Published online May 14, 2012. doi: 10.3748/wjg.v18.i18.2172
Revised: March 29, 2012
Accepted: April 9, 2012
Published online: May 14, 2012
Hepatitis C (HCV)-infected patients have a poorer survival post-liver transplantation compared to patients transplanted for other indications, since HCV recurrence post-transplant is universal and commonly follows an aggressive course. There is increasing evidence that in the non-transplant setting, induction of hepatocyte apoptosis is one of the main mechanisms by which HCV drives liver inflammation and fibrosis, and that HCV proteins directly promote apoptosis. Recent studies have shown that post-liver transplant, there is a link between high levels of HCV replication, enhanced hepatocyte apoptosis and the subsequent development of rapidly progressive liver fibrosis. Although the responsible mechanisms remain unclear, it is likely that immunosuppressive drugs play an important role. It is well known that immunosuppressants impair immune control of HCV, thereby allowing increased viral replication. However there is also evidence that immunosuppressants may directly induce apoptosis and this may be facilitated by the presence of high levels of HCV replication. Thus HCV and immunosuppressants may synergistically interact to further enhance apoptosis and drive more rapid fibrosis. These findings suggest that modulation of apoptosis within the liver either by changing immunosuppressive therapy or the use of apoptosis inhibitors may help prevent fibrosis progression in patients with post-transplant HCV disease.
- Citation: Lim EJ, Chin R, Angus PW, Torresi J. Enhanced apoptosis in post-liver transplant hepatitis C: Effects of virus and immunosuppressants. World J Gastroenterol 2012; 18(18): 2172-2179
- URL: https://www.wjgnet.com/1007-9327/full/v18/i18/2172.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i18.2172
Hepatitis C (HCV)-related liver failure is now the commonest indication for liver transplantation in the United States, Australia and Europe[1]. HCV-infected patients have a poorer survival post-transplantation compared to patients transplanted for other indications[2]. This is because HCV recurrence occurs in virtually all patients and commonly follows an aggressive course, with 20% or more of patients developing cirrhosis within 5 years of transplantation[3]. The cause of this accelerated disease has not been fully elucidated, but risk factors include advanced donor age, early high HCV viral load post-transplant[4], acute graft rejection and treatment thereof, and the degree of immunosuppression[5].
In the non-transplant setting, induction of hepatocyte apoptosis is one of the main mechanisms via which HCV drives liver inflammation and fibrosis[6]. Recent evidence suggests a link between high levels of HCV replication, high rates of apoptosis and the subsequent development of rapidly progressive graft injury and fibrosis after liver transplantation[7]. The mechanisms responsible for this high levels of apoptosis found in aggressive post-liver transplant HCV disease remain unclear. It is well known that immunosuppressants impair immune control of HCV, thereby allowing increased viral replication. There is also recent evidence that some commonly used immunosuppressants may directly induce apoptosis and this may be facilitated by the presence of high levels of HCV replication. This suggests that HCV and immunosuppressants may synergistically interact to enhance apoptosis and drive rapid fibrosis.
Apoptosis is a highly regulated physiological process that plays an important role in organogenesis and the maintenance of tissue homeostasis[8]. Cells posing a threat to the integrity of an organ, such as virus-infected cells, may be eliminated by apoptosis, which occurs by two major pathways - extrinsic and intrinsic. The extrinsic pathway is activated when death ligands [tumor necrosis factor (TNF), FasL/CD95L and TRAIL] secreted by cells of the immune system in response to foreign (for example, viral) antigens bind to their respective cell surface receptors, to trigger signaling pathways that result in the activation of caspases[9]. The caspases are a class of enzymes responsible for the execution of apoptosis within the cell. In the intrinsic pathway, intracellular apoptotic stimuli, such as viral antigens, cause disruption of mitochondrial membrane integrity, releasing cytochrome c that activates the caspase pathway[10]. The integrity of the outer mitochondrial membrane is predominantly maintained by anti-apoptotic members of the Bcl-2 family (e.g., Bcl-2 and Bcl-xL), which antagonize pro-apoptotic members (for example, Bax and Bak).
There are increasing amounts of experimental data implicating apoptosis as a driving force for fibrogenesis in a range of different liver diseases, including alcohol-related and cholestatic liver diseases and viral hepatitis[11]. Apoptotic hepatocytes are engulfed and cleared by both Kupffer cells and hepatic stellate cells (HSCs). Activated HSCs are the primary cell type responsible for promoting fibrogenesis within the damaged liver, and the uptake of apoptotic bodies by HSCs result in their activation and secretion of the key pro-fibrogenic cytokine transforming growth factor-β (TGF-β)[12]. In activated HSCs, TGF-β induces a marked upregulation of genes encoding fibrillar collagens and other extracellular matrix components, resulting in the abnormal deposition of collagen within the liver[13]. Kupffer cells, which are the resident liver macrophages, upon ingestion of apoptotic hepatocytes, also secrete TGF-β, thereby promoting a pro-fibrogenic response in activated HSCs[14]. Furthermore, TGF-β itself induces hepatocyte apoptosis via two independent pathways, SMAD and DAXX[15], thus providing a positive feedback loop that could further potentiate apoptosis-induced fibrosis. In support of these in vitro observations, inhibition of apoptosis reduces hepatic inflammation and fibrosis in experimental models of fibrotic liver disease[16].
