Published online Apr 14, 2012. doi: 10.3748/wjg.v18.i14.1635
Revised: May 28, 2011
Accepted: June 21, 2011
Published online: April 14, 2012
AIM: To determine the effectiveness of pancreatic duct (PD) stent placement for the prevention of pancreatitis after endoscopic retrograde cholangiopancreatography (ERCP) in high risk patients.
METHODS: Authors conducted a single-blind, randomized controlled trial to evaluate the effectiveness of a pancreatic spontaneous dislodgement stent against post-ERCP pancreatitis, including rates of spontaneous dislodgement and complications. Authors defined high risk patients as having any of the following: sphincter of Oddi dysfunction, difficult cannulation, prior history of post-ERCP pancreatitis, pre-cut sphincterotomy, pancreatic ductal biopsy, pancreatic sphincterotomy, intraductal ultrasonography, or a procedure time of more than 30 min. Patients were randomized to a stent group (n = 60) or to a non-stent group (n = 60). An abdominal radiograph was obtained daily to assess spontaneous stent dislodgement. Post-ERCP pancreatitis was diagnosed according to consensus criteria.
RESULTS: The mean age (± standard deviation) was 67.4 ± 13.8 years and the male: female ratio was 68:52. In the stent group, the mean age was 66 ± 13 years and the male: female ratio was 33:27, and in the non-stent group, the mean age was 68 ± 14 years and the male: female ratio was 35:25. There were no significant differences between groups with respect to age, gender, final diagnosis, or type of endoscopic intervention. The frequency of post-ERCP pancreatitis in PD stent and non-stent groups was 1.7% (1/60) and 13.3% (8/60), respectively. The severity of pancreatitis was mild in all cases. The frequency of post-ERCP pancreatitis in the stent group was significantly lower than in the non-stent group (P = 0.032, Fisher’s exact test). The rate of hyperamylasemia were 30% (18/60) and 38.3% (23 of 60) in the stent and non-stent groups, respectively (P = 0.05, χ2 test). The placement of a PD stent was successful in all 60 patients. The rate of spontaneous dislodgement by the third day was 96.7% (58/60), and the median (range) time to dislodgement was 2.1 (2-3) d. The rates of stent migration, hemorrhage, perforation, infection (cholangitis or cholecystitis) or other complicationss were 0% (0/60), 0% (0/60), 0% (0/60), 0% (0/60), 0% (0/60), respectively, in the stent group. Univariate analysis revealed no significant differences in high risk factors between the two groups. The pancreatic spontaneous dislodgement stent safely prevented post-ERCP pancreatitis in high risk patients.
CONCLUSION: Pancreatic stent placement is a safe and effective technique to prevent post-ERCP pancreatitis. Therefore authors recommend pancreatic stent placement after ERCP in high risk patients.
- Citation: Kawaguchi Y, Ogawa M, Omata F, Ito H, Shimosegawa T, Mine T. Randomized controlled trial of pancreatic stenting to prevent pancreatitis after endoscopic retrograde cholangiopancreatography. World J Gastroenterol 2012; 18(14): 1635-1641
- URL: https://www.wjgnet.com/1007-9327/full/v18/i14/1635.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i14.1635
Acute pancreatitis is a serious complication of endoscopic retrograde cholangiopancreatography (ERCP). The frequency of post-ERCP pancreatitis varies between 1% and 9%[1-6] in average risk patients, and its prevention remains a critical issue. Impaired drainage of the pancreatic duct (PD), leading to acinar injury, is a commonly accepted mechanism of injury[7,8]. Possible causes of impaired drainage include mechanical, chemical and thermal injury, as well as subsequent edema and spasm of the duodenal papilla.
Several prospective studies have confirmed that PD stent placement prevents post-ERCP pancreatitis, especially in high risk patients[9-13]. However, no consensus has yet been reached on the indications for prophylactic PD stent placement or on the type of stent which should be used. Therefore, we conducted a single-blind, randomized controlled trial (RCT) to evaluate the effectiveness of spontaneous dislodgement stents in preventing post-ERCP pancreatitis in high risk patients.
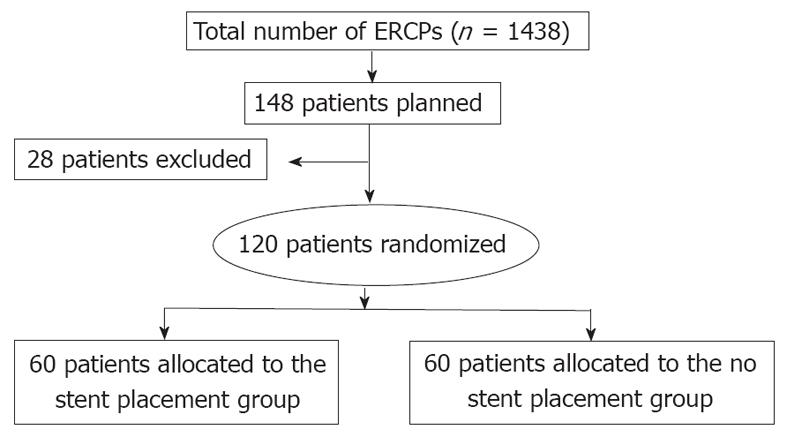
This RCT was conducted in Tokai University Hospital, Japan between April 2006 and June 2010. During this period, we performed 1438 ERCPs on patients with pancreatobiliary diseases. All procedures for this study were performed by one physician (Kawaguchi Y), an experienced surgeon with more than 2000 prior ERCP cases. Independent patient-related and procedure-related risk factors for post-ERCP pancreatitis have been previously published [3-5,14-16]. Patients at high risk of post-ERCP pancreatitis who met any of the following criteria were enrolled in this RCT: (1) A previous history of post-ERCP pancreatitis; (2) Sphincter of Oddi dysfunction; (3) Difficult cannulation; (4) Pre-cutting; (5) PD biopsy; (6) Intraductal PD ultrasonography; or (7) ERCP procedure time > 30 min prior to PD stent placement. Difficult cannulation was defined as > 10 min of attempted cannulation. We performed PD guidewire placement or pancreatic sphincterotomy in some cases of difficult cannulation. Exclusion criteria were as follows: (1) Inability to provide written informed consent; (2) Performance status of 4; (3) Age 19 years or younger; (4) Pregnancy or breastfeeding; (5) Inability to access duodenal papilla endoscopically; (6) Previous endoscopic sphincterotomy or endoscopic papillary balloon dilation; (7) Inability to insert a guidewire into the PD; (8) Patients requiring PD drainage; (9) Patients requiring endoscopic papillectomy; (10) Pancreatic head cancer; or (11) Pancreas divisum.
Of 148 patients screened, 120 patients who satisfied the inclusion criteria participated in this study. Patients were randomly assigned into a stent placement group (60 patients) and a non-stent placement group (60 patients) after the completion of diagnostic or therapeutic ERCP (Figure 1). Randomization was conducted by a simple randomization method without any stratification factor. After diagnostic or therapeutic ERCP was achieved and eligibility was confirmed, a research assistant assigned patients to either the stent or non-stent group, allocating patients using a uniform random number algorithm. All patients provided written informed consent before study entry. This study was approved by Institutional Review Board of Tokai University Hospital (Clinical Trials; University Hospital Medical Information Network Identification Number in Japan, 3995).
Each patient’s past medical history was obtained and physical examination was performed prior to ERCP. Lidocaine gel (4%) was used for pharyngeal anesthesia. Intravenous administration of midazolam with pethidine hydrochloride was used for conscious sedation. All patients received an intravenous drip infusion of 20 mg nafamostat mesilate during the examination, starting before ERCP, with the same dose of nafamostat mesilate as well as antibiotics administered afterwards.
ERCP was performed in standard fashion with JF-240, JF-260V or TJF240 video endoscopes (Olympus, Tokyo, Japan); PR-V216Q, PR-V234Q or PR-V220Q catheters (Olympus) and Jagwire guidewires (Boston Scientific Japan, Tokyo, Japan) were used. We used 5 Fr straight polyethylene stents, 3 cm in length, unflanged on the PD side, and with two flanges on the duodenal side (GPDS-5-3; Cook Endoscopy Inc., Winston-Salem, NC, United States). Stent dislodgment was confirmed by daily serial abdominal radiography. If the stent remained on the third day, it was removed endoscopically. In the stent group, we performed PD cannulation followed by contrast injection, guidewire insertion, and stent placement under fluoroscopy. All 120 patients were hospitalized for at least 3 d after the procedure for observation of potential pancreatitis or other complication, regardless of stent placement. On the assumption that the frequency of post-ERCP pancreatitis was 2.3% and 20% in the stent and non- stent group, respectively, 60 patients were needed in each group for 80% power and 5% typeIerror.
The definition of post-ERCP pancreatitis was based on Cotton’s criteria[17], with a modified definition of severity. Instead of the number of hospital days, we evaluated the degree of severity of pancreatitis by number of days before resuming feeding. Post-ERCP pancreatitis was defined as pancreatic pain and hyperamylasemia within 24 h post-procedure. Pancreatic pain was defined as persistent pain in the epigastric or periumbilical region. Hyperamylasemia was defined as an amylase level greater than three times the upper limit of normal in our institution.
The primary outcome was frequency and severity of post-ERCP pancreatitis. As secondary outcomes, we evaluated the frequency of serum hyperamylasemia, the success rate of stent placement, time to stent dislodgement, and other complications.
This trial was designed as a superiority study to detect differences in clinical effectiveness to prevent post-ERCP pancreatitis with the addition of PD stent placement. The effectiveness of pancreatic stenting was analyzed on an intention-to-treat basis.
The χ2 test or Fisher’s exact test were used to evaluate proportional differences. The Student t test was used for comparing continuous variables. Univariate evaluation was made for each potential risk factor. All statistical analyses were performed with StatView Ver. 5.0 (SAS Institute, Cary, NC, United States) and StatMate 4 (ATMS, Tokyo, Japan).
Tables 1 and 2 show basic patient characteristics including risk factors for post-ERCP pancreatitis and final diagnosis in both groups. The mean age (± standard deviation) of all patients was 67.4 ± 3.8 years and the male: female ratio was 68:52. In the stent group, the mean age was 66 ± 13 years and the male: female ratio was 33:27, whereas the mean age was 68 ± 14 years and the male: female ratio was 35:25 in the non-stent group. There were no significant differences between groups with respect to age, gender, final diagnosis, or type of endoscopic intervention (Table 1).
| Non-stent | Stent | P value | |
| No. of patients | 60 | 60 | - |
| Mean age (range) (yr) | 68 (27-92) | 66 (24-88) | 0.35 |
| Sex: female/male | 25/35 | 27/33 | 0.46 |
| Reasons of high risk | |||
| Previous post-ERCP pancreatitis | 5 (8%) | 5 (8%) | 0.65 |
| Sphincter of Oddi dysfunction | 0 (0%) | 0 (0%) | - |
| A difficult cannulation | 10 (17%) | 10 (17%) | 0.68 |
| Pre-cut | 0 (0%) | 0 (0%) | - |
| Pancreatic sphincterotomy | 5 (8%) | 4 (7%) | 1 |
| Pancreatic duct biopsy | 5 (8%) | 6 (10%) | 1 |
| IDUS for pancreatic duct | 27 (45%) | 25 (42%) | 0.15 |
| Procedure time greater than 30 min | 29 (48%) | 33 (55%) | 0.50 |
| Non-stent | Stent | P value | |
| Biliary disease | |||
| CBD stone | 16 (27) | 15 (25) | 0.68 |
| Cholangitis | 2 (3) | 2 (3) | 0.49 |
| Cholangiocarcinoma | 4 (7) | 3 (5) | 1 |
| Cholangiocellular carcinoma | 2 (3) | 1 (2) | 1 |
| Benign biliary stricture | 1 (2) | 2 (3) | 1 |
| Primary sclerosing cholangitis | 2 (3) | 1 (2) | 1 |
| GB stone | 1 (2) | 0 (0) | 1 |
| GB polyp | 1 (2) | 1 (2) | 1 |
| GB adenomyomatosis | 1 (2) | 1 (2) | 0.46 |
| Cholecystitis | 1 (2) | 1 (2) | 0.46 |
| GB carcinoma | 3 (5) | 3 (5) | 0.47 |
| Pancreatic disease | |||
| IPMN | 10 (17) | 11 (18) | 0.67 |
| MCN | 0 (0) | 1 (2) | 1 |
| SCN | 1 (2) | 0 (0) | 1 |
| Chronic pancreatitis | 3 (5) | 3 (5) | 0.47 |
| Pancreatic cyst | 1 (2) | 2 (3) | 1 |
| Pancreatic carcinoma | 11 (18) | 13 (22) | 0.65 |
The placement of the PD stent was successful in all 60 patients and no complications were observed (Table 3).
| No. of patients | 60 |
| Success rate in stent placement | 100% |
| Rate of spontaneous stent dislodgement | 96.7% |
| Duration time to dislodgement, d, (range) | 2.1 (23) |
| Complications | |
| Stent migration | 0% |
| Post-ERCP pancreatitis | 1.7% |
| Hyperamylasemia | 30% |
| Hemorrhage | 0% |
| Perforation | 0% |
| Infection (cholangitis, cholecystitis) | 0% |
| Others | 0% |
| Mean serum amylase level after procedures, U/L, (range) | 1246 (746-1964) |
The overall rate of post-ERCP pancreatitis was 7.5% (9/120). The rate of post-ERCP pancreatitis in the stent and non-stent groups was 1.7% (1/60) and 13.3% (8/60), respectively (P = 0.032, Fisher’s exact test). The severity of pancreatitis was mild in all nine patients (Table 4). The rate of hyperamylasemia was 30% (18/60) and 38.3% (23/60) in the stent and non- stent groups, respectively (P = 0.05, χ2 test).
| Non-stent | Stent | P value | |
| No. of patients | 60 | 60 | |
| Hyperamylasemia | 23 (38.3%) | 18 (30%) | 0.862 |
| Average serum amylase level (IU/L) (range) | 842.4 (381-2040) | 746.2 (420-1620) | 0.798 |
| Post-ERCP pancreatitis | 8 (13.3%) | 1 (1.7%) | 0.0322 |
| Mild | 8 | 1 | 0.0322 |
| Moderate | 0 | 0 | - |
| Severe | 0 | 0 | - |
| Average serum amylase level in pancreatitis cases (IU/L) (range) | 1720 (820-2040) | 1240 (746-1964) | 0.04 |
The rate of spontaneous dislodgement by the third day was 96.7% (58/60), and the median (range) time to dislodgement was 2.1 (range, 2-3) d (Table 3).
The rates of stent migration, hemorrhage, perforation, infection (cholangitis or cholecystitis) or other complications were 0% (0/120) in both groups (Table 3).
Table 5 shows the final diagnoses of those patients with post-ERCP pancreatitis patients in both groups. Regarding univariate analysis of risk factors for post-ERCP pancreatitis, there were no significant differences between groups with respect to various high risk factors studied (Table 6).
| Non-stent | Pancreatitis | Stent | Pancreatitis | |
| Biliary disease | ||||
| CBD stone | 16 | 3 (19%) | 15 | 0 |
| Cholangitis | 2 | 0 | 2 | 0 |
| Cholangiocarcinoma | 4 | 1 (25%) | 3 | 0 |
| Cholangiocellular carcinoma | 2 | 0 | 1 | 0 |
| Benign biliary stricture | 1 | 0 | 2 | 0 |
| Primary sclerosing cholangitis | 2 | 0 | 1 | 0 |
| GB stone | 1 | 0 | 0 | 0 |
| GB polyp | 1 | 0 | 1 | 0 |
| GB adenomyomatosis | 1 | 0 | 1 | 0 |
| Cholecystitis | 1 | 0 | 1 | 0 |
| GB carcinoma | 3 | 1 (33%) | 3 | 0 |
| Pancreatic disease | ||||
| IPMN | 10 | 1 (10%) | 11 | 0 |
| MCN | 0 | 0 | 1 | 0 |
| SCN | 1 | 0 | 0 | 0 |
| Chronic pancreatitis | 3 | 0 | 3 | 0 |
| Pancreatic cyst | 1 | 0 | 2 | 0 |
| Pancreatic carcinoma | 11 | 2 (18%) | 13 | 1 (8%) |
| Non-stent | Pancreatitis | Stent | Pancreatitis | Univariate P value | |
| No. of patients | 60 | 8 | 60 | 1 | |
| Age (< 60 yr) | 17 (28) | 3 (18) | 18 (30) | 0 (0) | 0.72 |
| Female | 25 (42) | 3 (12) | 27 (45) | 0 (0) | 0.78 |
| Previous post-ERCP pancreatitis | 5 (8) | 1 (20) | 5 (8) | 0 (0) | 0.68 |
| Sphincter of Oddi dysfunction | 0 (0) | 0 (0) | 0 (0) | 0 (0) | - |
| A difficult cannulation | 10 (17) | 2 (20) | 10 (17) | 0 (0) | 0.68 |
| Pre-cut | 0 (0) | 0 (0) | 0 (0) | 0 (0) | - |
| Pancreatic sphincterotomy | 5 (8) | 1 (20) | 4 (7) | 0 (0) | 0.68 |
| Pancreatic duct biopsy | 5 (8) | 1 (20) | 6 (10) | 1 (17) | 0.87 |
| IDUS for pancreatic duct | 27 (45) | 3 (11) | 25 (42) | 1 (4) | 0.74 |
| Procedure time greater than 30 min | 29 (48) | 4 (14) | 33 (55) | 0 (0) | 0.72 |
We conducted a single-blind, randomized controlled clinical study to evaluate the effectiveness of spontaneous dislodgement PD stents for preventing post-ERCP pancreatitis in high risk patients. The overall rate of post-ERCP pancreatitis was 7.5%. This was consistent with reported rates of post-ERCP pancreatitis, ranging from 1% to as high as 40% in patients with risk factors[1-5,7-13,18-22]. The rate of post-ERCP pancreatitis in the stent and non-stent groups was significantly different at 1.7% and 13.3%, respectively. Thus, prophylactic PD stenting may reduce the rate of post-ERCP pancreatitis from 13.3% to 1.7% in high risk patients. Our study suggested that PD stent placement prevented post-ERCP pancreatitis in high risk patients. However the severity of pancreatitis in this study was mild in all cases and we were therefore were unable to assess the efficacy of pancreatic stents for severe post-ERCP pancreatitis. Considering the sample size of this study and no cases of severe post-ERCP pancreatitis, this study had a limitation to determine any significant difference in severity between the two groups.
