and sporadic), and treatment of CRC. Despite the use of active targeted drugs for treatment of metastatic CRC in the past decade, and improvement of overall survival to nearly 2 years for nonresectable disease, the cure rate remains low[22].
5-Fluorouracil and oxaliplatin formed the mainstay of chemotherapeutic regimens for metastatic CRC. Oxaliplatin covalently binds to DNA, forming platinum-DNA adducts that cause prolonged G2 arrest and inhibition of growth, which lead to apoptotic cell death[23].
There is a large body of evidence that tumor cells that are resistant to chemotherapy represent a subpopulation of cells from the primary tumor, which is molecularly and phenotypically distinct. These cells are referred to by several names, including tumor-initiating cells, tumor-promoting cells, or more commonly, CSCs[16]. EMT is a highly conserved cellular process during embryonic development and a pathogenic feature in tumorigenesis[10,24].
During the process of EMT, epithelial cells lose the expression of E-cadherin and other components of epithelial cell junctions, adopt a mesenchymal cell phenotype, and acquire motility and invasive ability[25,26]. Furthermore, Mani et al[10] have induced EMT in nontumorigenic, immortalized human mammary epithelial cells (HMLEs) by ectopic expression of either the Twist or Snail transcription factors; these cells formed > 30-fold more mammospheres than did HMLEs infected with the corresponding control vector. They have concluded that the cells generated by EMT acquired yet another attribute of mammary stem cells. EMT, which enables cancer cell dissemination, also imparts a self-renewal capability to disseminating cancer cells.
There is no consensus as to the exact criteria that define a CSC, because markers might vary according to the tumor type. In our study, we suggested CD133 as a marker of tumor-initiating cells of CRC[14-18].
We evaluated the effect of nigericin and oxaliplatin on CRC cell lines, including invasion and metastasis, and growth on colon cancer spheres, or colonospheres. From the results of cell viability and flow cytometry assays, we could see that nigericin specifically targets CD133+ cell subpopulations within CRC cell lines. Moreover, nigericin induced inhibition of invasion and metastasis in HT29 cells. These effects may have been due to the fact that nigericin upregulated the expression of E-cadherin, while E-cadherin played an important role in cancer progression and EMT induction[27,28].
In a variety of human cancers, E-cadherin loss was closely related to poor prognosis, tumor progression, and metastasis[29,30]. Therefore, E-cadherin also could be a sign of drug efficiency of nigericin therapy in the future. Through analysis of the expression level of E-cadherin and vimentin, we may conclude that nigericin partly reverses EMT to affect the ability of CRC cells to invade and metastasize.
We further evaluated the effects of nigericin treatment on the characteristics of CSC phenotype. The nigericin treatment group had a decreased number of spheres or colonies relative to the vehicle control group. Our data led us to hypothesize further that nigericin treatment suppresses EMT-generating cells with the properties of stem cells. This hypothesis needs further studies using animal experiments and preclinical and clinical trials. Nigericin may prove to be the therapeutic strategy that is effective in patients with metastatic disease.
However, the molecular mechanisms involved in the effect of nigericin are poorly understood. Lu et al[13] have reported that nigericin, as a potassium ionophore, selectively inhibits Wnt1-mediated signaling in HEK293 cells.
The polyether ionophores like nigericin interfere with transmembrane potassium potential and promote mitochondrial and cell potassium efflux. We hypothesize that nigericin treatment antagonizes the Wnt signaling cascade, while Wnt signaling plays a crucial role in embryonic development and cancer[31-35]. Besides, certain other CSC markers and signaling pathways, including EZH2 and Hedgehog pathways may also play some important roles in the mechanism of nigericin treatment, and thus need further studies[36]. Further studies will focus on the relation between nigericin-induced EMT and Wnt signaling.
We showed for the first time that nigericin not only partly reversed the EMT process during cell invasion and metastasis, but also suppressed some of the CSC phenotypes generated by EMT. EMT plays a pivotal role in tumor invasion and metastasis; therefore, nigericin treatment may be of benefit in the future.
We thank Pei-Qin Yu, Xue-Hua Chen for their technical assistance, and also thank the members of Shanghai Institute of Digestive Surgery, Shanghai for useful advice and guidance.
Despite therapeutic innovations, metastatic colorectal cancer (CRC) often has a poor prognosis and high mortality.
Epithelial-mesenchymal transition (EMT) provides a new basis for understanding the progression of carcinoma towards dedifferentiated and more malignant states. The EMT program, which involves dissolution of adherens and tight junctions and a loss of cell polarity, dissociates the cells with epithelial cell sheets into individual cells that exhibit multiple mesenchymal attributes, including heightened invasiveness. EMT also generates cells with properties of stem cells. Nigericin has been reported recently to act as a selective breast cancer stem cell inhibitor. However, the effect of nigericin on CRC is unknown. In this study, the authors evaluated the anticancer effect of nigericin and its possible mechanisms.
