Published online Apr 7, 2012. doi: 10.3748/wjg.v18.i13.1479
Revised: February 9, 2012
Accepted: February 16, 2012
Published online: April 7, 2012
AIM: To investigate the effects of curcumin on gastric microcirculation and inflammation in rats with indomethacin-induced gastric damage.
METHODS: Male Sprague-Dawley rats were randomly divided into three groups. Group 1 (control group, n = 5) was fed with olive oil and 5% NaHCO3- (vehicle). Group 2 [indomethacin (IMN) group, n = 5] was fed with olive oil 30 min prior to indomethacin 150 mg/kg body weight (BW) dissolved in 5% NaHCO3- at time 0th and 4th h. Group 3 (IMN + Cur group, n = 4) was fed with curcumin 200 mg/kg BW dissolved in olive oil 0.5 mL, 30 min prior to indomethacin at 0th and 4th h. Leukocyte-endothelium interactions at postcapillary venules were recorded after acridine orange injection. Blood samples were determined for intercellular adhesion molecule (ICAM)-1 and tumor necrosis factor (TNF)-α levels using enzyme linked immunosorbent assay method. Finally, the stomach was removed for histopathological examination for gastric lesions and grading for neutrophil infiltration.
RESULTS: In group 2, the leukocyte adherence in postcapillary venules was significantly increased compared to the control group (6.40 ± 2.30 cells/frame vs 1.20 ± 0.83 cells/frame, P = 0.001). Pretreatment with curcumin caused leukocyte adherence to postcapillary venule to decline (3.00 ± 0.81 cells/frame vs 6.40 ± 2.30 cells/frame, P = 0.027). The levels of ICAM-1 and TNF-α increased significantly in the indomethacin-treated group compared with the control group (1106.50 ± 504.22 pg/mL vs 336.93 ± 224.82 pg/mL, P = 0.011 and 230.92 ± 114.47 pg/mL vs 47.13 ± 65.59 pg/mL, P = 0.009 respectively). Pretreatment with curcumin significantly decreased the elevation of ICAM-1 and TNF-α levels compared to treatment with indomethacin alone (413.66 ± 147.74 pg/mL vs 1106.50 ± 504.22 pg/mL, P = 0.019 and 58.27 ± 67.74 pg/mL vs 230.92 ± 114.47 pg/mL, P = 0.013 respectively). The histological appearance of the stomach in the control group was normal. In the indomethacin-treated group, the stomachs showed a mild to moderate neutrophil infiltration score. Gastric lesions were erosive and ulcerative. In rats treated with indomethacin and curcumin, stomach histopathology improved and showed only a mild neutrophil infiltration score and fewer erosive lesions in the gastric mucosa.
CONCLUSION: The results indicate that curcumin prevents indomethacin-induced gastropathy through the improvement of gastric microcirculation by attenuating the level of ICAM-1 and TNF-α.
- Citation: Thong-Ngam D, Choochuai S, Patumraj S, Chayanupatkul M, Klaikeaw N. Curcumin prevents indomethacin-induced gastropathy in rats. World J Gastroenterol 2012; 18(13): 1479-1484
- URL: https://www.wjgnet.com/1007-9327/full/v18/i13/1479.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i13.1479
Nonsteroidal anti-inflammatory drugs (NSAIDs) are among the most commonly prescribed medications worldwide. However, NSAIDs have adverse effects on the gastric mucosa, resulting in various clinical presentations, ranging from nonspecific dyspepsia to ulceration, upper gastrointestinal bleeding, and death, summarized by the term “NSAID gastropathy”[1]. NSAIDs-induced gastric damage is the major side effect of this kind of drug[2].
The main action of NSAIDs is to inhibit prostaglandin synthesis. There is substantial evidence supporting the view that the ulcerogenic effect of this medication correlates with its ability to suppress prostaglandin synthesis[3-5]. Endogenous prostaglandins normally regulate mucosal blood flow, epithelial cell proliferation, epithelial restitution, mucosal immunocyte function, mucus and bicarbonate secretion, and basal acid secretion[6]. Therefore, decreases in prostaglandins, protective factors for ulcer formation, lead to gastric mucosal injury.
Animal studies have shown that neutrophil adherence to the endothelium of the gastric microcirculation is critical in NSAIDs injury[7]. Neutrophil adherence damages the mucosa by producing oxygen-free radicals, releasing proteases, and obstructing capillary blood flow. NSAIDs might induce the synthesis of tumor necrosis factor (TNF)-α and leukotrienes[8,9]. These inflammatory mediators subsequently stimulate neutrophil adherence by the upregulation of adhesion molecules[10].
NSAID administration in rats caused a rapid and significant increase in adhesion between neutrophils and vascular endothelial cells in both gastric and mesenteric venules[11-13]. This was dependent on intercellular adhesion molecule (ICAM)-1 expression on the endothelium and CD11/CD18 expression on the leukocyte[14,15]. Interestingly, Andrews et al[10] recently reported that administration of aspirin or indomethacin to rats resulted in a significant increase in ICAM-1 expression in the gastric microcirculation.
Curcuma, a genus in the plant family of Zingiberacea, is the biological source for curcuminoids, including curcumin. Curcuma longa, the yellow tuberous root referred to as turmeric, was taken from India to Southeast Asia[16]. The yellow pigmented fraction of Curcuma longa contains curcuminoids, which are chemically related to its principal ingredient, curcumin[16]. It possesses a broad range of pharmacological activities, including antioxidant, anti-carcinogenic, wound-healing, and anti-inflammatory effects[17-19]. There are currently limited studies investigating the effect of curcumin on NSAIDs-induced gastric damage. The aim of this study was to investigate the anti-inflammation effect of curcumin on indomethacin-induced gastric damage in rats.
Male Sprague-Dawley rats weighing 180-220 g, purchased from the National Laboratory Animal Center, Mahidol University, Salaya (n = 18), Nakorn pathom, were used in this study. All rats were kept in a controlled temperature room at 25 ± 1 °C under standard conditions (12 h day-night rhythm). They were cared for in accordance with the Ethical Committee, Faculty of Medicine, Chulalongkorn University, Thailand. Curcumin powder (Cayman Chemical Company, United States) was suspended in olive oil.
All rats were fasted, with free access to water ad libitum, for 22-24 h before the experiment. They were randomly divided into three experimental groups. Group 1 (control, n = 6): Rats were fed with olive oil 30 min prior to 5% sodium bicarbonate 1 mL orally via an intragastric tube at time 0th and 4th h. Group 2 [indomethacin (IMN), n = 6]: Rats were fed with olive oil 30 min prior to indomethacin (150 mg/kg body weight in 5% sodium bicarbonate 1 mL orally via an intragastric tube) at time 0th and 4th h. Group 3 (IMN + Cur, n = 6): Rats were fed with curcumin (200 mg/kg body weight dissolved in olive oil 0.5 mL) 30 min prior to indomethacin [150 mg/kg body weight (BW) dissolved in 5% sodium bicarbonate 1 mL orally via an intragastric tube] at time 0th and 4th h.
After 8 h 30 min, animals were anesthetized with intraperitoneal injection of thiopental (50 mg/kg body weight). After tracheostomy, the carotid artery and jugular vein were cannulated for blood pressure measurement using a polygraph and for the administration of a fluorescent marker; acridine orange was infused intravenously (Sigma chemical Co., United States, 0.5 mg/kg BW/min). The abdominal wall was incised and the stomach was extended and fixed. Leukocyte adherence in the stomach was observed by intravital fluorescence microscopy. At the end of the experiment, blood samples were collected for ICAM-1 and TNF-α determination using enzyme linked immunosorbent assay (ELISA) methods. The stomach was cut and fixed in 10% formalin solution to inspect the histopathology.
It has been stated that NSAIDs-induced leukocyte adherence could contribute to the pathogenesis of gastric mucosal injury. To visualize leukocytes, acridine orange was infused intravenously (0.3 mg/kg body weight). The number of leukocyte adhesions was recorded using a video recorder. Videotape of each experiment was replayed and leukocyte adherence was monitored. Most leukocytes were adhered to the postcapillary venule (about 15-30 μm in diameter). Leukocytes were considered adherent to the vessel endothelium if they remained stationary for 30 s or longer. Adherent leukocytes were expressed as the number of leukocyte adherences per frame of view, as previously described[20].
After the experiment, blood samples were taken by cardiac puncture, and allowed to clot overnight before centrifuging at approximately 2000 ×g. Serum was stored at -80 °C for determining ICAM-1 and TNF-α levels by ELISA kit (R and D systems).
Samples of the stomach were excised and transferred to formalin. The samples were subsequently processed by routine techniques before embedding in paraffin. Sections were cut at the thickness of 5 μm and stained with hematoxylin and eosin (HE), as previously described[20]. One pathologist performed all the histopathological examinations. All histopathological changes were observed under a light microscope. The neutrophil infiltration score in each section was graded according previously determined criteria[21].
All data were presented as mean ± SD. To compare data among all groups of animals, one-way analysis of variance (one-way ANOVA) and Duncan comparisons were employed. All statistical tests were performed using SPSS for Windows version 13.0 (SPSS Inc., Chicago, IL, United States). Differences were considered statistically significant at P < 0.05.
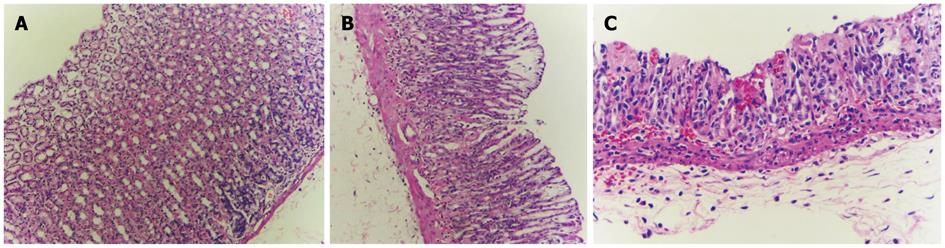
The histological appearance of the stomach in the control group (Figure 1) was normal. In the indomethacin treated group, the stomachs showed mild to moderate gastric mucosal injury. Gastric lesions were erosive and ulcerative. In rats treated with indomethacin and curcumin, the stomach histopathology improved and showed only mild gastric mucosal injury and reduced amounts of erosive lesions in the gastric mucosa. The summary of infiltration of inflammatory cells and gastric lesions are shown in Table 1.
| Experimental group | Neutrophil infiltration1 | Pathology | |||||
| 0 | 1 | 2 | 3 | No erosion | Erosion | Ulcer | |
| Control | 6 | - | - | - | 6 | - | - |
| IMN | - | 3 | 3 | - | - | 2 | 4 |
| IMN + Cur | 4 | 2 | - | - | 4 | 2 | - |
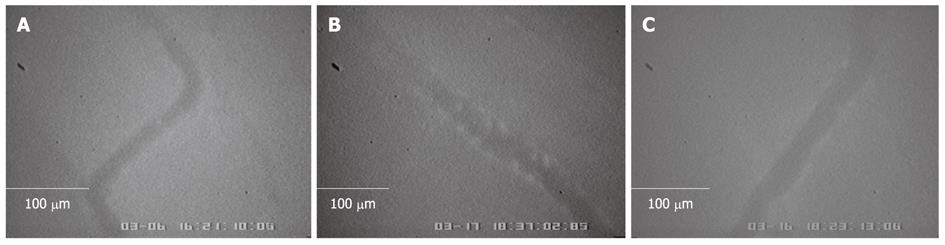
After gastric injury was induced by the administration of indomethacin, leukocyte adherence to endothelial cells of postcapillary venules (15-30 μm in diameter) was observed under intravital fluorescence microscopy, 10-15 min after acridine orange injection (Figure 2). The number of leukocytes adhering to postcapillary venules for 30 s or longer was counted. The mean number of leukocyte adherences in the IMN group without curcumin treatment was significantly higher than in the control group (6.40 ± 2.30 cells/frame vs 1.20 ± 0.83 cells/frame, P = 0.001).
The number of leukocyte adherences significantly decreased after pretreatment with curcumin as compared to the IMN group (3.00 ± 0.81 cells/frame vs 6.40 ± 2.30 cells/frame, P = 0.027).
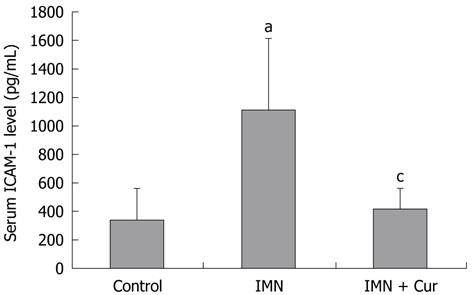
The levels of ICAM-1 increased significantly in the indomethacin treated group compared with the control group (1106.50 ± 504.22 pg/mL vs 336.93 ± 224.82 pg/mL, P = 0.011). Pretreatment with curcumin markedly decreased the elevation of the ICAM-1 level compared with the indomethacin treated group (413.66 ± 147.74 pg/mL vs 1106.50 ± 504.22 pg/mL, P = 0.019) (Figure 3).
Changes in TNF-αlevels
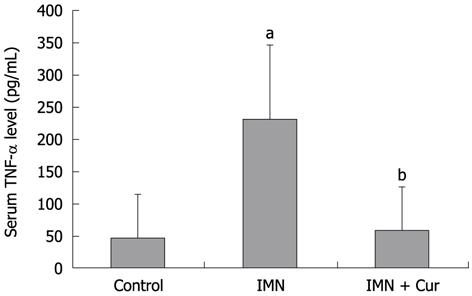
The level of TNF-α markedly increased in the indomethacin treated group compared with the control group (230.92 ± 114.47 pg/mL vs 47.13 ± 65.59 pg/mL, P = 0.009). Pretreatment with curcumin decreased the elevation of TNF-α levels compared with the indomethacin treated group (58.27 ± 67.74 pg/mL vs 230.92 ± 114.47 pg/mL, P = 0.013) (Figure 4).
In the present study, we investigated the effects of curcumin on indomethacin-induced gastric damage in rats. The results clearly demonstrated that curcumin administration prevented the ulcerogenic effect of indomethacin, possibly through its anti-inflammatory action. Evidence suggests that NSAIDs-induced gastric ulceration is a neutrophil-dependent process. NSAIDs administration to rats caused a rapid and significant increase in adhesion between neutrophils and vascular endothelial cells in both the gastric and mesenteric venules[11,12]. Indeed, monoclonal antibodies that blocked NSAID-induced neutrophil adherence to vascular endothelium could significantly alleviate NSAID- induced gastric mucosal injury[7,15,22]. Neutrophils play an important role in the development of inflammation and tissue injury by releasing a variety of inflammatory mediators[23,24]. These inflammatory mediators are capable of producing tissue injury; therefore, they may be involved in the pathogenesis of indomethacin-induced gastric mucosal injury[25]. Furthermore, adhesion molecules expressed on activated neutrophils, such as CD11b and CD18, have been shown to play an important role in neutrophil-induced tissue injury[14].
Moreover, NSAIDS are believed to have the effect on nuclear translocation of nuclear factor (NF)-κB, which modulates the expression of several adhesions molecules, including ICAM-1[26]. ICAM-1, one of the major adhesion molecules, plays a pivotal role in the inflammatory reaction by increasing leukocyte adhesion to endothelium and promoting transendothelial migration of leukocytes to inflammatory sites[27]. Another important mechanism that induces ICAM-1 expression is the increment of TNF-α levels[27,28]. The inhibitory effect of NSAIDs on COX-2 leads reduced prostaglandin E2 (PGE2) levels. Thus, TNF-α production, which is normally inhibited by PGE2, increases[28]. TNF-α is an important mediator causing NSAIDs induced gastropathy. Apart from its effect on adhesion molecules, TNF-α may have the ability to activate pro-apoptosis caspases, which regulate gastric epithelial cells apoptosis in NSAID-treated rats[27].
A previous study demonstrated that indomethacin administration caused significantly elevated TNF-α levels in rats[8]. Moreover, pretreatment with anti- TNF-α, dexamethasone, and PGE2 could prevent the increases in gastric mucosal injuries and TNF-α levels. Similarly, we found significant rises in TNF-α and ICAM-1 levels in the serum of the indomethacin-treated group compared to the control. Other formulations of NSAIDs could also increase TNF- α production. A study by Jainu et al[29] noted a significant increase in TNF-α, IL-1β and NOS-2 activity in aspirin-administered rats. The elevations of inflammatory mediators and adhesion molecules in serum correlated with the pathological findings of gastric mucosa and the numbers of adhered leukocytes in the gastric microcirculation. These were also true for curcumin-treated group. Pretreatment with curcumin could significantly reduce TNF-α and ICAM-1 levels in the serum, accompanied by an improvement in gastric mucosal inflammation and leukocyte adhesion.
Curcumin, a substance rich in phenolics, is known to possess antioxidant properties. Curcumin reduces gastric injury induced by NSAIDs[30]. It has been reported that curcumin can decrease gastric injury by preventing the peroxidase inactivation effect of indomethacin and scavenging reactive oxygen produced by this enzyme[30]. Several studies showed that curcumin is also an anti-inflammatory substance, with an inhibitory effect on transcription factor NF-κB activation. NF-κB is required for the expression of many genes linked with the host immune response, such as ICAM-1, TNF-α, IL-1β, and iNOS[31]. Cytoplasmic NF-κB is complexed with its inhibitor IkB and is, therefore, inactive. The cytokine-mediated activation of NF-κB requires activation of various kinases, which ultimately leads to the phosphorylation and degradation of IkB. Several beneficial effects of curcumin are consistent with its ability to inhibit the activity of NF-κB[32-34]. Singh et al[31] observed that curcumin inhibited NF-κB activation pathway after the convergence of various stimuli mediated by protein tyrosine kinase, protein kinase, and ubiquitin conjugation enzymes, but before the phosphorylation and subsequent release of IkB complexed to NF-κB. Jobin et al[35] and Plummer et al[36] examined the modulatory potential of curcumin on NF-κB signaling pathways and found that curcumin prevented phosphorylation of IkB by inhibiting the activation of IkB-kinase (IKKs). Our previous study demonstrated that Helicobacter pylori-induced gastric inflammation in rats is associated with increased NF-κB activation and macromolecular leakage, which can be reduced by curcumin[37]. In this study, 200 mg/kg curcumin was a sufficient dose for reducing gastric epithelial NF-κB p65 expression and mucosal macromolecular leakage. Despite its inconclusive mechanism of action, we clearly demonstrated that curcumin has a protective and beneficial effect on NSAIDs-induced gastropathy in rats. Further studies on the expression of inflammatory mediators and adhesion molecules in the gastric mucosa are necessary to demonstrate the exact curative effect of curcumin on NSAID-induced gastric pathology. Clinical studies might also be needed to verify the protective effect of curcumin in humans.
In conclusion, NSAIDs could induce gastric injury through increases in inflammatory cytokines and leukocyte adhesions. Curcumin, an anti-oxidant herbal substance, could prevent these adverse events and might be used as a preventive method for NSAIDs-induced gastropathy.
Nonsteroidal anti-inflammatory drugs (NSAIDs)-induced gastric damage is the major side effect of this kind of medication. Although the underlying pathogenesis of NSAIDs-induced gastric damage is unclear, neutrophils are believed to play an important role in the development of gastric inflammation and injury following NSAID administration. Curcumin possesses several biological activities, including an anti-inflammatory effect. Authors postulated that curcumin, acting through nuclear factor (NF)-κB inhibition, could reduce the production of adhesion molecules and inflammatory cytokines, resulting in the amelioration of gastric injury in NSAIDs-induced gastropathy in rats.
Curcumin (diferuloylmethane) is an active ingredient of Curcuma longa (turmeric), which exerts many biological activities by the inhibition of NF-κB-mediated reactions. NSAIDs can cause gastric mucosal damage through an increase in leukocyte-endothelial adhesions and the release of inflammatory mediators, leading to the free radical production. This study demonstrated an improvement in gastric mucosal damage and decreases in leukocyte adhesions, and intercellular adhesion molecule 1 and tumor necrosis factor (TNF)-α production after curcumin administration in the indomethacin-treated group.
The previous study showed that curcumin is an anti-inflammatory agent and can inhibit NF-κB activation in an in vitro study. However, it is not known whether curcumin’s anti-inflammatory effects will help prevent NSAIDs-induced gastropathy in vivo. In this study, authors investigated the protective effect of curcumin in indomethacin-induced gastric damage in rats. Authors found that curcumin could alleviate indomethacin-induced gastric injury via a decrease in leukocyte adhesions and TNF-α production.
Curcumin might be used as a new protective agent for NSAIDs-induced gastric damage in clinical use.
NSAIDs gastropathy: NSAIDs are well-known for their adverse effects on gastric mucosa, resulting in various clinical presentations, ranging from nonspecific dyspepsia to ulceration, upper gastrointestinal bleeding and death, summarized by the term “NSAID gastropathy“.
This is an interesting study of the effects of curcumin on indomethacin-induced gastric damage in rats. The results clearly demonstrated that curcumin administration prevented the ulcerogenic effect of indomethacin, possibly through its anti-inflammatory action.
Peer reviewers: Sang Kil Lee, Professor, Yonsei University College of Medicine, 250 Sung-san-Ro Seodaemun-gu, Seoul 120-752, South Korea; Vittorio Ricci, Professor, Physiology, Human Physiology Section, University of Pavia Medical School, Via Forlanini 6 Human Physiology Section, 27100 Pavia, Italy
S- Editor Gou SX L- Editor Stewart GJ E- Editor Zheng XM
| 1. | Becker JC, Domschke W, Pohle T. Current approaches to prevent NSAID-induced gastropathy--COX selectivity and beyond. Br J Clin Pharmacol. 2004;58:587-600. [PubMed] [Cited in This Article: ] |
| 2. | Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med. 1999;340:1888-1899. [PubMed] [Cited in This Article: ] |
| 3. | Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232-235. [PubMed] [Cited in This Article: ] |
| 4. | Whittle BJ. Temporal relationship between cyclooxygenase inhibition, as measured by prostacyclin biosynthesis, and the gastrointestinal damage induced by indomethacin in the rat. Gastroenterology. 1981;80:94-98. [PubMed] [Cited in This Article: ] |
| 5. | Rainsford KD, Willis C. Relationship of gastric mucosal damage induced in pigs by antiinflammatory drugs to their effects on prostaglandin production. Dig Dis Sci. 1982;27:624-635. [PubMed] [Cited in This Article: ] |
| 6. | Wallace JL. Nonsteroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology. 1997;112:1000-1016. [PubMed] [Cited in This Article: ] |
| 7. | Wallace JL, Keenan CM, Granger DN. Gastric ulceration induced by nonsteroidal anti-inflammatory drugs is a neutrophil-dependent process. Am J Physiol. 1990;259:G462-G467. [PubMed] [Cited in This Article: ] |
| 8. | Appleyard CB, McCafferty DM, Tigley AW, Swain MG, Wallace JL. Tumor necrosis factor mediation of NSAID-induced gastric damage: role of leukocyte adherence. Am J Physiol. 1996;270:G42-G48. [PubMed] [Cited in This Article: ] |
| 9. | Rainsford KD. Microvascular injury during gastric mucosal damage by anti-inflammatory drugs in pigs and rats. Agents Actions. 1983;13:457-460. [PubMed] [Cited in This Article: ] |
| 10. | Andrews FJ, Malcontenti-Wilson C, O'Brien PE. Effect of nonsteroidal anti-inflammatory drugs on LFA-1 and ICAM-1 expression in gastric mucosa. Am J Physiol. 1994;266:G657-G664. [PubMed] [Cited in This Article: ] |
| 11. | Wallace JL, McKnight W, Miyasaka M, Tamatani T, Paulson J, Anderson DC, Granger DN, Kubes P. Role of endothelial adhesion molecules in NSAID-induced gastric mucosal injury. Am J Physiol. 1993;265:G993-G998. [PubMed] [Cited in This Article: ] |
| 12. | Asako H, Kubes P, Wallace J, Gaginella T, Wolf RE, Granger DN. Indomethacin-induced leukocyte adhesion in mesenteric venules: role of lipoxygenase products. Am J Physiol. 1992;262:G903-G908. [PubMed] [Cited in This Article: ] |
| 13. | Asako H, Kubes P, Wallace J, Wolf RE, Granger DN. Modulation of leukocyte adhesion in rat mesenteric venules by aspirin and salicylate. Gastroenterology. 1992;103:146-152. [PubMed] [Cited in This Article: ] |
| 14. | Wallace JL, Arfors KE, McKnight GW. A monoclonal antibody against the CD18 leukocyte adhesion molecule prevents indomethacin-induced gastric damage in the rabbit. Gastroenterology. 1991;100:878-883. [PubMed] [Cited in This Article: ] |
| 15. | Wallace JL, McCafferty DM, Carter L, McKnight W, Argentieri D. Tissue-selective inhibition of prostaglandin synthesis in rat by tepoxalin: anti-inflammatory without gastropathy? Gastroenterology. 1993;105:1630-1636. [PubMed] [Cited in This Article: ] |
| 16. | Joe B, Vijaykumar M, Lokesh BR. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit Rev Food Sci Nutr. 2004;44:97-111. [PubMed] [Cited in This Article: ] |
| 17. | Toda S, Miyase T, Arichi H, Tanizawa H, Takino Y. Natural antioxidants. III. Antioxidative components isolated from rhizome of Curcuma longa L. Chem Pharm Bull (Tokyo). 1985;33:1725-1728. [PubMed] [Cited in This Article: ] |
| 18. | Srimal RC, Dhawan BN. Pharmacology of diferuloyl methane (curcumin), a non-steroidal anti-inflammatory agent. J Pharm Pharmacol. 1973;25:447-452. [PubMed] [Cited in This Article: ] |
| 19. | Satoskar RR, Shah SJ, Shenoy SG. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int J Clin Pharmacol Ther Toxicol. 1986;24:651-654. [PubMed] [Cited in This Article: ] |
| 20. | Eamlamnam K, Patumraj S, Visedopas N, Thong-Ngam D. Effects of Aloe vera and sucralfate on gastric microcirculatory changes, cytokine levels and gastric ulcer healing in rats. World J Gastroenterol. 2006;12:2034-2039. [PubMed] [Cited in This Article: ] |
| 21. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [PubMed] [Cited in This Article: ] |
| 22. | Alican I, Coşkun T, Corak A, Yeğen BC, Oktay S, Kurtel H. Role of neutrophils in indomethacin-induced gastric mucosal lesions in rats. Inflamm Res. 1995;44:164-168. [PubMed] [Cited in This Article: ] |
| 23. | Campbell EJ, Senior RM, McDonald JA, Cox DL. Proteolysis by neutrophils. Relative importance of cell-substrate contact and oxidative inactivation of proteinase inhibitors in vitro. J Clin Invest. 1982;70:845-852. [PubMed] [Cited in This Article: ] |
| 24. | Weiss SJ, LoBuglio AF. An oxygen-dependent mechanism of neutrophil-mediated cytotoxicity. Blood. 1980;55:1020-1024. [PubMed] [Cited in This Article: ] |
| 25. | Zimmerman BJ, Granger DN. Reperfusion-induced leukocyte infiltration: role of elastase. Am J Physiol. 1990;259:H390-H394. [PubMed] [Cited in This Article: ] |
| 26. | Lazzaroni M, Bianchi Porro G. Gastrointestinal side-effects of traditional non-steroidal anti-inflammatory drugs and new formulations. Aliment Pharmacol Ther. 2004;20 Suppl 2:48-58. [PubMed] [Cited in This Article: ] |
| 27. | Konturek PC, Duda A, Brzozowski T, Konturek SJ, Kwiecien S, Drozdowicz D, Pajdo R, Meixner H, Hahn EG. Activation of genes for superoxide dismutase, interleukin-1beta, tumor necrosis factor-alpha, and intercellular adhesion molecule-1 during healing of ischemia-reperfusion-induced gastric injury. Scand J Gastroenterol. 2000;35:452-463. [PubMed] [Cited in This Article: ] |
| 28. | Kast RE. Tumor necrosis factor has positive and negative self regulatory feed back cycles centered around cAMP. Int J Immunopharmacol. 2000;22:1001-1006. [PubMed] [Cited in This Article: ] |
| 29. | Jainu M, Devi CS. Gastroprotective action of Cissus quadrangularis extract against NSAID induced gastric ulcer: role of proinflammatory cytokines and oxidative damage. Chem Biol Interact. 2006;161:262-270. [PubMed] [Cited in This Article: ] |
| 30. | Chattopadhyay I, Bandyopadhyay U, Biswas K, Maity P, Banerjee RK. Indomethacin inactivates gastric peroxidase to induce reactive-oxygen-mediated gastric mucosal injury and curcumin protects it by preventing peroxidase inactivation and scavenging reactive oxygen. Free Radic Biol Med. 2006;40:1397-1408. [PubMed] [Cited in This Article: ] |
| 31. | Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected]. J Biol Chem. 1995;270:24995-25000. [PubMed] [Cited in This Article: ] |
| 32. | Bierhaus A, Zhang Y, Quehenberger P, Luther T, Haase M, Müller M, Mackman N, Ziegler R, Nawroth PP. The dietary pigment curcumin reduces endothelial tissue factor gene expression by inhibiting binding of AP-1 to the DNA and activation of NF-kappa B. Thromb Haemost. 1997;77:772-782. [PubMed] [Cited in This Article: ] |
| 33. | Kuner P, Schubenel R, Hertel C. Beta-amyloid binds to p57NTR and activates NFkappaB in human neuroblastoma cells. J Neurosci Res. 1998;54:798-804. [PubMed] [Cited in This Article: ] |
| 34. | Pendurthi UR, Williams JT, Rao LV. Inhibition of tissue factor gene activation in cultured endothelial cells by curcumin. Suppression of activation of transcription factors Egr-1, AP-1, and NF-kappa B. Arterioscler Thromb Vasc Biol. 1997;17:3406-3413. [PubMed] [Cited in This Article: ] |
| 35. | Jobin C, Bradham CA, Russo MP, Juma B, Narula AS, Brenner DA, Sartor RB. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J Immunol. 1999;163:3474-3483. [PubMed] [Cited in This Article: ] |
| 36. | Plummer SM, Holloway KA, Manson MM, Munks RJ, Kaptein A, Farrow S, Howells L. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013-6020. [PubMed] [Cited in This Article: ] |
| 37. | Sintara K, Thong-Ngam D, Patumraj S, Klaikeaw N, Chatsuwan T. Curcumin suppresses gastric NF-kappaB activation and macromolecular leakage in Helicobacter pylori-infected rats. World J Gastroenterol. 2010;16:4039-4046. [PubMed] [Cited in This Article: ] |