Several prospective studies have suggested that prophylactic PD stent placement decreases the risk of pancreatitis in high risk patients[9-14]. In contrast, Smithline et al[2] reported that PD stent insertion after ERCP did not have a significant beneficial effect in individuals undergoing biliary sphincterotomy for various indications. These differences may be attributed to variable levels of risk for pancreatitis in the individual study populations. However, two independent meta-analysis on the use of pancreatic stent placement for post-ERCP pancreatitis prophylaxis in patients at high risk of post-ERCP pancreatitis have demonstrated that stent placement significantly reduced the incidence of post-ERCP pancreatitis[10,23]. Based on these meta-analyses, European Society of Gastrointestinal Endoscopy guidelines recommend prophylactic pancreatic stent placement to prevent post-ERCP pancreatitis in high risk patients[24]. An updated meta-analysis of RCTs involving pancreatic stent placement also showed that pancreatic stent placement after ERCP reduced the risk of post-ERCP pancreatitis and was beneficial for patients at high risk compared to those who did not have stenting[25]. However, several areas requiring further clarification were noted, including the efficacy of pancreatic stents for severe post-ERCP pancreatitis, identification of risk factors and management of adverse events, optimal stent design and material, timing of both placement and removal, and comparison of stenting with wire-guided cannulation or pharmacoprophylaxis[25]. Several of these points warrant further investigation.
Regarding indications for stenting, prophylactic pancreatic stent placement has been shown to be cost-effective in patients at high risk of post-ERCP pancreatitis, but not in those at average risk[26]. Caution should be used when attempting prophylactic pancreatic stent placement due to the incidence of post-ERCP pancreatitis after failed attempts, which may be as high as 65%[27]. As such, prophylactic pancreatic stent placement in high risk patients is cost-effective only if the success rate of stent placement exceeds 75%. Careful selection of patients with risk factors for post-ERCP pancreatitis is therefore critical.
The type of stent used may also play an important role in prophylactic care. As for diameter of stents, most recent studies have used 3 Fr and 5 Fr diameter pancreatic stents. In two recent RCTs, 5 Fr stents proved equivalent to 3 Fr stents for most outcomes studied, though successful insertion of 5 Fr stents was achieved significantly more often[28,29]. Placement of 3-4 Fr stents require a small-caliber guidewire (0.018-0.025 inch), a procedure that is difficult and which requires a high level of experience[17,18,30,31]. In contrast, the 0.035-inch guidewire used for 5 Fr stents is relatively easy to use for stent placement. Regarding stent length, we used very short (3 cm) stents in this study. Long stents may be more difficult to place and may have higher rates of spontaneous dislodgement, causing damage to the intestines. We therefore recommend the use of shorter stents.
Regarding placement of PD stents, previous studies reported a 5%-10% failure rate and a low rate of complications (2%)[2,9,13]. It is difficult to cannulate the PD after all the procedures, and PD cannulation itself may cause pancreatitis. Unsuccessful cases were reported to be at a higher risk of pancreatitis[7,27]. In our study, we could place PD stents successfully in all patients. PD stent-related complications, such as migration or occlusion, did not occur in any patients. Based on the previous literature and our outcomes, we recommend 5 Fr diameter and very short (3 cm) stents.
As for optimal stent design, Sofuni et al[12] reported that the unflanged duodenal pigtail-type stent dislodged spontaneously at a higher rate, and that handling of the short duodenal pigtail stent may be complicated, such as the sudden forward movement of the stent on release, requiring close attention and experience. In addition, an internal flange may make spontaneous PD stent dislodgement difficult. In contrast, stents without an internal flange may dislodge spontaneously into the duodenum by pancreatic juice flow or friction with passing food. Since we used 5 Fr straight type stents without an internal flange, stents dislodged spontaneously in 1 d or 2 d in most cases. In our study, the spontaneous dislodgement rate was 96% at 3 d, with a median of 2.1 d. In the absence of spontaneous migration out of the PD at 5-10 d post-ERCP, prompt endoscopic stent removal is recommended due to the increased risk of post-ERCP pancreatitis (relative risk 5.2 in patients without vs with spontaneous stent elimination at 2 wk) and potential for stent-induced damage to the PD[28,29]. It is our recommendation to avoid the use of stents with an internal flange and to confirm stent dislodgement, or to remove stents in a timely interval, preferably within 1-2 wk after ERCP.
Finally, prophylactic pancreatic stent placement by operator and location warrants discussion. According to survey data, only a small percentage of endoscopists utilize prophylactic pancreatic stenting. The incidence of post-ERCP pancreatitis and a high ERCP volume were independently associated with the use of prophylactic pancreatic stenting[32-35]. From these surveys, endoscopists who did not place prophylactic pancreatic stents cited lack of experience in this technique as a primary reason[32]. We anticipate that a stent insertion success rate and low rate of complications can also be achieved in other institutions by ERCP specialists. Placement of pancreatic stents is quickly becoming standard practice. However, many endoscopists remain unfamiliar with the specific techniques required to achieve safe and effective PD guidewire access and stent placement. It is critically important that all endoscopists performing ERCP become proficient in techniques for safe and effective stent placement.
Our study has several limitations. First, this study is single-blind and observation bias is inevitable. Second, we could not evaluate the risk of post-ERCP pancreatitis in patients who failed PD stent placement. Third, we could not evaluate the efficacy of PD stenting alone because nafamostat mesilate was administered in both groups.
In conclusion, pancreatic spontaneous dislodgement stent placement decreases the risk of post-ERCP pancreatitis in patients who are likely to develop post-ERCP pancreatitis.
We thank the Research Committee of Intractable Pancreatic Diseases (Principal investigator: Tooru Shimosegawa) from the Ministry of Health, Labour, and Welfare of Japan. We thank Dr. Gautam A Deshpande for revising our English.
Acute pancreatitis is a serious complication of endoscopic retrograde cholangiopancreatography (ERCP) and its prevention remains a critical issue. Though several studies have confirmed that pancreatic duct (PD) stent placement prevents post-ERCP pancreatitis, no consensus has yet been reached on the indications for prophylactic PD stent placement or on the type of stent.
In this study, the authors demonstrated that pancreatic stent placement was a safe and effective technique to prevent post-ERCP pancreatitis. Authors recommend pancreatic stent placement after ERCP procedures in high risk patients.
All procedures in this study were performed by one endoscopist (Kawaguchi Y), an experienced operator with more than 2000 prior ERCP cases. Independent patient-related and procedure-related risk factors for post-ERCP pancreatitis have been previously published. This study suggests that PD stenting can significantly reduce post-ERCP pancreatitis in high risk patients.
By the general use of the pancreatic stent placement after ERCP procedures in high risk patients, post-ERCP pancreatitis may decrease in the future.
The authors used 5 Fr straight polyethylene stents, 3 cm in length, unflanged on the pancreatic duct side, and with two flanges on the duodenal side. As this stent dislodges spontaneously into the duodenum as a result of pancreatic juice flow or friction with passing food, it is termed a spontaneous dislodgement stent.
The pancreatic spontaneous dislodgement stent placement safely prevented post-ERCP pancreatitis in high risk patients. This result is very impressive.