In HCV infection, hepatocyte apoptosis is an important part of the host anti-viral defense mechanism since it interrupts viral replication and assists in the elimination of virus-infected cells. However, in keeping with the observed effects of apoptosis in laboratory studies, there is now evidence to suggest that the severity of liver damage in chronic HCV is associated with the degree of hepatocyte apoptosis[6]. Furthermore, the degree of apoptosis correlates with the level of viraemia[17]. Bantel and colleagues have studied a serum apoptosis biomarker, the proteolytic neoepitope of the caspase substrate cytokeratin-18, as a means of determining caspase activity to monitor liver injury and predict the progression of hepatic fibrosis in HCV-infected patients[18]. This biomarker was markedly elevated in the sera of HCV-infected patients compared to healthy controls, and in patients with normal transaminase levels, raised serum caspase activity was associated with advanced fibrosis on liver biopsy.
Hepatocyte apoptotic rates on liver biopsy are significantly greater in HCV-positive patients post-liver transplant compared to the non-transplant setting, with the severity of liver inflammation correlating with the level of hepatocyte apoptosis[7], and HCV viral load is known to be higher post-liver transplantation[19]. Thus one potential explanation for accelerated fibrosis post-transplantation is that the high levels of HCV replication that occurs due to impaired immune control of HCV replication may drive increased hepatocyte apoptosis.
How then does HCV affect apoptosis? One likely mechanism is that virus-specific cytotoxic T-cells may induce apoptosis of HCV-infected hepatocytes by upregulating death receptor ligands (TNF, FasL/CD95L and TRAIL), by producing antiviral cytokines (for example, interferon-γ), and by direct cell killing with perforins and granzymes[20]. HCV infection is also associated with an upregulation of death receptors on hepatocytes, and the levels of Fas/CD95 and FasL/CD95L have been shown to increase in parallel with the severity of inflammation and disease progression[21].
There is also considerable experimental evidence that HCV structural proteins can directly influence hepatocyte apoptosis. HCV core protein has been reported to sensitize hepatocytes to TNF-α-[22] and FasL/CD95L-[23] mediated apoptosis, by interacting with the cytoplasmic domains of TNFR1 and Fas/CD95 to enhance downstream signaling events. It also induces oxidative stress, enhances mitochondrial-mediated hepatocyte apoptosis[24] and upregulates TGF-β1 gene expression, thereby promoting apoptosis and fibrogenesis. However, the expression of core protein has also been shown to have a number of possible anti-apoptotic effects. These include inhibition of TNF-α- and Fas/CD95-mediated apoptosis through the upregulation NF-κB[25], and interaction with cFLIP, an endogenous caspase-8 inhibitor[26]. Core protein has also been reported to promote the anti-apoptotic Bcl-xL expression, inhibit interferon-α-mediated STAT1 signaling and activate STAT3, thereby protecting infected hepatocytes from T-cell-mediated apoptosis[27]. Both the E1 and E2 glycoproteins of HCV have been shown to induce hepatocyte apoptosis[28], with the E2 protein noted to activate the mitochondrial caspase pathway. However, E2 protein has also been shown to inhibit interferon-α-mediated STAT1 signaling and TRAIL-induced apoptosis, as well as enhance the proliferation of transfected Huh7 human hepatoma cells[29]. The data on the effect of HCV on caspase-independent apoptosis are lacking. One study showed that core protein expression promoted apoptosis-like caspase-independent cell death in osteosarcoma-derived cells[30], but the effect in liver cells is unknown.
The non-structural proteins of HCV have also been shown to affect hepatocyte apoptosis. By using a NS3-5B subgenomic replicon of HCV, Huh7.5 human hepatoma cells were shown to be sensitized to TRAIL-induced apoptosis[31]. Accumulation of NS4A on mitochondria has been found to promote mitochondrial-mediated apoptosis[32]. Similarly, the HCV protease NS3, can induce apoptosis in a caspase 8-dependent manner. On the other hand, NS2 has been found to inhibit the mitochondrial release of cytochrome c, thereby inhibiting mitochondrial-mediated apoptosis[33]. NS5A inhibits interferon-α-mediated STAT1 signaling[34] and protects hepatocytes against interferon-α- and TNF-α-mediated apoptosis. NS5A also prevents apoptosis by activating NF-κB, inhibiting TGF-β, and upregulating STAT3 expression to promote hepatocyte proliferation[35].
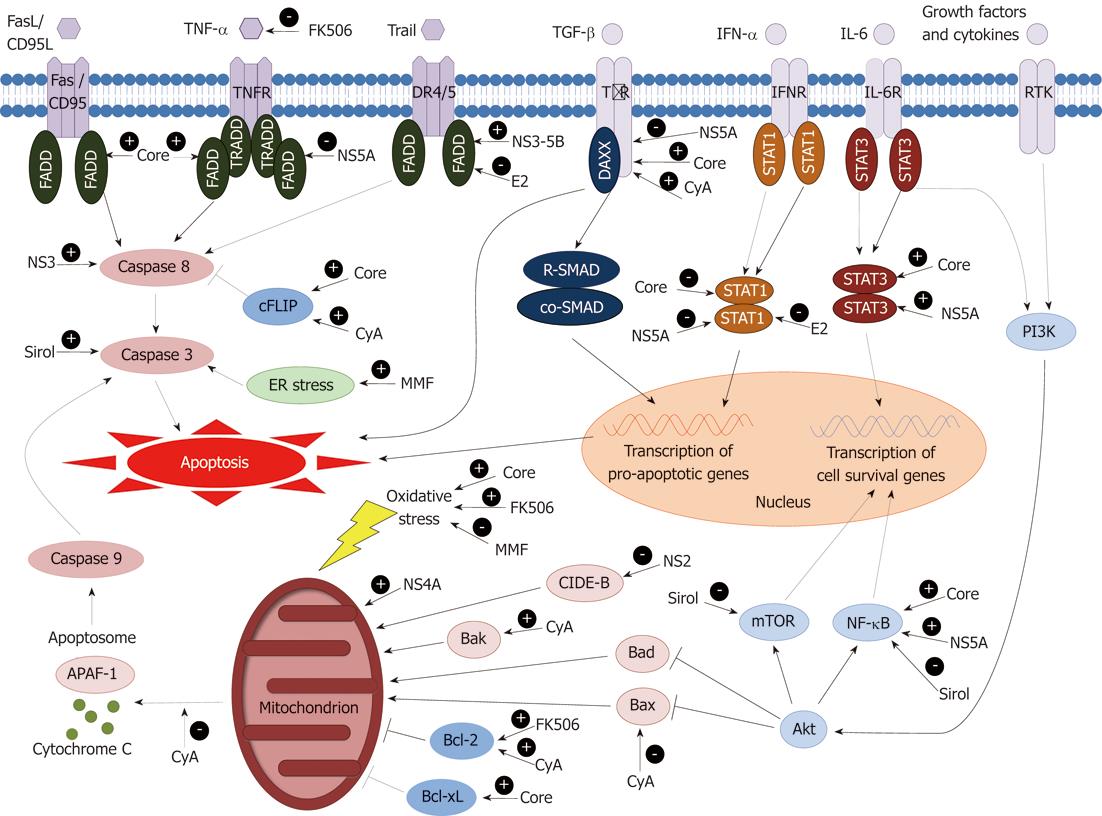
Thus HCV proteins have been shown to have a number of both pro- and anti-apoptotic effects in cultured hepatocytes but the net of contribution of these changes to hepatocyte apoptotic rates and liver fibrosis in vivo remains unclear. The discrepancies in these effects may be partly explained by differences in experimental conditions, cell types, apoptotic stimuli and HCV genotype-specific proteins expressed in various in vitro systems that may not mimic the true in vivo situation. Our current understanding of how the HCV proteins interact with apoptotic pathways within the hepatocyte is summarized in Figure 1.
Activated HSCs are the key cell type promoting fibrogenesis in the liver. HSC activation is increased in patients with chronic HCV infection and the degree of activation correlates with necroinflammatory grade and fibrosis stage[36]. Interestingly, patients with chronic HCV infection have elevated plasma levels of TGF-β1 and increased expression of TGF-β1 in the liver, while the clearance of HCV infection with anti-viral treatment is associated with normalization of plasma TGF-β1 levels[37]. This argues for an important role of TGF-β in HCV-mediated HSC activation and liver fibrogenesis.
Normally, hepatocytes do not express TGF-β, but hepatocytes exposed to HCV non-structural proteins upregulate TGF-β expression, resulting in the activation of HSCs[38]. HSCs express CD81 and LDL receptor, the putative receptors for HCV, and may perhaps be infected by HCV in vivo[39]. Expression of HCV core and non-structural proteins in HSCs was found to activate HSCs, resulting in upregulation of TGF-β and procollagen 1 expression[39]. The interaction of HCV E2 glycoprotein with HSCs is noted to upregulate HSC expression of matrix metalloproteinase 2, thus facilitating hepatic fibrogenesis.
Activated HSCs are primarily cleared by apoptosis, a process that would normally restrict the fibrogenic response within an inflamed liver. However, in patients with chronic HCV and advanced fibrosis, HSC apoptosis is reduced compared to patients with mild fibrosis[40]. This suggests that the inhibition of HSC apoptosis by HCV may contribute to the progression of liver fibrosis in this disease. Also, HCV-infected patients who are noted to have a high number of activated HSCs in liver biopsies done several months after liver transplantation developed advanced fibrosis within 2 years of transplantation, indicating that the degree of HSC activation may be an early predictor of post-transplant rapid fibrosis[41].
Kupffer cells have an integral role in the development of chronic liver inflammation in response to hepatocyte injury. Activated Kupffer cells contribute to HSC activation and thereby promote liver fibrosis. The interaction between HCV core protein and toll-like receptor (TLR) 2 on human Kupffer cells has been shown to upregulate cell surface programmed death-ligand 1 (PD-L1). The binding of Kupffer cell PD-L1 to PD-1 receptors on T-cells promotes T-cell apoptosis, thereby impairing the host adaptive anti-viral response[42]. HCV core protein has also been shown to inhibit TLR3-mediated induction of interferon-α, interferon-β and TRAIL, and this may impair the anti-viral activity of Kupffer cells[42]. HCV has not been shown to affect Kupffer cell apoptosis.
The aim of post-liver transplant immunosuppression is to dampen the adaptive immune response and prevent graft rejection. However, robust CD4+ and cytotoxic CD8+ T-cell responses play a central role in controlling HCV replication. The experimental evidence that the increased HCV viraemia that occurs post-transplant may directly drive higher rates of apoptosis suggests a likely link between immunosuppressive drug therapy, the resultant loss of immune control of HCV replication, and apoptosis-induced liver injury and fibrosis.
It is has been suggested that the overall level of immunosuppression, rather than the individual agent, is associated with the level of HCV viraemia and the degree of hepatic injury on liver biopsy in patients with post-transplant HCV recurrence[43]. Thus the use of pulse methylprednisolone for the treatment of acute graft rejection has been shown to dramatically elevate HCV viral load[43], while OKT3, another highly potent immunosuppressant used to treat steroid-refractory acute rejection, has been shown to accelerate HCV-associated liver fibrosis.
However, there is emerging evidence that individual immunosuppressive drugs used in long-term maintenance therapy may also have individual specific effects on both HCV replication and HCV-mediated liver injury. Some groups have shown that cyclosporine therapy is associated with less severe histological recurrence and improved graft survival post-liver transplantation compared to tacrolimus[44]. One possible explanation for this effect is that cyclosporine is known to inhibit HCV replication in vitro by the inhibition of NS2 and NS5A[45]. Tacrolimus, on the other hand exhibits no anti-viral effect in vitro and in fact impairs interferon-α activity by interfering with STAT-1 phosphorylation, and thus, may promote viral replication and persistence[46]. Mycophenolic acid (MPA), the active metabolite of mycophenolate mofetil (MMF), inhibits HCV replication in Huh7 human hepatoma cells without inhibiting cell proliferation or inducing apoptosis[47]. A synergistic inhibition of viral replication has also been shown when MPA was combined with cyclosporine or interferon-α[48].
In addition to their possible effects on viral replication, there is increasing evidence that some of the immunosuppressive agents may also directly contribute to apoptosis. Figure 1 summarizes our current understanding of where individual immunosuppressants interact with intracellular apoptotic pathways.
Cyclosporine has been shown to prevent hepatocyte necrosis in mice exposed to concanavalin A[49], but data on its effect on hepatocyte apoptosis are lacking. Cyclosporine is noted to cause apoptosis of renal vascular endothelial cells via endoplasmic reticulum stress, as well as fibrosis of the renal tubulointerstitium by upregulating TGF-β expression[50]. These findings raise concerns that similar effects may occur within the liver. Indeed, cyclosporine has been found to promote hepatocyte expression of pro-apoptotic Bak in a rat model of liver injury[51]. On the other hand, cyclosporine has also been shown to prevent apoptosis of human gingival fibroblasts by inhibiting Bax and upregulating anti-apoptotic Bcl-2[52], as well as reducing mitochondrial permeability and inhibiting cytochrome c release in human platelets and rat vascular endothelial cells in vitro[53]. In an animal model of colitis, cyclosporine was found to have a protective role against epithelial apoptosis through the upregulation of anti-apoptotic cFLIP and inhibition of caspase-8 activity[54].
Tacrolimus has also been shown to have both pro-apoptotic and anti-apoptotic effects in various cell lines in culture. Treatment with tacrolimus promotes Jurkat T-cell G0/G1 phase cell cycle arrest and the generation of reactive oxygen species, mitochondrial dysfunction and thereby apoptosis[55]. In contrast, in human islet cells exposed to pro-inflammatory cytokines such as IL-1 and interferon-γ, tacrolimus has an anti-apoptotic effect, causing a reduction in TNF-α and down-regulation of caspase-3, -8 and -9[56]. Tacrolimus has also been shown to promote hepatic expression of anti-apoptotic Bcl-2 in a rat model of liver injury[51]. However the effect of tacrolimus on apoptosis in human liver is unknown.
After solid organ transplantation, treatment with MMF has been associated with increased mucosal apoptosis in the upper gastrointestinal tract and colon, producing an appearance similar to graft-vs-host disease[57]. While MMF has been shown to induce apoptosis via promoting endoplasmic reticulum stress and increasing caspase-3 activity in human pancreatic islet cells[58], the opposite effect has been observed in renal transplant recipients, where reduced apoptosis of renal tubular epithelial, glomerular and interstitial cells was noted[59]. MMF has also been shown to reduce pancreatic β-cell apoptosis in a rodent model of diabetes, and reduce hepatocyte oxidative stress and apoptosis in a rat model of ischaemia/reperfusion injury[60]. The effect of MMF on human hepatocyte apoptosis is currently unknown.
Sirolimus has been found to induce apoptosis in acute lymphoblastic leukemia cells by inhibiting the PI3K/Akt pathway[61]. It also induces apoptosis in vascular smooth muscle cells by activating caspase-3 and inhibiting NF-κB nuclear translocation[62]. However, sirolimus is known to inhibit HSC proliferation in vitro, reduce TGF-β expression and inhibit collagen deposition, thereby reducing hepatic fibrosis in a rat model of liver injury[63]. Indeed, sirolimus has also been shown to reduce liver fibrogenesis, improve liver function and enhance survival in rats with established cirrhosis[64]. Huh7 hepatoma cells transfected with the HCV-1b genome have upregulated PI3K-Akt-mTOR signaling[65], possibly rendering HCV-infected cells more resistant to apoptosis. Sirolimus, by inhibiting the mTOR pathway, has been shown to inhibit NS5A phosphorylation, thereby inhibiting HCV replication[66]. Sirolimus-based maintenance immunosuppression has been associated with lower HCV RNA levels at 12 mo following liver transplantation and improved patient survival at 6 years compared to calcineurin inhibitors[67].
Understanding the role of hepatocyte apoptosis in the pathogenesis of post-transplant HCV-mediated liver injury and the likely contributing role of the immunosuppressive agents has a number of important therapeutic implications. It is hoped that increased knowledge of the pro- or anti-apoptotic effects of different immunosuppressive agents and whether they exacerbate HCV-induced apoptosis may allow the development of immunosuppressive regimes that minimize this aspect of HCV-mediated liver injury. In this regard, sirolimus is of particular interest given its possible anti-apoptotic and anti-fibrotic effects both in vitro and in animal models.
These findings also suggest a possible therapeutic role for apoptosis inhibitors in post-transplant HCV. There is increasing experimental and clinical experience with the use of this class of compounds in liver disease. The pan-caspase inhibitor IDN-6556 was found to reduce hepatocyte apoptosis and liver fibrosis in bile duct-ligated mice[16], and improve liver function tests in patients with hepatic dysfunction[68]. VX-166, another pan-caspase inhibitor, has been shown to reduce hepatocyte caspase-3 expression and apoptosis, thereby decreasing hepatic fibrosis in a murine model of non-alcoholic steatohepatitis[69]. Given the evidence linking HCV-induced hepatocyte apoptosis with liver fibrosis, 2 randomized, double-blind, placebo-controlled studies have been conducted using pan-caspase inhibitors in patients with chronic HCV, one using PF-03491390[70] and the other using IDN-6556[71]. In both studies, the orally administered pan-caspase inhibitors were well tolerated with minimal adverse effects and showed significant reductions in serum transaminases. Besides directly targeting caspases, compounds that inhibit other components of the apoptotic pathway upstream to caspases are currently in development. There are currently no drugs that inhibit the caspase-independent apoptotic pathway in the literature.
Conversely, the promotion of HSC apoptosis may also act to reduce hepatic fibrosis. Cortex Dictamni extract was noted to induce apoptosis of activated HSCs, resulting in decreased hepatic collagen deposition and attenuated fibrosis in a murine model of liver injury[72]. Another compound, 2’,4’,6’-tris(methoxymethoxy) chalcone, is noted to induce apoptosis of activated HSCs by enhancing FasL/CD95L expression without affecting hepatocyte apoptosis[73]. The tyrosine kinase inhibitor sorafenib has also been found to increase HSC expression of caspase-3 and induce HSC apoptosis resulting in reduced hepatic collagen deposition and fibrosis in bile duct-ligated rats[74]. These compounds raise the possibility of treatment to reduce the population of activated HSCs within the transplanted liver in HCV-recurrence.
In conclusion, the management of post-liver transplant HCV disease remains one of the major challenges in transplant medicine. Enhanced hepatocyte apoptosis appears to contribute to much of the liver injury that drives rapid liver fibrosis in this disease, and in the near future clinically useful serum biomarkers of apoptosis may be available to monitor for this. The precise mechanisms that drive this accelerated hepatocyte apoptosis post-transplant require further study, but it appears that both HCV itself and immunosuppressants play contributory and possibly synergistic roles. In the future as the effects of various immunosuppressive agents on HCV-induced liver cell apoptosis are clarified, a combination of fine-tuning immunosuppressive regimens as well as the manipulation of apoptosis within the liver represents novel therapeutic possibilities for the management of this complex disease.
Peer reviewers: Mara Massimi, PhD, Department of Basic and Applied Biology, University of L’Aquila, Via Vetoio, 67010 Coppito, Italy; Pedro Lorenzo Majano Rodriguez, PhD, Unidad de Biología Molecular, Hospital Universitario de la Princesa, Diego de Léon 62, 28006 Madrid, Spain
S- Editor Lv S L- Editor A E- Editor Xiong L
| 1. | Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver Transpl. 2003;9:331-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 442] [Cited by in F6Publishing: 456] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 2. | Berenguer M. Recurrent hepatitis C: worse outcomes established, interventions still inadequate. Liver Transpl. 2007;13:641-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Neumann UP, Berg T, Bahra M, Seehofer D, Langrehr JM, Neuhaus R, Radke C, Neuhaus P. Fibrosis progression after liver transplantation in patients with recurrent hepatitis C. J Hepatol. 2004;41:830-836. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 289] [Cited by in F6Publishing: 295] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 4. | Shackel NA, Jamias J, Rahman W, Prakoso E, Strasser SI, Koorey DJ, Crawford MD, Verran DJ, Gallagher J, McCaughan GW. Early high peak hepatitis C viral load levels independently predict hepatitis C-related liver failure post-liver transplantation. Liver Transpl. 2009;15:709-718. [PubMed] [Cited in This Article: ] |
| 5. | Samonakis DN, Triantos CK, Thalheimer U, Quaglia A, Leandro G, Teixeira R, Papatheodoridis GV, Sabin CA, Rolando N, Davies S. Immunosuppression and donor age with respect to severity of HCV recurrence after liver transplantation. Liver Transpl. 2005;11:386-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Bantel H, Schulze-Osthoff K. Apoptosis in hepatitis C virus infection. Cell Death Differ. 2003;10 Suppl 1:S48-S58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Ballardini G, De Raffele E, Groff P, Bioulac-Sage P, Grassi A, Ghetti S, Susca M, Strazzabosco M, Bellusci R, Iemmolo RM. Timing of reinfection and mechanisms of hepatocellular damage in transplanted hepatitis C virus-reinfected liver. Liver Transpl. 2002;8:10-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Prindull G. Apoptosis in the embryo and tumorigenesis. Eur J Cancer. 1995;31A:116-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305-1308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4271] [Cited by in F6Publishing: 4145] [Article Influence: 159.4] [Reference Citation Analysis (0)] |