Recent reports have highlighted the important role of EMT during the invasion-metastasis cascade. In particular, EMT can be seen at the edges of colon carcinomas that are invading adjacent tissues. This is the first study to report that nigericin could suppress CRC metastasis. Furthermore, our studies indicated the possible mechanisms of action of nigericin.
Through understanding the effect of nigericin on CRC cells, this study may indicate a future promising therapeutic strategy in the treatment of patients with metastatic colorectal carcinoma.
E-cadherin is a hallmark of epithelial cell protein expression; vimentin is an intermediate filament component of the mesenchymal cell cytoskeleton. CD133 protein, a pentaspan cell surface receptor, is a putative CRC stem cell marker.
The study investigated the antitumor activity of nigericin on CRC stem cells. The authors found that nigericin selectively targeted cancer stem cells, and inhibited EMT. It is well designed and well presented.
Peer reviewer: Dr. Francesco Crea, Department of Pharmacology, University of Pisa, Via Roma 55, 56100 Pisa, Italy
S- Editor Gou SX L- Editor Kerr C E- Editor Xiong L
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 2. | Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol. 2005;16:481-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 3. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [PubMed] [Cited in This Article: ] |
| 4. | Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 2010;70:5649-5669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 5. | Klymkowsky MW, Savagner P. Epithelial-mesenchymal transition: a cancer researcher's conceptual friend and foe. Am J Pathol. 2009;174:1588-1593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 6. | Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265-273. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442-454. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15-33. [PubMed] [Cited in This Article: ] |
| 9. | Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151-3161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1000] [Cited by in F6Publishing: 1029] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 10. | Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704-715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 11. | Morel AP, Lièvre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3:e2888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1116] [Cited by in F6Publishing: 1203] [Article Influence: 75.2] [Reference Citation Analysis (1)] |
| 12. | Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 13. | Lu D, Choi MY, Yu J, Castro JE, Kipps TJ, Carson DA. Salinomycin inhibits Wnt signaling and selectively induces apoptosis in chronic lymphocytic leukemia cells. Proc Natl Acad Sci USA. 2011;108:13253-13257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 14. | O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 15. | Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111-115. [PubMed] [Cited in This Article: ] |
| 16. | Dallas NA, Xia L, Fan F, Gray MJ, Gaur P, van Buren G, Samuel S, Kim MP, Lim SJ, Ellis LM. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res. 2009;69:1951-1957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 17. | Ferrand A, Sandrin MS, Shulkes A, Baldwin GS. Expression of gastrin precursors by CD133-positive colorectal cancer cells is crucial for tumour growth. Biochim Biophys Acta. 2009;1793:477-488. [PubMed] [Cited in This Article: ] |
| 18. | Haraguchi N, Ohkuma M, Sakashita H, Matsuzaki S, Tanaka F, Mimori K, Kamohara Y, Inoue H, Mori M. CD133+CD44+ population efficiently enriches colon cancer initiating cells. Ann Surg Oncol. 2008;15:2927-2933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 19. | Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Suri P, Wicha MS. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063-6071. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 20. | Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68:3645-3654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 21. | Yilmaz M, Christofori G, Lehembre F. Distinct mechanisms of tumor invasion and metastasis. Trends Mol Med. 2007;13:535-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 22. | Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, Starling N. Colorectal cancer. Lancet. 2010;375:1030-1047. [PubMed] [Cited in This Article: ] |
| 23. | Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573-584. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548-558. [PubMed] [Cited in This Article: ] |
| 25. | Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420-1428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 26. | Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438-1449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 27. | Hajra KM, Fearon ER. Cadherin and catenin alterations in human cancer. Genes Chromosomes Cancer. 2002;34:255-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 28. | Jeanes A, Gottardi CJ, Yap AS. Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene. 2008;27:6920-6929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 29. | Kowalski PJ, Rubin MA, Kleer CG. E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res. 2003;5:R217-R222. [PubMed] [Cited in This Article: ] |
| 30. | Dorudi S, Sheffield JP, Poulsom R, Northover JM, Hart IR. E-cadherin expression in colorectal cancer. An immunocytochemical and in situ hybridization study. Am J Pathol. 1993;142:981-986. [PubMed] [Cited in This Article: ] |
| 31. | Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469-480. [PubMed] [Cited in This Article: ] |
| 32. | Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 33. | Willert K, Jones KA. Wnt signaling: is the party in the nucleus? Genes Dev. 2006;20:1394-1404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 34. | Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644-1646. [PubMed] [Cited in This Article: ] |
| 35. | Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781-810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 36. | Crea F, Fornaro L, Paolicchi E, Masi G, Frumento P, Loupakis F, Salvatore L, Cremolini C, Schirripa M, Graziano F. An EZH2 polymorphism is associated with clinical outcome in metastatic colorectal cancer patients. Ann Oncol. 2012;23:1207-1213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |