Published online Mar 14, 2012. doi: 10.3748/wjg.v18.i10.1110
Revised: December 7, 2011
Accepted: January 22, 2012
Published online: March 14, 2012
AIM: To compare the clinical characteristics of pyogenic liver abscess (PLA) in patients with and without hepatic neoplasm (HN).
METHODS: Authors performed a retrospective analysis involving patients with PLA. The demographic, clinical features, laboratory and imaging findings, management and outcome of patients with and without HN were studied.
RESULTS: From January 2000 to December 2009 inclusive, 318 patients (35 with HN) had PLA, and mean age and comorbidity were comparable between the two groups. More patients with HN experienced right upper quadrant pain (68.6% vs 52.7%, P < 0.04), developed jaundice (14.3% vs 5.7%, P < 0.03) and hepatomegaly (17.1% vs 3.9%, P < 0.01), and had higher serum total bilirubin level (43.3 μmol/L vs 30.0 μmol/L, P = 0.05). Most patients in both groups had PLAs in the right hepatic lobe, and biliary tract disorder was the most common underlying cause (71.4% and 61.8%). However, more PLAs in the HN group were associated with thicker abscess wall (37.1% vs 19.4%, P < 0.01), septal lobulation (77.1% vs 58%, P < 0.02), gaseous cavitation (17% vs 7.8%, P = 0.03), portal thrombophlebitis (11.4% vs 1.8%, P < 0.01) and aerobilia (25.9% vs 5.5%, P < 0.01). Mixed bacterial growth (40% vs 15.2%, P < 0.01) and Gram-negative bacilli (22.8% vs 60.4%, P < 0.01) were dominant isolates in PLAs with and without HN, respectively. Although incidence of the complications was comparable between the two groups, patients with HN had a higher mortality rate than those without (71.4% vs 8.8%, P < 0.01). Multivariate logistic regression analysis revealed underlying active malignancy [odds ratio (OR): 40.45, 95% CI: 14.76-111.65], hypoalbuminemia (OR: 1.22, 95% CI: 1.14-1.38), disseminated intravascular coagulation (OR: 3.32, 95% CI: 1.19-9.69) and acute coronary syndrome (OR: 4.48, 95% CI: 1.08-17.8) were independent risk factors associated with mortality. However, several HN cases, presented concurrently with PLAs, were found to have curative resectable tumors and had good prognosis after surgery.
CONCLUSION: PLA associated with HN tends to form a distinct clinical syndrome with a different extent of clinical manifestations, radiological and microbiological features and complications.
- Citation: Law ST, Li KK. Is hepatic neoplasm-related pyogenic liver abscess a distinct clinical entity? World J Gastroenterol 2012; 18(10): 1110-1116
- URL: https://www.wjgnet.com/1007-9327/full/v18/i10/1110.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i10.1110
Pyogenic liver abscess (PLA) has been recognized since the time of Hippocrates[1-4]. It is the most common type of visceral abscess; in a report of 540 cases of intra-abdominal abscesses, PLA accounted for 48% of visceral abscesses. This condition is potentially life threatening, with a mortality rate ranging from 10% to 40%[5,6]. The previous reports have indicated that hepatic malignancy is the major risk and poor prognostic factor for PLA[7,8]. Clinically, it is mandatory for clinicians to rule in/out any coexisting tumor component in the PLA, so that appropriate neoadjuvant therapy or even surgical resection if localized can be offered promptly after treatment of the infection. However, to the best of our knowledge, the issue of the prediction of the presence of hepatic malignancy in PLA has never been investigated.
Thus, the aim of this study was to review the characteristics of patients with and without hepatic neoplasm (HN)-associated PLA in the following areas: risk factors, clinical features, characteristics features of liver abscess, treatment and outcome.
The records of patients discharged from Tuen Mun Hospital in Hong Kong with a diagnosis of PLA (International Classification of Diseases code 572.0) between January 2000 and December 2009 were reviewed. Cases were identified through searching the hospital database (Clinical Management System of Hospital Authority of Hong Kong). Underestimation of actual caseload is possible because coding might not have included patients suffering from an underlying disease.
The case definition of PLA required patients to have one or more filling defects on liver imaging [either ultrasound or computed tomography (CT)], together with either (1) complete resolution of radiological abnormalities following antimicrobial therapy with/without positive blood/pus culture, or (2) histological proof of microbiological infection without underlying neoplasm.
The diagnosis of hepatic tumor abscess was based on the presence of hepatic tumor of primary or secondary origin plus one or both of the following: (1) positive pus culture; and (2) partial resolution of radiological abnormalities following antimicrobial therapy.
The clinical records of these patients were retrospectively reviewed to obtain the demographic characteristics, clinical features, laboratory, imaging findings, treatment methods and final outcomes. Recurrence was defined as the development of new clinical and radiological changes subsequent to clinical and/or radiological resolution.
The data were compiled and analyzed using SPSS for Windows version 17.0 (SPSS Inc., Chicago, IL, United States). All continuous variables were expressed as mean ± SD. Categorical variables were reported as percentages. Student’s t test, χ2 test, Fisher’s exact test and Mann-Whitney U test were used when appropriate. P≤ 0.05 was considered statistically significant.
From January 2000 to December 2009 inclusive, a total of 318 patients were diagnosed with PLA, 35 (11%) of which were associated with HN. There were 29 cases of hepatopancreatobiliary (HPB) malignant disease and causes identified for the other patients were as follows: colonic cancers in three patients, and hepatocellular, gastric and breast cancer in one patient each. Among 29 cases of HPB neoplasms, there were 20 cases of cholangiocarcinoma (13 intrahepatic and seven extrahepatic cholangiocarcinoma), six cases of gallbladder cancer, and three of pancreatic cancer. In the HN group, 10 patients had neoplasms that presented concomitantly with the development of PLA, and they included four gallbladder cancers, two intrahepatic cholangiocarcinomas, two cancers of the head of the pancreas, and one each of breast and cecal cancer. Although they presented concurrently, most of them were found to be widely metastatic by imaging, and unable to undergo curative resection. Only four cases had localized tumors on presentation: two cases each of intrahepatic cholangiocarcinoma and cancer of the head of the pancreas. They remained stable after the resection of the tumors. For the patients whose HNs presented prior to PLAs, the median period of time was 5 mo (range: 2-17 mo).
The demographic characteristics of these patients are shown in Table 1. There was no sex dominance in the HN group (male to female ratio: 1.06). The mean age was 67.7 years (range: 24-91 years; median: 72 years) in the HN group and 65 years (range: 24-97 years; median: 65 years) in the non-HN group (data not shown). There was no significant difference in age and sex between the two groups (P = 0.40). Major comorbidities of the two groups were similar and included hypertension, ischemic heart disease, diabetes mellitus and stroke.
| Variable | HN group(n = 35) | Non-HN group(n = 283) | P value |
| Age (yr) | 67.7 ± 16.2 | 65.4 ± 15.13 | 0.40 |
| Sex (male: female) | 18:17 | 163:120 | |
| Comorbidity | |||
| Diabetes mellitus | 9 (25.7) | 81 (28.6) | 0.36 |
| Hypertension | 12 (34.3) | 78 (27.6) | 0.20 |
| Ischemic heart disease | 1 (2.9) | 23 (8.1) | 0.14 |
| Stroke | 2 (5.7) | 31 (11.0) | 0.17 |
| Duration of symptoms before presentation (d) | 3.7 ± 2.8 | 4.9 ± 4.9 | 0.18 |
| Symptoms and signs | |||
| Fever and chill | 34 (97.1) | 268 (94.7) | 0.27 |
| Right upper quadrant pain | 24 (68.6) | 149 (52.7) | < 0.04 |
| Diarrhea | 1 (2.9) | 21 (7.5) | 0.16 |
| Cough and sputum | 6 (17.1) | 59 (20.8) | 0.41 |
| Jaundice | 5 (14.3) | 16 (5.7) | < 0.03 |
| Hepatomegaly | 6 (17.1) | 11 (3.9) | < 0.01 |
| Right pleural effusion/ consolidation (on admission) | 3 (8.6) | 39 (13.8) | 0.20 |
The clinical features of PLA in patients with and without HN are shown in Table 1. Patients with HN tended to have more acute onset of symptoms than did patients without HN. As shown in Table 1, the most common presenting features were fever, chills and right upper quadrant pain. The HN group experienced right upper quadrant pain (68.6% vs 52.7%, P < 0.04), jaundice (14.3% vs 5.7%, P < 0.03) and hepatomegaly (17.1% vs 3.9%, P < 0.01) more than did the non-HN group, with a significant difference between them. About 20% of patients in both groups experienced respiratory symptoms but only half of these patients had abnormalities in chest radiography.
The laboratory findings are summarized in Table 2. Anemia, leukocytosis, high erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), hypoalbuminemia, and elevated total bilirubin and alanine aminotransferase were common in the two groups. The HN group tended to have a higher serum bilirubin level (43.3 μmol/L vs 30.0 μmol/L, P = 0.05). About 40% of the patients had positive bacterial growth in blood culture on admission. Ultrasonography (USG) and CT of the abdomen were performed in two (5.7%) and nine (25.7%) patients with HN, and 81 (28.6%) and 20 (7.1%) patients without HN, respectively. Combined imaging was performed in 24 (68.6%) and 182 (64.3%) patients with and without HN, respectively. The characteristics of PLA found by radiological imaging are shown in Table 2. The majority of the patients in both groups had right lobe PLA of comparable size. Most PLAs in the two groups appeared as hypoechoic nodules on USG imaging. Both groups had the following common features: rim enhancement, septal lobulation and fluid cavitation in CT imaging, with greater frequency in the HN group. In contrast, more patients in the HN group had thicker (37.1% vs 19.4%, P < 0.01) abscess walls (i.e., thickened abscess wall is defined as wall thickness > 1 cm[9]) than those in the non-HN group had, but both carried a similar risk of rupture. Moreover, aerobilia, gaseous cavitation and portal thrombophlebitis were experienced more in the HN group, with a significant difference between the two groups (P < 0.05).
| Laboratory parameters | HN group(n = 35) | Non-HN group(n = 283) | P value |
| Hemoglobin (mean, g/dL) | 10.0 | 11.3 | 0.22 |
| White cell count (mean, 109/L) | 18.9 | 16.8 | 0.13 |
| ESR (mean, mm/h) | 82.0 | 78.9 | 0.84 |
| CRP (mean, mg/L) | 120.0 | 141.8 | 0.56 |
| Albumin (mean, g/L) | 27.1 | 29.6 | 0.56 |
| Total bilirubin (mean, mol/L) | 43.3 | 30.0 | 0.05 |
| Alanine aminotransferase (mean, U/L) | 64.1 | 84.1 | 0.16 |
| Hba1c (mean, %) | 10.3 | 9.1 | 0.41 |
| Bacteremia | 13 (37.1) | 120 (42.4) | 0.27 |
| Radiological features | |||
| Size (cm, mean ± SD) | 6.8 ± 3.1 | 6.1 ± 3.0 | 0.19 |
| Site | |||
| Right lobe | 22 (62.9) | 156 (55.1) | 0.19 |
| Solitary/multiple | 15 (43)/ 7 (20) | 133 (47)/ 23 (8.1) | |
| Left lobe | 7 (20) | 82 (29) | 0.13 |
| Solitary/multiple | 5 (14.3)/ 2 (5.7) | 73 (25.8)/ 9 (3.2) | |
| Bilobar | 3 (8.6) | 37 (13.1) | 0.22 |
| Echogenicity | |||
| Hypoechoic | 31 (88.6) | 256 (90.5) | 0.36 |
| Hyperechoic/heterogenous | 4 (11.4) | 27 (9.5) | 0.37 |
| Thicken wall | 13 (37.1) | 55 (19.4) | < 0.01 |
| Rim enhancement | 26 (74.3) | 176 (62.2) | 0.08 |
| Septal lobulation | 27 (77.1) | 164 (58.0) | < 0.02 |
| Aerobilia | 7 (25.9) | 16 (5.5) | < 0.01 |
| Fluid/gaseous cavitation | 33 (94.3)/ 6 (17.1) | 243 (85.9)/ 21 (7.8) | 0.08/ 0.03 |
| Portal thrombophlebitis | 4 (11.4) | 5 (1.8) | < 0.01 |
| Subcapsular rupture of the abscess | 2 (5.7) | 16 (5.6) | 0.49 |
The etiologies of the two groups are summarized in Table 3. The pattern of the causative organisms was entirely different between the two groups. Most of the microorganisms isolated from the HN group were mixed growth (40%), followed by Gram-negative and Gram-positive isolates, whereas Gram-negative isolates were dominant in the non-HN group, followed by polymicrobial isolates and Gram-positive cocci. Klebsiella spp. were the dominant Gram-negative organisms in both groups.
| Bacteriology | HN group(n = 35) | Non-HN group(n = 283) | P value |
| Gram-positive organism | 6 (17.2) | 23 (8.1) | 0.04 |
| Streptococcus milleri | 3 (8.6) | 18 (6.4) | |
| Others | 3 (8.6) | 5 (1.7) | |
| Gram-negative organism | 8 (22.8) | 171 (60.4) | < 0.01 |
| Escherichia coli | 2 (5.7) | 27 (9.5) | |
| Klebsiella spp | 5 (14.2) | 135 (47.7) | |
| Others | 1 (2.9)1 | 9 (3.2)2 | |
| Mixed growth | 14 (40.0) | 43 (15.2) | < 0.01 |
| Unknown | 7 (20.0) | 46 (16.3) | < 0.05 |
| Antibiotic resistance | 10 (28.6) | 49 (17.3) | 0.05 |
| Pathogenesis | |||
| Biliary tract disorder | 25 (71.4) | 175 (61.8) | 0.14 |
| Portal pyemia | 3 (8.6) | 11 (3.9) | 0.10 |
| Direct spread | 5 (14.3) | 0 (0) | < 0.01 |
| Hematogenous | 2 (5.7) | 5 (1.8) | 0.07 |
| Cryptogenic | 0 (0) | 15 (5.3) | 0.08 |
| Not investigated | 0 (0) | 77 (27.2) | < 0.01 |
Biliary tract disorder was the most common cause of disease in the two groups. In 14 (4.4%) patients PLA was due to portal pyemia: three patients were from the HN group and all had advanced colon cancer with multiple hepatic metastasis. The remaining 11 were from the non-HN group: seven with colonic diverticulitis shown by abdominal CT scan; two with active inflammatory bowel disease; and two with a recent history of acute appendicitis. Five patients from the HN group had PLAs caused by the direct spread of infection. One patient developed PLA after transarterial chemoembolization for hepatocellular carcinoma and the organism involved was Staphylococcus aureus, thus the infection was probably due to catheter delivery of the skin contaminant. The other four cases had advanced carcinoma of the gallbladder with local invasion into the liver parenchyma. Seven cases (two from the HN group) were caused by hematogenous spread: In the HN group, these were cases of advanced gastric and breast cancer and both had multiple liver metastases. Causes identified in the other group were as follows: urinary tract infection in two patients, and one each with pneumonia, right psoas abscess and continuous ambulatory peritoneal dialysis peritonitis. Lastly, > 20% of patients in the non-HN group had not been investigated for the underlying causes of PLA.
The management and outcomes of the two groups are summarized in Table 4. All patients received intravenous broad-spectrum antibiotics after assessment for sepsis. Antibiotic resistance was detected in 10 (28.6%) isolates from the HN group, but in only 49 (17.3%) from patients without HN, with a significant difference between the two groups (P = 0.05). Antibiotic therapy was the only treatment for seven and 32 patients in the HN and non-HN groups, respectively, and the reason for not performing image-guided aspiration and drainage was the small size of the lesion (< 3 cm in diameter). Among the HN group, USG-guided needle aspiration was performed in 27 (77.1%) patients and continuous catheter drainage in six (17.1%). In the non-HN group, 233 (82.3%) patients had USG-guided needle aspiration and among these, 206 (72.8%) patients had continuous catheter drainage, with a significant difference between the two groups. A pigtail catheter was not inserted in these 48 patients after percutaneous aspiration because the abscess was too solid or it was completely collapsed after aspiration, thus, further catheter drainage was not considered useful. Surgical drainage was required in one (2.9%) and 18 (6.4%) patients with and without HN, respectively. The main reason for laparotomy among these patients was the presentation of acute abdomen. There were no patients who failed to respond to antibiotics with/without percutaneous drainage.
| HN group(n = 35) | Non-HN group(n = 283) | P value | |
| Intervention | |||
| Imaging-guided aspiration ± drainage | 27 (77.1)/ 6 (17.1) | 233 (82.3)/ 206 (72.8) | 0.23/ < 0.01 |
| Surgical drainage | 1 (2.9) | 18 (6.4) | 0.20 |
| Complications and outcomes | |||
| Metastatic infection | 0 (0) | 17 (5.7) | 0.07 |
| Septic shock | 14 (40) | 77 (27.2) | 0.06 |
| DIC1 | 6 (17.1) | 44 (15.5) | 0.39 |
| Acute coronary syndrome | 2 (5.7) | 14 (4.9) | 0.42 |
| Respiratory/renal failure | 1 (2.9) | 14 (4.9) | 0.29 |
| ICU care2 | 1 (2.9) | 31 (11) | 0.07 |
| Recurrence | 1 (2.9) | 16 (5.7) | 0.25 |
| Death | 25 (71.4) | 25 (8.8) | < 0.01 |
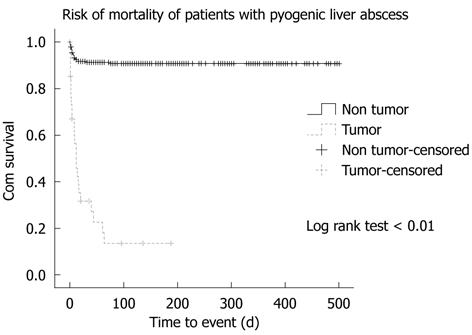
No patients with HN had detectable metastatic infections, whereas 16 (5.7%) patients without HN experienced distant spread of the infection: urinary tract infection in eight, endophthalmitis in three, pneumonia in two, and right empyema, right psoas abscess and simultaneous endophthalmitis and urinary tract infection in one patient each. Septic shock developed in 40% of patients with HN but in only 25% of those without HN, with no significant difference between the two groups. Other complications, such as disseminated intravascular coagulation (DIC), acute coronary syndrome and respiratory/renal failure had comparable incidence between the two groups. The intensive care unit was offered to 31 (11%) patients without HN but only one (2.9%) patient with HN, with no significant difference between the two groups. Recurrence rate of PLA was low in both groups. Twenty-five (71.4%) patients with HN died during the management, all of which was attributed to the underlying HNs, in which all except one had the septic process under control. Only one case died due to uncontrolled Clostridium perfringens septicemia on day 7 of hospitalization. The non-HN group had only 25 (8.8%) deaths and causes were as follows: 17 with liver abscesses, six with hospital-acquired pneumonia, two with cerebrovascular accident, and one with uncontrolled bleeding duodenal ulcer, with a significant difference between the two groups (log rank test < 0.01) (Figure 1). By using multivariate logistic regression analysis, underlying active malignancy, hypoalbuminemia, DIC and acute coronary syndrome were independent risk factors associated with mortality for these two groups (Table 5). These combined risk factors accounted for 49% (Nagelkerke r2: 0.49) of the mortality risk and HN was the most significant clinical variable in this model.
| Variable1 | Wald estimate2 | βcoefficient (B) | OR3 (95% CI) | P value |
| Hepatic malignancy | 51.5 | 3.7 | 40.45 (14.76-111.65) | 0.00 |
| Hypoalbuminemia | 15.2 | 0.2 | 1.22 (1.14-1.38) | 0.00 |
| DIC | 5.2 | 1.2 | 3.32 (1.19-9.69) | < 0.05 |
| Acute coronary syndrome | 4.3 | 1.5 | 4.48 (1.08-17.80) | 0.02 |
| Impaired renal function | 0.1 | 0 | 1.00 (0.82-1.12) | 0.76 |
| Total bilirubin | 0.3 | 0 | 1.00 (0.71-1.51) | 0.48 |
| Septic shock | 0.9 | 0.03 | 1.03 (0.82-2.67) | 0.39 |
| Surgical intervention | 0.11 | 0.13 | 1.14 (0.71-3.90) | 0.74 |
PLA has been recognized since the time of Hippocrates. During recent decades, despite advances in microbiology, imaging-guided intervention and antibiotic therapy, PLA is still a potential life-threatening condition with a mortality rate ranging from 10% to 40%, and one of the reasons is the increasing number of patients with primary or secondary HNs[5-8]. In general, only 5% of malignant liver tumors are of primary origin, whereas the rest are assumed to be metastases[10]. In fact, the liver is the second most common site of metastasis next to lymph nodes. Our study revealed two important findings. First, the primary HNs, instead of liver metastases, were dominant in those PLAs associated with HN. The liver is resistant to infection because it is one of the reticuloendothelial systems in the body and harbors numerous lymphoid cells. Second, the primary site of the HN is also crucial for the development of PLAs because HPB malignant disease was dominant within the HN group.
In the present study, the mean age of the patients and the prevalence of their comorbidity were comparable with previous studies. Men and women were equally affected in patients with HN, whereas there was male dominance in patients without HN, as in most previous studies[11-17]. The duration of symptoms before admission was shorter in the HN group than the non-HN group. This is because a higher proportion of the cases in HN groups were due to the biliary tract pathology. Seeto et al[18] have found that PLA presents most acutely (3 d) if it is due to biliary tract disorder.
The patients in both groups presented with similar clinical features: fever, chill and right upper quadrant pain were frequent symptoms. Because of the presence of hepatic tumors of either primary or secondary, there was a higher incidence of right upper quadrant pain, jaundice and hepatomegaly on physical examination in patients with HN.
Abnormalities in laboratory parameters were similar in both groups, mainly anemia, leukocytosis, high ESR and CRP, deranged liver function test, with elevated serum bilirubin and alkaline phosphatase. However, the change in serum alanine aminotransferase was less pronounced in patients with HN, which may relate to the dominance of cancer of the biliary system among the HNs, thus reflecting the probable secondary biliary cirrhotic change in liver parenchyma. Right lobe involvement was dominant in both groups of patients, which is consistent with prior reports[19]. The right lobe dominance is due to its size and propensity to receive most of the portal blood flow. Bilobar involvement was only seen in 8.6% of the HN group. This is because bilobar tumors are more common in hepatic metastases of extra-HPB origin, which only contributed five (14.3%) cases in our series.
PLAs with and without HN had several similar radiological features in our study. USG appearance of both groups might range from hyperechoic to hypoechoic and this variation has a close relationship to the pathological stage of PLA. During the very early stage of abscess formation, the hepatocytes/tumor cells undergo acute inflammation and thus the abscess might appear solid, i.e., hyperechoic. When these cells start to become necrotic, the abscess liquefies with increasing fluid content and surrounding edema. This is also the stage most patients with PLA present clinically, and thus most PLAs, with or without HN, are hypoechoic by USG and are fluid-containing lesions with rim enhancement in CT contrast studies. In contrast, our study demonstrated that PLAs with and without HNs had several contrast features in imaging. First, within the hepatic tumor tissue, the tumor cells will secrete cytokine to stimulate the adjacent fibrous stroma tissue to grow, therefore, thickened abscess wall and septation were more commonly seen in PLAs with HN[20]. Second, there was a higher incidence of aerobilia and portal thrombophlebitis in patients with HN that might be related to the high incidence of HPB malignancies in the HN group. Third, the gaseous cavitation was more commonly seen in PLAs associated with HN. This might be explained by the fact that the tumor cells have high energy demand for rapid duplication, and this leads to accumulation of lactic acid through their glycolytic pathway. Subsequently, the acidic environment stimulates the formic hydrogen lyase of the bacteria to produce carbon dioxide and hydrogen through mixed acid fermentation[21]. The comparable risk of rupture of PLA associated with/without HN might be explained by the combined counteracting effects of the wall thickness and the gaseous cavitation inside the abscess.
The bacteriological pattern in PLA was entirely different between the two groups in which there was a higher incidence of polymicrobial isolates in the abscesses of the HN group. This may be related to the mixture of microorganisms in the normal flora in the gastrointestinal and biliary tract. There are three possible mechanisms for the bacterial flora to develop PLA in the presence of HN: malignant biliary obstruction caused by HPB malignancies, portal venous suppuration, which usually happens in colorectal tumor, and bacterial seeding via hepatic artery following systemic chemotherapy[22]. Although only some patients were investigated by colonoscopy, endoscopic retrograde cholangiopancreatography and endoscopic ultrasound were performed, the biliary disease was still the most common identifiable cause of PLA in patients without HN as in other recent studies[18,23,24].
In our study, most of the patients were managed by combination of antibiotic and imaging-guided aspiration with/without drainage. There was no difference in the complications between the two groups during the imaging-guided intervention. No tumor seedlings were reported in the HN group, and this might have been due to only minute quantities of tumor cells in the central region of the tumor mass. Thus, imaging-guided aspiration of the hepatic tumor abscess is regarded as a relatively safe procedure. Although both groups of patients responded similarly in the initial phase of management, the patients with HN tended to have a more fulminant clinical course with a higher incidence of septic shock, DIC and acute coronary syndrome. There was no detected metastatic infection in patients with HN and this might be explained by the difficulty for the bacteria to disseminate within the tumor tissue. Surprisingly, underlying active hepatic malignancy was less likely to be associated with the recurrence of PLA, because a considerable proportion of the patients with HN died. This would have made an apparently negative association between malignancy and recurrence.
In the present study, the overall mortality rate of PLA was 16%, which is comparable to other studies[25]. The higher mortality rate of patients with HN was due to underlying active malignancy and higher incidence of the complications mentioned previously. Half of the deaths in the HN group happened within the first 12 wk. Although there were two cases each of intrahepatic cholangiocarcinoma and cancer of the head of the pancreas that presented concurrently with PLAs, these four cases were found to have localized tumors and thus curative resection could be performed after treatment of PLA. Thus, it is justifiable to have early follow-up imaging with/without biopsy for those PLAs that have a suspicion of malignancy. Despite the advanced stage of the disease, early diagnosis of the neoplastic component is warranted because neoadjuvant target therapy might modify its natural course. In our study, the most important determinants of mortality rate were underlying active hepatic malignancy, hypoalbuminemia, DIC and acute coronary syndrome. The prognostic value of underlying malignancy has been recognized in other studies[24,26]. Hypoalbuminemia reflects the duration of the growth of the liver abscess and the degree of impairment of hepatic synthetic function. DIC and acute coronary syndrome may relate to the severity of the septic process.
PLA associated with HN tends to have a distinct clinical syndrome with a different extent of clinical manifestations, radiological and microbiological features, and complications. The initial antibiotic of choice should be broad-spectrum with coverage of both Gram-positive and -negative microorganisms. These PLAs can be safely treated with image-guided aspiration with/without drainage, with negligible risk of tumor seedlings. Early follow-up imaging should be considered for those PLAs with potential risk of malignancy.
Hepatic neoplasm (HN) is a recognized cause of pyogenic liver abscess (PLA). It is well-known that HN can mimic PLA on presentation and it is difficult to identify the coexisting hepatic tumor component in PLA, thus, PLA associated with HN is a diagnostic challenge to clinicians. There have been few studies focused on this subject.
Due to the better understanding of carcinogenesis and the advancement of molecular medicine, treatments based on molecular targets are being developed and these might have the potential to modify the clinical course of these neoplasms.
PLA associated with HN tends to have a distinct clinical syndrome with a different extent of clinical manifestations, radiological and microbiological features, and complications. Several cases, presented concurrently with PLAs, were found to have curative resectable tumors and had good prognosis after surgery.
The initial antibiotic of choice should be broad-spectrum with coverage of both Gram-positive and -negative microorganisms. These PLAs can be safely treated with image-guided aspiration with/without drainage, with negligible risk of tumor seedlings. Early follow-up imaging should be considered for those PLAs with potential risk of malignancy.
This is a unique manuscript documenting the clinical course of liver abscess associated with/without malignancy. The authors report differences between two groups (liver abscess with and without malignancy) and liver abscess with malignancy was associated with unfavorable prognosis. The viewpoint of this manuscript is interesting, but the clinical impact of this study is not fully discussed.
Peer reviewer: Taku Aoki, Dr., Division of Hepato-Biliary-Pancreatic and Transplantation Surgery, Department of Surgery, Graduate School of Medicine, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan
S- Editor Shi ZF L- Editor Kerr C E- Editor Zheng XM
| 1. | Altemeier WA, Culbertson WR, Fullen WD, Shook CD. Intra-abdominal abscesses. Am J Surg. 1973;125:70-79. [PubMed] [Cited in This Article: ] |
| 2. | Pang TC, Fung T, Samra J, Hugh TJ, Smith RC. Pyogenic liver abscess: an audit of 10 years' experience. World J Gastroenterol. 2011;17:1622-1630. [PubMed] [Cited in This Article: ] |
| 3. | Malik AA, Bari SU, Rouf KA, Wani KA. Pyogenic liver abscess: Changing patterns in approach. World J Gastrointest Surg. 2010;2:395-401. [PubMed] [Cited in This Article: ] |
| 4. | Cerwenka H. Pyogenic liver abscess: differences in etiology and treatment in Southeast Asia and Central Europe. World J Gastroenterol. 2010;16:2458-2462. [PubMed] [Cited in This Article: ] |
| 5. | Wong WM, Wong BC, Hui CK, Ng M, Lai KC, Tso WK, Lam SK, Lai CL. Pyogenic liver abscess: retrospective analysis of 80 cases over a 10-year period. J Gastroenterol Hepatol. 2002;17:1001-1007. [PubMed] [Cited in This Article: ] |
| 6. | Wang JH, Liu YC, Lee SS, Yen MY, Chen YS, Wang JH, Wann SR, Lin HH. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis. 1998;26:1434-1438. [PubMed] [Cited in This Article: ] |
| 7. | Petri A, Höhn J, Hódi Z, Wolfárd A, Balogh A. Pyogenic liver abscess -- 20 years' experience. Comparison of results of treatment in two periods. Langenbecks Arch Surg. 2002;387:27-31. [PubMed] [Cited in This Article: ] |
| 8. | Mischinger HJ, Hauser H, Rabl H, Quehenberger F, Werkgartner G, Rubin R, Deu E. Pyogenic liver abscess: studies of therapy and analysis of risk factors. World J Surg. 1994;18:852-87; discussion 858. [PubMed] [Cited in This Article: ] |
| 9. | Mortelé KJ, Segatto E, Ros PR. The infected liver: radiologic-pathologic correlation. Radiographics. 2004;24:937-955. [PubMed] [Cited in This Article: ] |
| 10. | Kuntz E, Kuntz HD. Hepatology: principle and practice. 2nd ed. Heidelberg: Springer Medizin Verlag 2006; 773. [Cited in This Article: ] |
| 11. | Chu KM, Fan ST, Lai EC, Lo CM, Wong J. Pyogenic liver abscess. An audit of experience over the past decade. Arch Surg. 1996;131:148-152. [PubMed] [Cited in This Article: ] |
| 12. | Yang CC, Chen CY, Lin XZ, Chang TT, Shin JS, Lin CY. Pyogenic liver abscess in Taiwan: emphasis on gas-forming liver abscess in diabetics. Am J Gastroenterol. 1993;88:1911-1915. [PubMed] [Cited in This Article: ] |
| 13. | Kuo CM, Kuo CH, Changchien CS. Liver abscess in patients with cirrhosis of the liver: a 12-year experience. J Gastroenterol. 2001;36:552-556. [PubMed] [Cited in This Article: ] |
| 14. | Kung JS, Lin HH. Clinical study of pyogenic liver abscess. J Med Sci. 1995;15:257-266. [Cited in This Article: ] |
| 15. | Kaplan GG, Gregson DB, Laupland KB. Population-based study of the epidemiology of and the risk factors for pyogenic liver abscess. Clin Gastroenterol Hepatol. 2004;2:1032-1038. [PubMed] [Cited in This Article: ] |
| 16. | Lee KT, Wong SR, Sheen PC. Pyogenic liver abscess: an audit of 10 years' experience and analysis of risk factors. Dig Surg. 2001;18:459-65; discussion 465-6. [PubMed] [Cited in This Article: ] |
| 17. | Chiu CT, Lin DY, Wu CS, Chang-Chien CS, Sheen IS, Liaw YF. [A clinical study on pyogenic liver abscess]. Taiwan Yi Xue Hui Za Zhi. 1987;86:405-412. [PubMed] [Cited in This Article: ] |
| 18. | Seeto RK, Rockey DC. Pyogenic liver abscess. Changes in etiology, management, and outcome. Medicine (Baltimore). 1996;75:99-113. [PubMed] [Cited in This Article: ] |
| 19. | Jain M, Agarwal A. MRCP findings in recurrent pyogenic cholangitis. Eur J Radiol. 2008;66:79-83. [PubMed] [Cited in This Article: ] |
| 20. | Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA. 2003;100:2645-2650. [PubMed] [Cited in This Article: ] |
| 21. | Lee HL, Lee HC, Guo HR, Ko WC, Chen KW. Clinical significance and mechanism of gas formation of pyogenic liver abscess due to Klebsiella pneumoniae. J Clin Microbiol. 2004;42:2783-2785. [PubMed] [Cited in This Article: ] |
| 22. | Yeh TS, Jan YY, Jeng LB, Chen TC, Hwang TL, Chen MF. Hepatocellular carcinoma presenting as pyogenic liver abscess: characteristics, diagnosis, and management. Clin Infect Dis. 1998;26:1224-1226. [PubMed] [Cited in This Article: ] |
| 23. | Alvarez Pérez JA, González JJ, Baldonedo RF, Sanz L, Carreño G, Junco A, Rodríguez JI, Martínez MD, Jorge JI. Clinical course, treatment, and multivariate analysis of risk factors for pyogenic liver abscess. Am J Surg. 2001;181:177-186. [PubMed] [Cited in This Article: ] |
| 24. | Branum GD, Tyson GS, Branum MA, Meyers WC. Hepatic abscess. Changes in etiology, diagnosis, and management. Ann Surg. 1990;212:655-662. [PubMed] [Cited in This Article: ] |
| 25. | Yang CC, Yen CH, Ho MW, Wang JH. Comparison of pyogenic liver abscess caused by non-Klebsiella pneumoniae and Klebsiella pneumoniae. J Microbiol Immunol Infect. 2004;37:176-184. [PubMed] [Cited in This Article: ] |