| 10. | Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell. 2002;9:459-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1238] [Cited by in F6Publishing: 1235] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 11. | Canbay A, Friedman S, Gores GJ. Apoptosis: the nexus of liver injury and fibrosis. Hepatology. 2004;39:273-278. [PubMed] [Cited in This Article: ] |
| 12. | Canbay A, Taimr P, Torok N, Higuchi H, Friedman S, Gores GJ. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab Invest. 2003;83:655-663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 287] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 13. | Pinzani M, Rombouts K. Liver fibrosis: from the bench to clinical targets. Dig Liver Dis. 2004;36:231-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 246] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 14. | Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 686] [Cited by in F6Publishing: 702] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 15. | Lee KY, Bae SC. TGF-beta-dependent cell growth arrest and apoptosis. J Biochem Mol Biol. 2002;35:47-53. [PubMed] [Cited in This Article: ] |
| 16. | Canbay A, Feldstein A, Baskin-Bey E, Bronk SF, Gores GJ. The caspase inhibitor IDN-6556 attenuates hepatic injury and fibrosis in the bile duct ligated mouse. J Pharmacol Exp Ther. 2004;308:1191-1196. [PubMed] [Cited in This Article: ] |
| 17. | Delladetsima I, Psichogiou M, Alexandrou P, Nikolopoulos G, Revenas K, Hatzakis A, Boletis J. Apoptosis and hepatitis C virus infection in renal transplant recipients. Am J Clin Pathol. 2008;129:744-748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Bantel H, Lügering A, Heidemann J, Volkmann X, Poremba C, Strassburg CP, Manns MP, Schulze-Osthoff K. Detection of apoptotic caspase activation in sera from patients with chronic HCV infection is associated with fibrotic liver injury. Hepatology. 2004;40:1078-1087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 214] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 19. | McCaughan GW, Zekry A. Pathogenesis of hepatitis C virus recurrence in the liver allograft. Liver Transpl. 2002;8:S7-S13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Fischer R, Baumert T, Blum HE. Hepatitis C virus infection and apoptosis. World J Gastroenterol. 2007;13:4865-4872. [PubMed] [Cited in This Article: ] |
| 21. | Zylberberg H, Rimaniol AC, Pol S, Masson A, De Groote D, Berthelot P, Bach JF, Bréchot C, Zavala F. Soluble tumor necrosis factor receptors in chronic hepatitis C: a correlation with histological fibrosis and activity. J Hepatol. 1999;30:185-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 140] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Kang SM, Kim SJ, Kim JH, Lee W, Kim GW, Lee KH, Choi KY, Oh JW. Interaction of hepatitis C virus core protein with Hsp60 triggers the production of reactive oxygen species and enhances TNF-alpha-mediated apoptosis. Cancer Lett. 2009;279:230-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Ruggieri A, Harada T, Matsuura Y, Miyamura T. Sensitization to Fas-mediated apoptosis by hepatitis C virus core protein. Virology. 1997;229:68-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 194] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, Weinman SA. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 690] [Cited by in F6Publishing: 667] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 25. | Marusawa H, Hijikata M, Chiba T, Shimotohno K. Hepatitis C virus core protein inhibits Fas- and tumor necrosis factor alpha-mediated apoptosis via NF-kappaB activation. J Virol. 1999;73:4713-4720. [PubMed] [Cited in This Article: ] |
| 26. | Saito K, Meyer K, Warner R, Basu A, Ray RB, Ray R. Hepatitis C virus core protein inhibits tumor necrosis factor alpha-mediated apoptosis by a protective effect involving cellular FLICE inhibitory protein. J Virol. 2006;80:4372-4379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Kawamura H, Govindarajan S, Aswad F, Machida K, Lai MM, Sung VM, Dennert G. HCV core expression in hepatocytes protects against autoimmune liver injury and promotes liver regeneration in mice. Hepatology. 2006;44:936-944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Ciccaglione AR, Marcantonio C, Costantino A, Equestre M, Rapicetta M. Expression of HCV E1 protein in baculovirus-infected cells: effects on cell viability and apoptosis induction. Intervirology. 2003;46:121-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Lee SH, Kim YK, Kim CS, Seol SK, Kim J, Cho S, Song YL, Bartenschlager R, Jang SK. E2 of hepatitis C virus inhibits apoptosis. J Immunol. 2005;175:8226-8235. [PubMed] [Cited in This Article: ] |
| 30. | Berg CP, Schlosser SF, Neukirchen DK, Papadakis C, Gregor M, Wesselborg S, Stein GM. Hepatitis C virus core protein induces apoptosis-like caspase independent cell death. Virol J. 2009;6:213. [PubMed] [Cited in This Article: ] |
| 31. | Lan L, Gorke S, Rau SJ, Zeisel MB, Hildt E, Himmelsbach K, Carvajal-Yepes M, Huber R, Wakita T, Schmitt-Graeff A. Hepatitis C virus infection sensitizes human hepatocytes to TRAIL-induced apoptosis in a caspase 9-dependent manner. J Immunol. 2008;181:4926-4935. [PubMed] [Cited in This Article: ] |