Peer reviewer: Pascal Bucher, MD, Chef de Clinique, Service de Chirurgie Viscérale et Transplantation, University Hospital Geneva, 4 Rue Gabrielle Perret Gentile, 1211 Geneva, Switzerland
S- Editor Yang XC L- Editor Cant MR E- Editor Li JY
| 1. | Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383-393. [PubMed] [Cited in This Article: ] |
| 2. | Smithline A, Silverman W, Rogers D, Nisi R, Wiersema M, Jamidar P, Hawes R, Lehman G. Effect of prophylactic main pancreatic duct stenting on the incidence of biliary endoscopic sphincterotomy-induced pancreatitis in high-risk patients. Gastrointest Endosc. 1993;39:652-657. [PubMed] [Cited in This Article: ] |
| 3. | Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, Fennerty MB, Ryan ME, Shaw MJ. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1716] [Cited by in F6Publishing: 1607] [Article Influence: 57.4] [Reference Citation Analysis (2)] |
| 4. | Masci E, Toti G, Mariani A, Curioni S, Lomazzi A, Dinelli M, Minoli G, Crosta C, Comin U, Fertitta A. Complications of diagnostic and therapeutic ERCP: a prospective multicenter study. Am J Gastroenterol. 2001;96:417-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 625] [Cited by in F6Publishing: 584] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 5. | Freeman ML, DiSario JA, Nelson DB, Fennerty MB, Lee JG, Bjorkman DJ, Overby CS, Aas J, Ryan ME, Bochna GS. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001;54:425-434. [PubMed] [Cited in This Article: ] |
| 6. | Lawrence C, Romagnuolo J, Cotton PB, Payne KM, Hawes RH. Post-ERCP pancreatitis rates do not differ between needle-knife and pull-type pancreatic sphincterotomy techniques: a multiendoscopist 13-year experience. Gastrointest Endosc. 2009;69:1271-1275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Freeman ML, Guda NM. Prevention of post-ERCP pancreatitis: a comprehensive review. Gastrointest Endosc. 2004;59:845-864. [PubMed] [Cited in This Article: ] |
| 8. | Sherman S, Ruffolo TA, Hawes RH, Lehman GA. Complications of endoscopic sphincterotomy. A prospective series with emphasis on the increased risk associated with sphincter of Oddi dysfunction and nondilated bile ducts. Gastroenterology. 1991;101:1068-1075. [PubMed] [Cited in This Article: ] |
| 9. | Fazel A, Quadri A, Catalano MF, Meyerson SM, Geenen JE. Does a pancreatic duct stent prevent post-ERCP pancreatitis? A prospective randomized study. Gastrointest Endosc. 2003;57:291-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 232] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 10. | Singh P, Das A, Isenberg G, Wong RC, Sivak MV, Agrawal D, Chak A. Does prophylactic pancreatic stent placement reduce the risk of post-ERCP acute pancreatitis? A meta-analysis of controlled trials. Gastrointest Endosc. 2004;60:544-550. [PubMed] [Cited in This Article: ] |
| 11. | Tsuchiya T, Itoi T, Sofuni A, Itokawa F, Kurihara T, Ishii K, Tsuji S, Kawai T, Moriyasu F. Temporary pancreatic stent to prevent post endoscopic retrograde cholangiopancreatography pancreatitis: a preliminary, single-center, randomized controlled trial. J Hepatobiliary Pancreat Surg. 2007;14:302-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Sofuni A, Maguchi H, Itoi T, Katanuma A, Hisai H, Niido T, Toyota M, Fujii T, Harada Y, Takada T. Prophylaxis of post-endoscopic retrograde cholangiopancreatography pancreatitis by an endoscopic pancreatic spontaneous dislodgement stent. Clin Gastroenterol Hepatol. 2007;5:1339-1346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Tarnasky PR, Palesch YY, Cunningham JT, Mauldin PD, Cotton PB, Hawes RH. Pancreatic stenting prevents pancreatitis after biliary sphincterotomy in patients with sphincter of Oddi dysfunction. Gastroenterology. 1998;115:1518-1524. [PubMed] [Cited in This Article: ] |
| 14. | Kennedy PT, Russo E, Kumar N, Powell N, Bansi D, Thillainayagam A, Vlavianos P, Westaby D. The safety and utility of prophylactic pancreatic duct stents in the prevention of post-ERCP pancreatitis: an analysis of practice in a single UK tertiary referral center. Surg Endosc. 2010;24:1923-1928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Masci E, Mariani A, Curioni S, Testoni PA. Risk factors for pancreatitis following endoscopic retrograde cholangiopancreatography: a meta-analysis. Endoscopy. 2003;35:830-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 261] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 16. | Loperfido S, Angelini G, Benedetti G, Chilovi F, Costan F, De Berardinis F, De Bernardin M, Ederle A, Fina P, Fratton A. Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest Endosc. 1998;48:1-10. [PubMed] [Cited in This Article: ] |
| 17. | Williams EJ, Taylor S, Fairclough P, Hamlyn A, Logan RF, Martin D, Riley SA, Veitch P, Wilkinson ML, Williamson PR. Risk factors for complication following ERCP; results of a large-scale, prospective multicenter study. Endoscopy. 2007;39:793-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 253] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 18. | Cheng CL, Sherman S, Watkins JL, Barnett J, Freeman M, Geenen J, Ryan M, Parker H, Frakes JT, Fogel EL. Risk factors for post-ERCP pancreatitis: a prospective multicenter study. Am J Gastroenterol. 2006;101:139-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 414] [Cited by in F6Publishing: 404] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 19. | Mehta SN, Pavone E, Barkun JS, Bouchard S, Barkun AN. Predictors of post-ERCP complications in patients with suspected choledocholithiasis. Endoscopy. 1998;30:457-463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 83] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Vandervoort J, Soetikno RM, Tham TC, Wong RC, Ferrari AP, Montes H, Roston AD, Slivka A, Lichtenstein DR, Ruymann FW. Risk factors for complications after performance of ERCP. Gastrointest Endosc. 2002;56:652-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 152] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 21. | Elton E, Howell DA, Parsons WG, Qaseem T, Hanson BL. Endoscopic pancreatic sphincterotomy: indications, outcome, and a safe stentless technique. Gastrointest Endosc. 1998;47:240-249. [PubMed] [Cited in This Article: ] |
| 22. | Catalano MF, Linder JD, Chak A, Sivak MV, Raijman I, Geenen JE, Howell DA. Endoscopic management of adenoma of the major duodenal papilla. Gastrointest Endosc. 2004;59:225-232. [PubMed] [Cited in This Article: ] |
| 23. | Andriulli A, Forlano R, Napolitano G, Conoscitore P, Caruso N, Pilotto A, Di Sebastiano PL, Leandro G. Pancreatic duct stents in the prophylaxis of pancreatic damage after endoscopic retrograde cholangiopancreatography: a systematic analysis of benefits and associated risks. Digestion. 2007;75:156-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Dumonceau JM, Andriulli A, Deviere J, Mariani A, Rigaux J, Baron TH, Testoni PA. European Society of Gastrointestinal Endoscopy (ESGE) Guideline: prophylaxis of post-ERCP pancreatitis. Endoscopy. 2010;42:503-515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 202] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 25. | Mazaki T, Masuda H, Takayama T. Prophylactic pancreatic stent placement and post-ERCP pancreatitis: a systematic review and meta-analysis. Endoscopy. 2010;42:842-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 26. | Das A, Singh P, Sivak MV, Chak A. Pancreatic-stent placement for prevention of post-ERCP pancreatitis: a cost-effectiveness analysis. Gastrointest Endosc. 2007;65:960-968. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Freeman ML, Overby C, Qi D. Pancreatic stent insertion: consequences of failure and results of a modified technique to maximize success. Gastrointest Endosc. 2004;59:8-14. [PubMed] [Cited in This Article: ] |
| 28. | Chahal P, Tarnasky PR, Petersen BT, Topazian MD, Levy MJ, Gostout CJ, Baron TH. Short 5Fr vs long 3Fr pancreatic stents in patients at risk for post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin Gastroenterol Hepatol. 2009;7:834-839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Fehmi SMA, Schoenfeld PS, Scheiman JM, Kwon RS, Piraka CR, Wamsteker EJ, Korsnes S, Anderson MA, Elta GH. 5Fr prophylactic pancreatic stents are easier to place and require fewer guide wires than 3Fr stents. Gastrointest Endosc. 2008;67:AB328-AB329. [DOI] [Cited in This Article: ] |
| 30. | Sherman S, Hawes RH, Savides TJ, Gress FG, Ikenberry SO, Smith MT, Zaidi S, Lehman GA. Stent-induced pancreatic ductal and parenchymal changes: correlation of endoscopic ultrasound with ERCP. Gastrointest Endosc. 1996;44:276-282. [PubMed] [Cited in This Article: ] |
| 31. | Smith MT, Sherman S, Ikenberry SO, Hawes RH, Lehman GA. Alterations in pancreatic ductal morphology following polyethylene pancreatic stent therapy. Gastrointest Endosc. 1996;44:268-275. [PubMed] [Cited in This Article: ] |
| 32. | Brackbill S, Young S, Schoenfeld P, Elta G. A survey of physician practices on prophylactic pancreatic stents. Gastrointest Endosc. 2006;64:45-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Dumonceau JM, Rigaux J, Kahaleh M, Gomez CM, Vandermeeren A, Devière J. Prophylaxis of post-ERCP pancreatitis: a practice survey. Gastrointest Endosc. 2010;71:934-939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 34. | Freeman ML. Pancreatic stents for prevention of post-ERCP pancreatitis: for everyday practice or for experts only? Gastrointest Endosc. 2010;71:940-944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Freeman ML. Pancreatic stents for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin Gastroenterol Hepatol. 2007;5:1354-1365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |