Published online Apr 7, 2012. doi: 10.3748/wjg.v18.i13.1525
Revised: January 16, 2012
Accepted: February 8, 2012
Published online: April 7, 2012
AIM: To evaluate the noninvasive parameters and hepatic fibrosis scores in obese children with nonalcoholic fatty liver disease (NAFLD).
METHODS: A total of 77 children diagnosed with NAFLD via liver biopsy were included and divided into 2 subgroups according to the histopathologic staging of hepatic fibrosis: mild (stage 0-1) vs significant fibrosis (stage 2-4). Clinical and laboratory parameters were evaluated in each patient. The aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio, AST/platelet ratio index (APRI), PGA index, Forns index, FIB-4, NAFLD fibrosis score, and pediatric NAFLD fibrosis index (PNFI) were calculated.
RESULTS: No clinical or biochemical parameter exhibited a significant difference between patients with mild and significant fibrosis. Among noninvasive hepatic fibrosis scores, only APRI and FIB4 revealed a significant difference between patients with mild and significant fibrosis (APRI: 0.67 ± 0.54 vs 0.78 ± 0.38, P = 0.032 and FIB4: 0.24 ± 0.12 vs 0.31 ± 0.21, P = 0.010). The area under the receiving operating characteristic curve of FIB4 was 0.81, followed by Forns index (0.73), APRI (0.70), NAFLD fibrosis score (0.58), AST/ALT ratio (0.53), PGA score (0.45), and PNFI (0.41).
CONCLUSION: APRI and FIB4 might be useful noninvasive hepatic fibrosis scores for predicting hepatic fibrosis in children with NAFLD.
- Citation: Yang HR, Kim HR, Kim MJ, Ko JS, Seo JK. Noninvasive Parameters and hepatic fibrosis scores in children with nonalcoholic fatty liver disease. World J Gastroenterol 2012; 18(13): 1525-1530
- URL: https://www.wjgnet.com/1007-9327/full/v18/i13/1525.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i13.1525
The disease spectrum of nonalcoholic fatty liver disease (NAFLD) ranges from simple steatosis to nonalcoholic steatohepatitis (NASH), fibrosis, and cirrhosis; NASH progresses towards cirrhosis, even in children[1,2]. Therefore, accurate diagnosis and early detection of hepatic fibrosis are required in obese children suspicious of NAFLD.
Percutaneous liver biopsy is the gold standard for the diagnosis of NASH[3]. However, histopathologic investigation has some limitations because of its invasiveness and high costs, especially in children; moreover, the histopathologic findings of NAFLD in children are somewhat different from those in adults, revealing portal inflammation and portal fibrosis mainly in pediatric NASH in contrast to lobular inflammation, perisinusoidal fibrosis, ballooning, and Mallory’s hyaline in adult NASH[4,5].
Noninvasive markers of hepatic fibrosis and noninvasive hepatic fibrosis scores have been evaluated in previous studies, mostly in adult patients with NAFLD[6]. Although pediatric NAFLD shows peculiar histopathologic features as described above[4,5], there have been only limited studies of noninvasive biochemical markers of hepatic fibrosis related to NAFLD in pediatric populations[7-10], and no validation studies in children on the previously suggested noninvasive hepatic fibrosis scores excluding 1 study on the enhanced liver fibrosis panel[11]. Only 1 pediatric noninvasive score has been developed up to date[12].
Therefore, the present study aimed to evaluate the noninvasive clinical and laboratory parameters and noninvasive hepatic fibrosis scores indicating the presence of hepatic fibrosis and its severity in obese children with NAFLD.
A total of 77 obese children with NAFLD under the age of 18 years who visited the Pediatric Obesity Clinic were included and divided into 2 subgroups according to the grading and staging of NAFLD. Obesity was defined as a body mass index (BMI) value higher than the 95th percentile for the child’s age and sex, and overweight as a BMI between the 85th and 95th percentiles. NAFLD was diagnosed on the basis of histopathologic findings compatible with NAFLD on liver biopsy[13]. This study was approved by the Institutional Review Board of the Seoul National University Bundang Hospital.
Serum levels of fasting glucose, insulin, total cholesterol, TGs, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and apoprotein A1 and B levels were measured after a 12-h fast at the same time of liver biopsy. Insulin resistance was determined by the homeostatic model assessment of insulin resistance. Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, albumin, γ-glutamyl transpeptidase (γGT), alkaline phosphatase (ALP) levels, prothrombin time (PT), and tumor necrosis factor-α were also measured.
For the differential diagnosis of chronic hepatitis, creatine phosphokinase, lactate dehydrogenase, ammonia, lactate, pyruvate, anti-HAV IgM antibody, HBs antigen and anti-HBs antibody, anti-HCV antibody, EBV VCA IgM antibody, CMV IgM antibody, serum ceruloplasmin, and anti-nuclear antibody were evaluated.
The presence of fatty liver was evaluated in each patient using either abdominal sonography or noncontrast abdominal computed tomography (CT). Using abdominal sonography, fatty liver was detected and the degree of fatty liver was defined as mild, moderate, and severe fatty liver[14]. Regarding abdominal CT, fatty liver was diagnosed when the difference between CT numbers of the liver and spleen was greater than 10[15].
Percutaneous needle liver biopsy was performed in all patients. Histopathologic grades of steatosis, lobular and portal inflammation, and hepatocyte ballooning and the histopathologic stages of fibrosis were evaluated in each patient to diagnose NAFLD and to assess the stages of hepatic fibrosis[16]. The histological scoring system of Kleiner et al[13] was also applied to the liver biopsy specimens to assess the histologic stages of hepatic fibrosis and the NAFLD activity score (NAS). Fibrosis was staged as follows: stage 0: none; stage 1: perisinusoidal or periportal fibrosis (stage 1a: mild perisinusoidal; stage 1b: moderate perisinusoidal; stage 1c: portal/periportal; stage 2: perisinusoidal and portal/periportal fibrosis; stage 3: bridging fibrosis; stage 4: cirrhosis)[14].
The AST/ALT ratio was calculated as the ratio of AST to ALT[10]; AST/platelet ratio index (APRI) as follows: (AST level/AST upper level of normal/platelet counts) × 100[17]; PGA index as the sum of 3 scores based on the test results of PT, γGT activity, and apoprotein A1 and ranged from 0 to 12[18]; Forns index as follows: [7.811 - 3.131 × ln(platelet count) + 0.781 × ln(γGT) + 3.467 × ln(age) - 0.014 × cholesterol][19]; FIB-4 as (age × AST level/platelet count ×√ALT)[20]; NAFLD fibrosis score as follows: [-1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × impaired fasting glucose/diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio - 0.013 × platelet count - 0.66 × albumin][21]. Pediatric NAFLD fibrosis index (PNFI) was calculated using a formula suggested by Nobili et al[12].
The results are expressed as mean ± SD. The data were analyzed using the SPSS 18.0 software program (SPSS Inc., Chicago, IL, United States). Frequency data were compared using Fisher’s exact test for nonparametric analysis. The Mann-Whitney U test was used for comparisons of means between 2 groups. Multivariate logistic regression analysis was performed to determine potential variables predicting significant hepatic fibrosis. P-values less than 0.05 were considered statistically significant.
Receiver operating characteristic (ROC) curves and the area under the ROC curve (AUROC) were applied to assess and compare the diagnostic accuracy of each noninvasive hepatic fibrosis scores.
A total of 77 children (M:F = 66:11; mean age 12.2 ± 2.2 years, range 8-18 years) diagnosed as NAFLD were included. The clinical characteristics of children with NAFLD are shown in Table 1.
| Parameters | Total (n = 77) | Mild fibrosis(stage 0-1) (n = 51) | Significant fibrosis(stage 2-3) (n = 26) | P value |
| Clinical characteristics | ||||
| Gender (M:F) | 66:11 | 42:9 | 24:2 | 0.316 |
| Age (yr) | 12.2 ± 2.3 | 12.0 ± 2.4 | 12.7 ± 1.9 | 0.132 |
| AC (cm) | 91.1 ± 10.1 | 92.1 ± 11.6 | 89.7 ± 7.8 | 0.637 |
| Weight (kg) | 68.9 ± 19.3 | 68.3 ± 21.3 | 70.2 ± 15.1 | 0.448 |
| Height (cm) | 155.5 ± 12.2 | 155.1 ± 12.1 | 156.3 ± 12.6 | 0.407 |
| BMI (kg/m2) | 28.1 ± 5.1 | 27.8 ± 5.2 | 28.7 ± 5.1 | 0.425 |
| Biochemical parameters | ||||
| AST (IU/L) | 82.1 ± 46.6 | 77.4 ± 46.3 | 91.3 ± 46.8 | 0.063 |
| ALT (IU/L) | 167.4 ± 99.1 | 158.7 ± 95.4 | 184.6 ± 105.6 | 0.206 |
| ALP (IU/L) | 283.7 ± 110.0 | 273.0 ± 113.8 | 304.7 ± 101.1 | 0.185 |
| γGT (IU/L) | 53.9 ± 29.1 | 51.1 ± 25.9 | 59.4 ± 34.4 | 0.410 |
| Total bilirubin (mg/dL) | 0.76 ± 0.37 | 0.75 ± 0.30 | 0.77 ± 0.50 | 0.438 |
| Albumin (g/dL) | 4.54 ± 0.31 | 4.54 ± 0.32 | 4.55 ± 0.30 | 1.000 |
| PT INR | 1.02 ± 0.07 | 1.01 ± 0.06 | 1.03 ± 0.08 | 0.090 |
| Total cholesterol (mg/dL) | 180.2 ± 32.6 | 176.8 ± 31.2 | 186.9 ± 34.7 | 0.139 |
| Triglyceride (mg/dL) | 132.8 ± 53.6 | 126.6 ± 52.4 | 142.7 ± 55.0 | 0.235 |
| LDL cholesterol (mg/dL) | 97.2 ± 24.9 | 93.5 ± 20.6 | 103.1 ± 30.2 | 0.198 |
| HDL cholesterol (mg/dL) | 48.1 ± 11.4 | 48.9 ± 12.2 | 46.9 ± 10.2 | 0.995 |
| Apoprotein A1 (mg/dL) | 120.2 ± 15.9 | 121.6 ± 15.7 | 118.1 ± 16.8 | 0.543 |
| Apoprotein B (mg/dL) | 83.5 ± 17.7 | 85.6 ± 17.5 | 80.0 ± 18.3 | 0.418 |
| Fasting glucose (mg/dL) | 98.1 ± 34.2 | 95.4 ± 11.9 | 103.4 ± 56.7 | 0.311 |
| Insulin (μIU/mL) | 24.6 ± 14.6 | 23.2 ± 15.5 | 26.2 ± 13.5 | 0.244 |
| HOMA-IR | 5.8 ± 4.3 | 6.1 ± 4.8 | 5.3 ± 3.2 | 0.586 |
| TNF-α (pg/mL) | 18.2 ± 15.0 | 15.7 ± 12.3 | 21.7 ± 18.0 | 0.400 |
| HbA1C (%) | 5.7 ± 1.1 | 5.5 ± 0.3 | 5.9 ± 1.6 | 0.806 |
| Platelet (× 109/L) | 307.9 ± 69.2 | 313.2 ± 71.1 | 297.3 ± 65.4 | 0.416 |
Histopathologic findings of the liver in children with NAFLD are listed in Table 2. Staging for hepatic fibrosis revealed the prevalence of stage 0 (n = 12), stage 1A (n = 12), stage 1B (n = 7), stage 1C (n = 20), stage 2 (n = 21), and stage 3 (n = 5). There was no patient with stage 4 of hepatic histology. Fibrosis stages were grouped into 2 subgroups: mild hepatic fibrosis (stage 0 to 1) (n = 51); and significant hepatic fibrosis (stage 2 to 3) (n = 26). The histopathologic findings for these 2 groups are compared in Table 2. There were no differences in steatosis, lobular inflammation, and NAS between 2 fibrosis groups (Table 2). Only hepatocyte ballooning was significantly different between the 2 groups (P = 0.018) (Table 2).
| Parameters and hepatic fibrosis scores | Total (n = 77) | Mild fibrosis(stage 0-1) (n = 51) | Significant fibrosis(stage 2-3) (n = 26) | P value |
| Invasive histopathologic NAFLD scores1 | ||||
| Steatosis (grade 0/1/2/3) | 0/13/36/28 | 0/7/22/22 | 0/6/14/6 | 0.085 |
| Inflammation (grade 0/1/2/3) | 9/43/25/0 | 7/29/15/0 | 2/14/10/0 | 0.325 |
| Ballooning (grade 0/1/2) | 27/33/17 | 22/21/8 | 5/12/9 | 0.018 |
| NAS | 4.3 ± 1.4 | 4.18 ± 1.48 | 4.46 ± 1.14 | 0.470 |
| Noninvasive hepatic fibrosis scoring systems | ||||
| AST/ALT ratio | 0.53 ± 0.22 | 0.52 ± 0.16 | 0.57 ± 0.31 | 0.802 |
| AST/platelet ratio index | 0.71 ± 0.49 | 0.67 ± 0.54 | 0.78 ± 0.38 | 0.032 |
| PGA index | 3.78 ± 1.80 | 3.85 ± 1.89 | 3.68 ± 1.70 | 0.710 |
| Forns index | -0.94 ± 1.18 | -1.06 ± 0.21 | -0.69 ± 1.09 | 0.097 |
| FIB4 score | 0.27 ± 0.16 | 0.24 ± 0.12 | 0.31 ± 0.21 | 0.010 |
| NAFLD fibrosis score | -4.95 ± 1.32 | -5.07 ± 1.27 | -4.73 ± 1.41 | 0.532 |
| Pediatric NAFLD fibrosis index | 7.67 ± 2.48 | 7.71 ± 2.79 | 7.61 ± 2.08 | 0.314 |
Clinical data of the patients and the results of laboratory tests for obesity and obesity-related complications are listed in Table 1. None of these noninvasive clinical and biochemical parameters exhibited a significant difference between the 2 fibrosis groups based on histopathologic findings (Table 1).
The result of multivariate logistic regression analysis for clinical or biochemical factors to predict significant hepatic fibrosis in children with NAFLD is shown in Table 3.
| Variables | OR | 95% CI | P value |
| Age | 1.357 | 1.027-1.793 | 0.032 |
| ALP | 1.006 | 1.000-1.011 | 0.035 |
The results of hepatic fibrosis scores including the AST/ALT ratio, APRI, PGA index, Forns index, FIB4, NAFLD fibrosis score, and PNFI were compared between the 2 groups based on the stage of hepatic fibrosis (Table 2).
Among the hepatic fibrosis scores, APRI and FIB4 revealed statistically significant differences between patients with mild fibrosis and significant fibrosis (APRI, P = 0.032; FIB4, P = 0.010) (Table 2). The other hepatic fibrosis scores were not significantly different between the 2 groups (AST/ALT ratio, P = 0.808; PGA index, P = 0.710; Forns index, P = 0.097; NAFLD fibrosis score, P = 0.532; PNFI, P = 0.314) (Table 2).
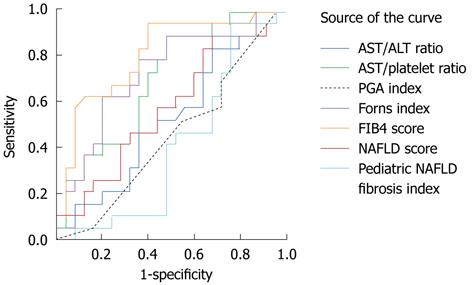
ROC curves of the hepatic fibrosis scoring systems are shown in Figure 1.
When the AUROC of each scoring system was compared, the AUROC of FIB4 was 0.81 (95% CI: 0.68-0.94), followed by the Forns index (AUROC = 0.73, 95% CI: 0.58-0.88), APRI (AUROC = 0.70, 95% CI: 0.55-0.86), NAFLD fibrosis score (AUROC = 0.58, 95% CI: 0.41-0.75), AST/ALT ratio (AUROC = 0.53, 95% CI: 0.35-0.70), PGA score (AUROC = 0.45, 95% CI: 0.28-0.62), and PNFI (AUROC = 0.41, 95% CI: 0.24-0.58).
Due to the invasiveness of histopathologic diagnosis based on liver biopsy, noninvasive methods including the measurement of various clinical parameters or laboratory markers have been applied to clinical practice and research, mostly in adults, to facilitate the diagnosis of NAFLD and to predict hepatic fibrosis in obese patients.