| 32. | Nomura-Takigawa Y, Nagano-Fujii M, Deng L, Kitazawa S, Ishido S, Sada K, Hotta H. Non-structural protein 4A of Hepatitis C virus accumulates on mitochondria and renders the cells prone to undergoing mitochondria-mediated apoptosis. J Gen Virol. 2006;87:1935-1945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Erdtmann L, Franck N, Lerat H, Le Seyec J, Gilot D, Cannie I, Gripon P, Hibner U, Guguen-Guillouzo C. The hepatitis C virus NS2 protein is an inhibitor of CIDE-B-induced apoptosis. J Biol Chem. 2003;278:18256-18264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Podevin P, Sabile A, Gajardo R, Delhem N, Abadie A, Lozach PY, Beretta L, Bréchot C. Expression of hepatitis C virus NS5A natural mutants in a hepatocytic cell line inhibits the antiviral effect of interferon in a PKR-independent manner. Hepatology. 2001;33:1503-1511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Gong G, Waris G, Tanveer R, Siddiqui A. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kappa B. Proc Natl Acad Sci USA. 2001;98:9599-9604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 485] [Cited by in F6Publishing: 486] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 36. | Chu CM, Shyu WC, Liaw YF. Comparative studies on expression of alpha-smooth muscle actin in hepatic stellate cells in chronic hepatitis B and C. Dig Dis Sci. 2008;53:1364-1369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Flisiak R, Jaroszewicz J, Lapinski TW, Flisiak I, Prokopowiczi D. Effect of pegylated interferon alpha 2b plus ribavirin treatment on plasma transforming growth factor-beta1, metalloproteinase-1, and tissue metalloproteinase inhibitor-1 in patients with chronic hepatitis C. World J Gastroenterol. 2005;11:6833-6838. [PubMed] [Cited in This Article: ] |
| 38. | Schulze-Krebs A, Preimel D, Popov Y, Bartenschlager R, Lohmann V, Pinzani M, Schuppan D. Hepatitis C virus-replicating hepatocytes induce fibrogenic activation of hepatic stellate cells. Gastroenterology. 2005;129:246-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 39. | Bataller R, Paik YH, Lindquist JN, Lemasters JJ, Brenner DA. Hepatitis C virus core and nonstructural proteins induce fibrogenic effects in hepatic stellate cells. Gastroenterology. 2004;126:529-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 207] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 40. | Gonzalez SA, Fiel MI, Sauk J, Canchis PW, Liu RC, Chiriboga L, Yee HT, Jacobson IM, Talal AH. Inverse association between hepatic stellate cell apoptosis and fibrosis in chronic hepatitis C virus infection. J Viral Hepat. 2009;16:141-148. [PubMed] [Cited in This Article: ] |
| 41. | Russo MW, Firpi RJ, Nelson DR, Schoonhoven R, Shrestha R, Fried MW. Early hepatic stellate cell activation is associated with advanced fibrosis after liver transplantation in recipients with hepatitis C. Liver Transpl. 2005;11:1235-1241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Tu Z, Pierce RH, Kurtis J, Kuroki Y, Crispe IN, Orloff MS. Hepatitis C virus core protein subverts the antiviral activities of human Kupffer cells. Gastroenterology. 2010;138:305-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 43. | Gane EJ, Naoumov NV, Qian KP, Mondelli MU, Maertens G, Portmann BC, Lau JY, Williams R. A longitudinal analysis of hepatitis C virus replication following liver transplantation. Gastroenterology. 1996;110:167-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 383] [Cited by in F6Publishing: 393] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 44. | Villamil F, Levy G, Grazi GL, Mies S, Samuel D, Sanjuan F, Rossi M, Lake J, Munn S, Mühlbacher F. Long-term outcomes in liver transplant patients with hepatic C infection receiving tacrolimus or cyclosporine. Transplant Proc. 2006;38:2964-2967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Fernandes F, Ansari IU, Striker R. Cyclosporine inhibits a direct interaction between cyclophilins and hepatitis C NS5A. PLoS One. 2010;5:e9815. [PubMed] [Cited in This Article: ] |
| 46. | Hirano K, Ichikawa T, Nakao K, Matsumoto A, Miyaaki H, Shibata H, Eguchi S, Takatsuki M, Ikeda M, Yamasaki H. Differential effects of calcineurin inhibitors, tacrolimus and cyclosporin a, on interferon-induced antiviral protein in human hepatocyte cells. Liver Transpl. 2008;14:292-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Henry SD, Metselaar HJ, Lonsdale RC, Kok A, Haagmans BL, Tilanus HW, van der Laan LJ. Mycophenolic acid inhibits hepatitis C virus replication and acts in synergy with cyclosporin A and interferon-alpha. Gastroenterology. 2006;131:1452-1462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 48. | Jain A, Kashyap R, Demetris AJ, Eghstesad B, Pokharna R, Fung JJ. A prospective randomized trial of mycophenolate mofetil in liver transplant recipients with hepatitis C. Liver Transpl. 2002;8:40-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 49. | Zhang XL, Quan QZ, Sun ZQ, Wang YJ, Jiang XL, Wang D, Li WB. Protective effects of cyclosporine A on T-cell dependent ConA-induced liver injury in Kunming mice. World J Gastroenterol. 2001;7:569-571. [PubMed] [Cited in This Article: ] |
| 50. | Sun BK, Li C, Lim SW, Jung JY, Lee SH, Kim IS, Kim YS, Kim J, Bang BK, Yang CW. Expression of transforming growth factor-beta-inducible gene-h3 in normal and cyclosporine-treated rat kidney. J Lab Clin Med. 2004;143:175-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Tannuri U, Tannuri AC, Coelho MC, Mello ES, dos Santos AS. Effect of the immunosuppressants on hepatocyte cells proliferation and apoptosis during liver regeneration after hepatectomy - molecular studies. Pediatr Transplant. 2008;12:73-79. [PubMed] [Cited in This Article: ] |
| 52. | Jung JY, Jeong YJ, Jeong TS, Chung HJ, Kim WJ. Inhibition of apoptotic signals in overgrowth of human gingival fibroblasts by cyclosporin A treatment. Arch Oral Biol. 2008;53:1042-1049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | Tharakan B, Holder-Haynes JG, Hunter FA, Smythe WR, Childs EW. Cyclosporine A prevents vascular hyperpermeability after hemorrhagic shock by inhibiting apoptotic signaling. J Trauma. 2009;66:1033-1039. [PubMed] [Cited in This Article: ] |
| 54. | Satoh Y, Ishiguro Y, Sakuraba H, Kawaguchi S, Hiraga H, Fukuda S, Nakane A. Cyclosporine regulates intestinal epithelial apoptosis via TGF-beta-related signaling. Am J Physiol Gastrointest Liver Physiol. 2009;297:G514-G519. [PubMed] [Cited in This Article: ] |
| 55. | Choi SJ, You HS, Chung SY. Tacrolimus-induced apoptotic signal transduction pathway. Transplant Proc. 2008;40:2734-2736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Del Castillo JM, García-Martín MC, Arias-Díaz J, Giné E, Vara E, Cantero JL. Antiapoptotic effect of tacrolimus on cytokine-challenged human islets. Cell Transplant. 2009;18:1237-1246. [PubMed] [Cited in This Article: ] |
| 57. | Nguyen T, Park JY, Scudiere JR, Montgomery E. Mycophenolic acid (cellcept and myofortic) induced injury of the upper GI tract. Am J Surg Pathol. 2009;33:1355-1363. [PubMed] [Cited in This Article: ] |
| 58. | Johnson JD, Ao Z, Ao P, Li H, Dai LJ, He Z, Tee M, Potter KJ, Klimek AM, Meloche RM. Different effects of FK506, rapamycin, and mycophenolate mofetil on glucose-stimulated insulin release and apoptosis in human islets. Cell Transplant. 2009;18:833-845. [PubMed] [Cited in This Article: ] |
| 59. | Pardo-Mindán FJ, Errasti P, Panizo A, Sola I, de Alava E, Lozano MD. Decrease of apoptosis rate in patients with renal transplantation treated with mycophenolate mofetil. Nephron. 1999;82:232-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 60. | Liu YX, Jin LM, Zhou L, Xie HY, Jiang GP, Wang Y, Feng XW, Chen H, Yan S, Zheng SS. Mycophenolate mofetil attenuates liver ischemia/reperfusion injury in rats. Transpl Int. 2009;22:747-756. [PubMed] [Cited in This Article: ] |
| 61. | Avellino R, Romano S, Parasole R, Bisogni R, Lamberti A, Poggi V, Venuta S, Romano MF. Rapamycin stimulates apoptosis of childhood acute lymphoblastic leukemia cells. Blood. 2005;106:1400-1406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 62. | Giordano A, Avellino R, Ferraro P, Romano S, Corcione N, Romano MF. Rapamycin antagonizes NF-kappaB nuclear translocation activated by TNF-alpha in primary vascular smooth muscle cells and enhances apoptosis. Am J Physiol Heart Circ Physiol. 2006;290:H2459-H2465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 63. | Zhu J, Wu J, Frizell E, Liu SL, Bashey R, Rubin R, Norton P, Zern MA. Rapamycin inhibits hepatic stellate cell proliferation in vitro and limits fibrogenesis in an in vivo model of liver fibrosis. Gastroenterology. 1999;117:1198-1204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 167] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 64. | Neef M, Ledermann M, Saegesser H, Schneider V, Reichen J. Low-dose oral rapamycin treatment reduces fibrogenesis, improves liver function, and prolongs survival in rats with established liver cirrhosis. J Hepatol. 2006;45:786-796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 65. | Mannová P, Beretta L. Activation of the N-Ras-PI3K-Akt-mTOR pathway by hepatitis C virus: control of cell survival and viral replication. J Virol. 2005;79:8742-8749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 143] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 66. | Coito C, Diamond DL, Neddermann P, Korth MJ, Katze MG. High-throughput screening of the yeast kinome: identification of human serine/threonine protein kinases that phosphorylate the hepatitis C virus NS5A protein. J Virol. 2004;78:3502-3513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Wagner D, Kniepeiss D, Schaffellner S, Jakoby E, Mueller H, Fahrleitner-Pammer A, Stiegler P, Tscheliessnigg KH, Iberer F. Sirolimus has a potential to influent viral recurrence in HCV positive liver transplant candidates. Int Immunopharmacol. 2010;10:990-993. [PubMed] [Cited in This Article: ] |
| 68. | Valentino KL, Gutierrez M, Sanchez R, Winship MJ, Shapiro DA. First clinical trial of a novel caspase inhibitor: anti-apoptotic caspase inhibitor, IDN-6556, improves liver enzymes. Int J Clin Pharmacol Ther. 2003;41:441-449. [PubMed] [Cited in This Article: ] |
| 69. | Witek RP, Stone WC, Karaca FG, Syn WK, Pereira TA, Agboola KM, Omenetti A, Jung Y, Teaberry V, Choi SS. Pan-caspase inhibitor VX-166 reduces fibrosis in an animal model of nonalcoholic steatohepatitis. Hepatology. 2009;50:1421-1430. [PubMed] [Cited in This Article: ] |
| 70. | Shiffman ML, Pockros P, McHutchison JG, Schiff ER, Morris M, Burgess G. Clinical trial: the efficacy and safety of oral PF-03491390, a pancaspase inhibitor - a randomized placebo-controlled study in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2010;31:969-978. [PubMed] [Cited in This Article: ] |
| 71. | Pockros PJ, Schiff ER, Shiffman ML, McHutchison JG, Gish RG, Afdhal NH, Makhviladze M, Huyghe M, Hecht D, Oltersdorf T. Oral IDN-6556, an antiapoptotic caspase inhibitor, may lower aminotransferase activity in patients with chronic hepatitis C. Hepatology. 2007;46:324-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 72. | Wu XX, Wu LM, Fan JJ, Qin Y, Chen G, Wu XF, Shen Y, Sun Y, Xu Q. Cortex Dictamni extract induces apoptosis of activated hepatic stellate cells via STAT1 and attenuates liver fibrosis in mice. J Ethnopharmacol. 2011;135:173-178. [PubMed] [Cited in This Article: ] |
| 73. | Lee SH, Zhao YZ, Park EJ, Che XH, Seo GS, Sohn DH. 2',4',6'-Tris(methoxymethoxy) chalcone induces apoptosis by enhancing Fas-ligand in activated hepatic stellate cells. Eur J Pharmacol. 2011;658:9-15. [PubMed] [Cited in This Article: ] |