In our study performed in children with biopsy-proven NAFLD, both clinical and biochemical parameters were evaluated on the basis of the histopathologic findings of liver biopsy specimens, particularly focused on biochemical parameters. However, all of these clinical and biochemical parameters failed to distinguish significant fibrosis from no/mild fibrosis in children with NAFLD by univariate analysis, although the result of multivariate analysis suggested age and ALP as possible prognostic factors that predict significant hepatic fibrosis.
Regarding the clinical markers in children with NAFLD, 1 multicenter study reached the same conclusion as our study, revealing no significantly different clinical parameters for significant fibrosis (stage 2 or more), and the serum AST level was the only biochemical parameter associated with the severity of hepatic fibrosis[9]. In contrast, another pediatric study reported that BMI was the only clinical parameter that significantly differentiated NAFLD with hepatic fibrosis from NAFLD without fibrosis, and there were no biochemical parameters predictive of hepatic fibrosis[10]. From these results, it appears that more developed noninvasive tools beyond simple parameters are needed to detect hepatic fibrosis in children suspicious of NAFLD.
To this point, a number of noninvasive hepatic fibrosis scores have been developed and applied to chronic liver diseases, such as hepatitis B, hepatitis C, alcoholic fatty liver disease, and NAFLD[22,23]. These scores include indices based on indirect biochemical markers and/or clinical parameters developed for adults, such as the AST/ALT ratio[10], APRI[17], PGA index[18], Forns index[19], FIB-4[20], and NAFLD fibrosis score[21]. These scores have been validated in a number of previously published studies in adults[22], but no validation studies have been performed in children with NAFLD, excluding the AST/ALT ratio, which did not differentiate hepatic fibrosis from no fibrosis in children[10].
In our study, only APRI and FIB4 exhibited statistically significant differences between patients with mild fibrosis and those with significant fibrosis among these noninvasive scores. Regarding APRI, no studies have been reported in children with NAFLD. However, a study on APRI in children with chronic viral hepatitis revealed that the AUROC of APRI was 0.71 for hepatic fibrosis, and the sensitivity and the specificity of APRI were 47% and 90%, respectively, at the cutoff of 0.5[24]. In addition, another study comparing APRI with FibroScan and FibroTest in children with chronic liver disease reported the AUROC of APRI to be 0.73[25]. In our study on children with NAFLD, the AUROC of APRI was 0.70, which was similar to those of previous studies on children with chronic liver diseases.
FIB4, comprising age in addition to AST, ALT, and platelet counts, also differentiated significant hepatic fibrosis from mild fibrosis in our study, with the highest AUROC of 0.81. In previous studies on FIB4 in adult patients with NAFLD, the AUROCs of FIB4 were 0.802 and 0.86, respectively, which were higher than those of the AST/ALT ratio, APRI, NAFLD fibrosis score, and the BMI, AST/ALT ratio, diabetes score in both studies[26,27]. FIB4 was significant in distinguishing advanced fibrosis (stage 3 to 4) from mild fibrosis (stage 0 to 2) in these 2 studies[26,27]. These results were similar to those of our study, indicating that FIB4 had the highest AUROC among a variety of hepatic fibrosis scores using standard laboratory tests. Thus, FIB4 also appears to be a desirable noninvasive hepatic fibrosis score, even in children, with the application of age-appropriate cutoffs.
Up to date, PNFI was the only noninvasive hepatic fibrosis scoring system developed for children with NAFLD, which revealed the AUROC for significant fibrosis (stage 2 to 3) was 0.663[28], and according to previous studies, PNFI was significant in differentiating children with hepatic fibrosis from children without fibrosis[12,28]. However, in our study, PNFI failed to distinguish significant hepatic fibrosis from no/mild fibrosis in children with the lowest AUROC of 0.41.
Because histopathologic findings of pediatric NASH are to some extent distinct from adult NASH[4,5], the application of noninvasive hepatic fibrosis scores to children with NAFLD should be considered from a different point of view. More validation studies may be required in the future to apply noninvasive hepatic fibrosis scoring systems to clinical fields in pediatric population.
In conclusion, it appears that no single clinical or laboratory parameter can reflect the presence of hepatic fibrosis or the severity of fibrosis in children with NAFLD. Therefore, APRI and FIB4 might be regarded as useful noninvasive methods to evaluate hepatic fibrosis even in children with NAFLD.
Although liver biopsy is the gold standard for the diagnosis of nonalcoholic fatty liver disease (NAFLD), it has some limitations in children because of its invasiveness and high costs, and moreover, the histopathologic findings of pediatric nonalcoholic steatohepatitis (NASH) are somewhat different from adult NASH. Thus, noninvasive methods to predict hepatic fibrosis are required in children suspicious of NAFLD.
The application of noninvasive hepatic fibrosis scores to detect liver fibrosis may be useful in detecting and predicting significant hepatic fibrosis in pediatric NAFLD.
There have been only limited studies of noninvasive markers or hepatic fibrosis scores of hepatic fibrosis related to NAFLD in pediatric populations. This is the first study applying noninvasive clinical or biochemical markers and various noninvasive hepatic fibrosis scores together to the detection of hepatic fibrosis. Our study suggests that AST/platelet ratio index and FIB4, among hepatic fibrosis scores, might be useful to detect hepatic fibrosis even in children with NAFLD.
The study may represent a future diagnostics for NAFLD in pediatric population, suggesting APRI and FIB4 as noninvasive tools to evaluate hepatic fibrosis in children possibly instead of invasive liver biopsy.
NAFLD is a form of chronic liver disease with histopathologic features of alcoholinduced liver disease occurs in persons who do not consume a significant amount of alcohol. Because the main etiology of NAFLD is obesity, the prevalence of NAFLD is increasing in children as the prevalence of pediatric obesity is increasing.
In this study, authors investigate several non-invasive scores for liver fibrosis in children with established and histology-proven NAFLD. This study is of interest, as today only view reports about the diagnostic accuracy of these scores in children are available.
Peer reviewer: Matthias Ocker, Professor, Institute for Surgical Research, Philipps University Marburg, Baldingerstrasse, Marburg 35033, Germany
S- Editor Gou SX L- Editor A E- Editor Zheng XM
| 1. | Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1029] [Cited by in F6Publishing: 1056] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 2. | Molleston JP, White F, Teckman J, Fitzgerald JF. Obese children with steatohepatitis can develop cirrhosis in childhood. Am J Gastroenterol. 2002;97:2460-2462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 267] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 3. | Straub BK, Schirmacher P. Pathology and biopsy assessment of non-alcoholic fatty liver disease. Dig Dis. 2010;28:197-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Schwimmer JB, Behling C, Newbury R, Deutsch R, Nievergelt C, Schork NJ, Lavine JE. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:641-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 537] [Cited by in F6Publishing: 501] [Article Influence: 26.4] [Reference Citation Analysis (1)] |
| 5. | Ko JS, Yoon JM, Yang HR, Myung JK, Kim H, Kang GH, Cheon JE, Seo JK. Clinical and histological features of nonalcoholic fatty liver disease in children. Dig Dis Sci. 2009;54:2225-2230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Guha IN, Parkes J, Roderick PR, Harris S, Rosenberg WM. Non-invasive markers associated with liver fibrosis in non-alcoholic fatty liver disease. Gut. 2006;55:1650-1660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Nobili V, Alkhouri N, Alisi A, Ottino S, Lopez R, Manco M, Feldstein AE. Retinol-binding protein 4: a promising circulating marker of liver damage in pediatric nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:575-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Nobili V, Alisi A, Torre G, De Vito R, Pietrobattista A, Morino G, De Ville De Goyet J, Bedogni G, Pinzani M. Hyaluronic acid predicts hepatic fibrosis in children with nonalcoholic fatty liver disease. Transl Res. 2010;156:229-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Carter-Kent C, Yerian LM, Brunt EM, Angulo P, Kohli R, Ling SC, Xanthakos SA, Whitington PF, Charatcharoenwitthaya P, Yap J. Nonalcoholic steatohepatitis in children: a multicenter clinicopathological study. Hepatology. 2009;50:1113-1120. [PubMed] [Cited in This Article: ] |
| 10. | Iacobellis A, Marcellini M, Andriulli A, Perri F, Leandro G, Devito R, Nobili V. Non invasive evaluation of liver fibrosis in paediatric patients with nonalcoholic steatohepatitis. World J Gastroenterol. 2006;12:7821-7825. [PubMed] [Cited in This Article: ] |
| 11. | Nobili V, Parkes J, Bottazzo G, Marcellini M, Cross R, Newman D, Vizzutti F, Pinzani M, Rosenberg WM. Performance of ELF serum markers in predicting fibrosis stage in pediatric non-alcoholic fatty liver disease. Gastroenterology. 2009;136:160-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 190] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 12. | Nobili V, Alisi A, Vania A, Tiribelli C, Pietrobattista A, Bedogni G. The pediatric NAFLD fibrosis index: a predictor of liver fibrosis in children with non-alcoholic fatty liver disease. BMC Med. 2009;7:21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6807] [Cited by in F6Publishing: 7387] [Article Influence: 388.8] [Reference Citation Analysis (5)] |
| 14. | Strauss S, Gavish E, Gottlieb P, Katsnelson L. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. AJR Am J Roentgenol. 2007;189:W320-W323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 292] [Article Influence: 17.2] [Reference Citation Analysis (1)] |
| 15. | Ricci C, Longo R, Gioulis E, Bosco M, Pollesello P, Masutti F, Crocè LS, Paoletti S, de Bernard B, Tiribelli C. Noninvasive in vivo quantitative assessment of fat content in human liver. J Hepatol. 1997;27:108-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 244] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467-2474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2702] [Cited by in F6Publishing: 2730] [Article Influence: 109.2] [Reference Citation Analysis (0)] |
| 17. | Loaeza-del-Castillo A, Paz-Pineda F, Oviedo-Cárdenas E, Sánchez-Avila F, Vargas-Vorácková F. AST to platelet ratio index (APRI) for the noninvasive evaluation of liver fibrosis. Ann Hepatol. 2008;7:350-357. [PubMed] [Cited in This Article: ] |
| 18. | Teare JP, Sherman D, Greenfield SM, Simpson J, Bray G, Catterall AP, Murray-Lyon IM, Peters TJ, Williams R, Thompson RP. Comparison of serum procollagen III peptide concentrations and PGA index for assessment of hepatic fibrosis. Lancet. 1993;342:895-898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 85] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, Bruguera M, Sánchez-Tapias JM, Rodés J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 672] [Cited by in F6Publishing: 751] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 20. | Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1288] [Cited by in F6Publishing: 1425] [Article Influence: 83.8] [Reference Citation Analysis (0)] |
| 21. | Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1917] [Cited by in F6Publishing: 2018] [Article Influence: 118.7] [Reference Citation Analysis (1)] |
| 22. | Poynard T, Morra R, Ingiliz P, Imbert-Bismut F, Thabut D, Messous D, Munteanu M, Massard J, Benhamou Y, Ratziu V. Biomarkers of liver fibrosis. Adv Clin Chem. 2008;46:131-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Adams LA. Biomarkers of liver fibrosis. J Gastroenterol Hepatol. 2011;26:802-809. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | McGoogan KE, Smith PB, Choi SS, Berman W, Jhaveri R. Performance of the AST-to-platelet ratio index as a noninvasive marker of fibrosis in pediatric patients with chronic viral hepatitis. J Pediatr Gastroenterol Nutr. 2010;50:344-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | de Lédinghen V, Le Bail B, Rebouissoux L, Fournier C, Foucher J, Miette V, Castéra L, Sandrin L, Merrouche W, Lavrand F. Liver stiffness measurement in children using FibroScan: feasibility study and comparison with Fibrotest, aspartate transaminase to platelets ratio index, and liver biopsy. J Pediatr Gastroenterol Nutr. 2007;45:443-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 26. | Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104-1112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 864] [Cited by in F6Publishing: 947] [Article Influence: 63.1] [Reference Citation Analysis (1)] |
| 27. | McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59:1265-1269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 572] [Cited by in F6Publishing: 587] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 28. | Alkhouri N, Carter-Kent C, Lopez R, Rosenberg WM, Pinzani M, Bedogni G, Feldstein AE, Nobili V. A combination of the pediatric NAFLD fibrosis index and enhanced liver fibrosis test identifies children with fibrosis. Clin Gastroenterol Hepatol. 2011;9:150-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |