Published online Mar 14, 2012. doi: 10.3748/wjg.v18.i10.1009
Revised: August 18, 2011
Accepted: August 27, 2011
Published online: March 14, 2012
Neuroendocrine tumors (NETs) are diagnosed with increasing frequency and patients often present with liver metastases at the time of diagnosis. Apart from treatment of the metastases, resection of the primary tumor at an early phase is recommended to prevent complications, although it may be difficult to locate, especially in patients with functionally inactive NETs. Small and multifocal tumors in the jejunum and ileum represent a particular challenge. Primary hepatic neuroendocrine carcinoma is extremely rare and is diagnosed only after exclusion of other primary tumors. Therefore, some uncertainty may remain, as small non-hepatic primary tumors may escape detection. Diagnostic work-up in these patients includes biochemical assays and imaging modalities (also comprising specific techniques of scintigraphy and positron emission tomography). This editorial highlights the contributions of endoscopy and operative exploration to the search for the primary tumor. Besides esophago-gastro-duodenoscopy, endoscopic ultrasonography, colonoscopy and bronchoscopy, special endoscopic techniques such as balloon enteroscopy or capsule endoscopy are used with growing experience. Compared with balloon enteroscopy, capsule endoscopy is non-invasive and better tolerated, but it cannot localize a lesion precisely and does not allow biopsy or removal of lesions. Before proceeding to surgery, a discussion of the findings by a tumor board should be a standard procedure. Improvements in diagnostic tools have created new perspectives for the detection of obscure primary tumors in patients with neuroendocrine liver metastases, and these searches are best coordinated by a multidisciplinary team.
- Citation: Cerwenka H. Neuroendocrine liver metastases: Contributions of endoscopy and surgery to primary tumor search. World J Gastroenterol 2012; 18(10): 1009-1014
- URL: https://www.wjgnet.com/1007-9327/full/v18/i10/1009.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i10.1009
Neuroendocrine tumors (NETs)[1-9] are diagnosed with increasing frequency. The overall annual incidence of gastrointestinal NETs was recently reported to be 1.32 per 100 000 in men and 1.33 per 100 000 in women, with 12% of tumors in the stomach, 29% in the small intestine, 38% in the appendix, 13% in the colon, and 8% in the rectum[10]. After the lymph nodes, the liver is the second most common site of neuroendocrine metastases; in the study by Hlatky et al[11], 39% of patients had liver metastases at the time of diagnosis. Resection of neuroendocrine liver metastases, if feasible, is the treatment of choice. In patients with advanced disease, an aggressive surgical approach, including staged procedures if required, is warranted as part of multimodal treatment[12-15]. Although resection of the primary tumor in an early phase is recommended to prevent complications[16,17], the primary tumor may be difficult to locate[18], especially in patients with functionally inactive NETs. If curative resection of liver metastases is not possible, however, it is unclear whether the primary tumor should also be removed. When possible, debulking, leaving < 10% of residual tumor volume, has proven helpful[19]. One argument for removing the primary tumor from patients with unresectable liver metastases is that these hepatic foci can be treated by interventional techniques, whereas the primary tumor is best treated by resection. Indeed, recent studies[20,21] have suggested that resection of the primary tumors, even from patients with unresectable neuroendocrine liver metastases, may be associated with improved survival.
About 11% to 14% of NET patients have metastatic disease with an unknown primary tumor[22], and these primary tumors may also be multifocal. The most common primary location is the gastrointestinal tract, followed by respiratory and thymic carcinoids[23]. Primary tumors in the jejunum and ileum may be small, but they often cause a characteristic fibrosis in the mesentery (desmoplastic reaction), resulting in bowel obstruction, ischemia or perforation in approximately one-third of patients[24,25]. Primary hepatic neuroendocrine carcinoma is extremely rare[26,27] and is diagnosed only after exclusion of other primary tumors. Therefore, some uncertainty may remain, because small non-hepatic primary tumors may escape detection. As with metastases, the therapy of choice is resection[28].
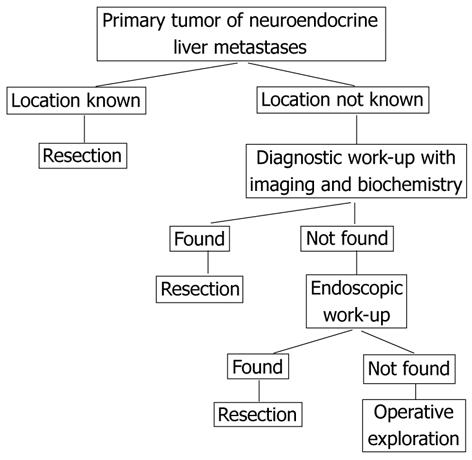
Diagnostic work-up in these patients includes biochemical assays and imaging modalities (Figure 1). Although ultrasonography is an important screening method with easy access and low costs, it remains operator dependent and its capacity for detecting primary tumors, especially in the small intestine, is limited. On contrast-enhanced ultrasonography using microbubbles, NETs of the small intestine and pancreas may present as hypoechoic, isoechoic, and/or hyperechoic liver lesions. Necrotic areas can also be identified, especially after treatment[29]. Computed tomography (CT) scans are very helpful in diagnosing NETs, especially larger foci in the mesentery or in the liver, but may not be useful in diagnosing small primary tumors. Magnetic resonance (MR) imaging is performed to determine the extent, anatomical relationships and resectability of the liver metastases but may also contribute to the detection and localization of intestinal primary tumors. On post-gadolinium T1-weighted fat-suppressed images, these primary tumors can be visualized as nodular masses originating from the bowel wall, or as regional uniform bowel wall thickening with moderately intense enhancement[30]. Less is known about the uses of CT/MR enteroclysis. For example, CT enteroclysis in a small number of patients (n = 8) had a sensitivity for detection of primary NETs of 50%, but false-positive results were also reported[31]. MR enteroclysis is an evolving technique that has shown promise in initial studies[30].
Selective receptor-targeting radiopeptides have been developed for molecular imaging and therapy of NETs that overexpress peptide receptors on their cell membranes. The somatostatin analog 111In-octreotide can be used to localize NETs expressing somatostatin receptors. Newer modified somatostatin analogs, including Tyr(3)-octreotide and Tyr(3)-octreotate, have been successfully used for diagnosis of these tumors, including the localization of primary tumors, and for radionuclide therapy and have therefore been named “theragnostics”. Radiopeptides targeting other receptors, including radiolabeled analogs of cholecystokinin, gastrin, bombesin, substance P, vasoactive intestinal peptide, and neuropeptide Y, may also be used[32]. As scintigraphy detects tumors not seen on CT and vice versa, these two imaging techniques can be seen as complementing each other. Imaging by 18F-Dopa positron emission tomography (PET) is based on the decarboxylase contained in these tumors and is especially useful in patients with negative scintigraphy results. Due to better anatomical allocation of “hot spots”, PET can be combined with CT. Fluorodeoxyglucose (FDG) is taken up primarily by poorly differentiated tumors due to their enhanced glucose metabolism, making FDG-PET useful in depicting and identifying this group of tumors. 68Ga-DOTATOC PET/CT is presently being investigated and has yielded promising results[33-35]. The combination of 68Ga-DOTATOC with PET/CT was found to have an impact on the therapeutic management of 38% of patients with NET, including identifying the primary tumor for resection in about 8%; moreover, in 50% of these patients, the relevant findings were detected by a single imaging modality (i.e., CT or PET)[36]. These findings indicate that CT and PET have comparable sensitivity, provide complementary information and contribute equally to therapeutic decision making[36].
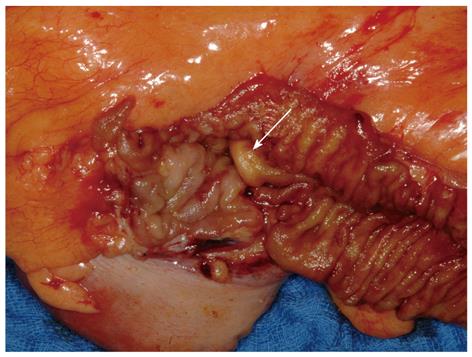
Upper and lower gastrointestinal endoscopy should be performed not only to identify the primary tumor, but also to rule out concomitant malignancies, which are present in a higher percentage (about 20%) of NET patients than in the normal population[37]. The importance of esophago-gastro-duodenoscopy (EGD) is emphasized by findings showing that the incidence of stomach NETs is increasing. Although one study[38] reported a 10-fold increase, this applies mainly to well-differentiated NETs and is probably due to increased rates of screening and associated diagnosis. Endoscopic ultrasonography (EUS) is the method of choice for the assessment of tumor size and depth of infiltration[38] and is very helpful in the diagnosis of pancreatic NETs. A study of the EUS characteristics of cystic pancreatic NETs found that these tumors constitute 0.67% of all cystic pancreatic lesions and 9.5% of all confirmed pancreatic NETs[39]. Primary tumors in the colon can be detected by colonoscopy, and these examinations should include the terminal ileum, as far as possible. Figure 2 shows a resected small primary tumor in the terminal ileum that was detected by colonoscopy extending beyond the ileocecal valve. Intraoperatively, this tumor was not detectable, either by inspection from the serosal side or by palpation; rather, its removal was based on its endoscopic localization.
Bronchoscopy, in combination with thoracic CT or PET/CT, can help identify primary tumors in the bronchi/lungs.
Capsule endoscopy[40] is a practical technique, but analysis of the films requires expertise and time. Extraluminal tumor growth may limit this method. A comparison of CT, enteroclysis, nuclear imaging, and video capsule endoscopy (VCE) of the small bowel showed that CT and enteroclysis were unable to detect a primary tumor, whereas nuclear imaging identified abnormalities in the abdominal area in about two-thirds of the patients but was unable to relate this to an intestinal localization[16]. In contrast, VCE had a high diagnostic yield (45%) in identifying primary tumors in the small intestine. Although nuclear imaging had a comparable diagnostic yield, it could not differentiate between intestinal and mesenterial localization[16]. A study of 300 patients who underwent VCE for obscure bleeding following non-diagnostic EGD and colonoscopy showed that one had a neuroendocrine carcinoma, and nine had other small-bowel masses. Capsule retention occurred in two patients, with one requiring urgent surgery[41].
An alternative for examination of the small bowel is balloon endoscopy, using either a single or double balloon technique[42,43]. This method, however, is time-consuming, and complications, including small bowel perforation, ileus, and pancreatitis, have been reported in 0.8%-4.0% of patients[44]. A recent study[45] has shown that a single-balloon push-and-pull enteroscopy system was safe, useful, and highly effective in the diagnosis and treatment of several small-bowel diseases. Moreover, double balloon enteroscopy (DBE) in 12 consecutive patients with suspected NETs resulted in the detection of submucosal tumors in the ileum or jejunum in seven patients; in two patients with a submucosal ileum protrusion suspicious for NET, however, laparotomy and intraoperative endoscopy did not confirm that the protrusion was a tumor. The diagnostic yield of DBE for primary tumors in patients with metastatic or suspected NET was 33%, suggesting that DBE should only be performed in selected patients, possibly based on a previous positive work-up[46].
Compared with balloon enteroscopy, capsule endoscopy is non-invasive and better tolerated, but it cannot localize a lesion precisely and does not allow biopsy or removal of lesions. The velocity of the transport of the capsule by peristalsis is irregular and there is no possibility of rinsing and suction; thus, a lesion may be missed. New technologies may expand the role of capsule endoscopy by, for example, controlling capsule movement, enabling this method to be used therapeutically or to obtain tissue biopsies and providing a transcutaneous power delivery system[47]. A recent meta-analysis[48] found that capsule endoscopy and DBE had similar diagnostic yields in patients with obscure gastrointestinal bleeding.
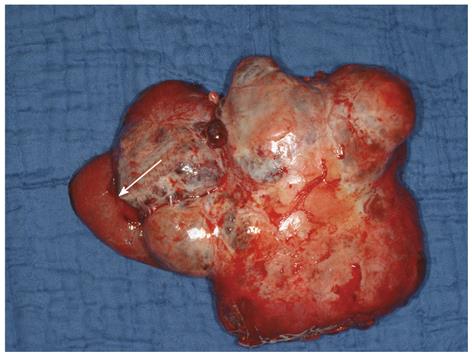
Intraoperative exploration may be performed as a self-contained procedure or in combination with other surgical goals such as resection of liver metastases or mesenteric masses, or cholecystectomy. Prophylactic removal of the gallbladder from NET patients during abdominal operations is recommended for two reasons. First, anticipating future long-term somatostatin analog therapy of these patients, removal of the gallbladder will prevent gallstone-associated complications associated with this treatment. Second, removal of the gallbladder will prevent gallbladder necrosis from hepatic artery embolization for liver metastases. In a recent study, preoperative identification of the primary tumor failed in 21% of patients, whereas laparoscopic exploration was successful in locating the primary tumor in 87%[49]. The presence of a mesenteric mass may indicate a primary tumor in the adjacent small bowel loop. Figure 3 shows the operative specimen from an NET patient with bulky disease in the mesentery; no primary tumor could be found pre- or intraoperatively. The adjacent loop of the ileum was resected en bloc with the mesenteric tumor (for reasons of blood supply), and its meticulous histopathological work-up yielded two foci of neuroendocrine carcinoma.
In contrast, if a small primary tumor is present in the small intestine without visible disease in the mesentery, a wide mesenteric dissection would be preferable, given the propensity of these tumors to cause mesenteric spread and desmoplastic masses.
During the course of laparoscopic exploration, exteriorization of the bowel and thorough palpation are recommended. Although these tumors may cause dimpling of the serosa, this is not a reliable feature and will not always be visible during laparoscopic exploration. Careful palpation, however, also allows identification of small and multifocal primary tumors.
Intraoperative ultrasonography (IOUS) is very helpful for liver resections and may be used to screen the pancreas, if no primary tumor is found in the intestine or at other locations. IOUS is also useful for measuring the distance between a pancreatic primary tumor and the pancreatic duct[50]. The pancreas was recently found to be the most common primary site (35%) for NET liver metastases but, as pancreatic tumors are well visualized on CT, all of them were detected preoperatively by CT[49]. Thus, occult primary tumors in the pancreas are highly unlikely in patients with NET liver metastases, limiting the use of EUS/IOUS of the pancreas in patients previously examined by CT.
During the course of the exploration, a biopsy can be taken of the liver metastases if they have not been identified histopathologically. Due to the rarity of these tumors, there have been few studies on the frequency and success of operative exploration, suggesting the need for multicenter studies using a standardized protocol. It is also unclear whether conversion to an open procedure is warranted if no primary tumor is found during laparoscopic exploration. If all laparoscopically visible parts of the abdominal cavity have been thoroughly inspected and the intestine has been exteriorized and meticulously palpated, open access by laparotomy will probably not yield any additional diagnostic benefit, although multiple tight adhesions may be an indication for conversion. To date, however, this has not been studied.
Before proceeding to surgery, a discussion of the findings by a tumor board should be a standard procedure.
In conclusion, improvements in diagnostic tools have created new perspectives for the detection of obscure primary tumors in patients with neuroendocrine liver metastases, and these searches are best coordinated by a multidisciplinary team.
Peer reviewers: Zenichi Morise, MD, PhD, Professor and Chairman, Department of Surgery, Banbuntane Houtokukai Hospital, Fujita Health University School of Medicine, 3-6-10 Otobashi Nakagawa-ku, Nagoya, 454-8509 Aichi, Japan; Dr. Bernardo Frider, MD, Professor, Department of Hepatology, Hospital General de Agudos Cosme Argerich, Alte Brown 240, 1155 Buenos Aires, Argentina; Rui Marinho, Professor, Department of Gastroenterology and Hepatology, Hospital Santa Maria, Rua Prof. Aires de Sousa, 1 r/c A, 1600-590 Lisboa, Portugal; Metin Basaranoglu, MD, PhD, Associate Professor, Gastroenterology Division, Ankara Hospital, Sihhiye, Tr-06000 Ankara, Turkey
S- Editor Lv S L- Editor Kerr C E- Editor Li JY
| 1. | Kvols LK, Brendtro KL. The North American Neuroendocrine Tumor Society (NANETS) guidelines: mission, goals, and process. Pancreas. 2010;39:705-706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Vinik AI, Woltering EA, Warner RR, Caplin M, O'Dorisio TM, Wiseman GA, Coppola D, Go VL. NANETS consensus guidelines for the diagnosis of neuroendocrine tumor. Pancreas. 2010;39:713-734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 189] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 3. | Kulke MH, Anthony LB, Bushnell DL, de Herder WW, Goldsmith SJ, Klimstra DS, Marx SJ, Pasieka JL, Pommier RF, Yao JC. NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas. 2010;39:735-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 387] [Cited by in F6Publishing: 357] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 4. | Strosberg JR, Coppola D, Klimstra DS, Phan AT, Kulke MH, Wiseman GA, Kvols LK. The NANETS consensus guidelines for the diagnosis and management of poorly differentiated (high-grade) extrapulmonary neuroendocrine carcinomas. Pancreas. 2010;39:799-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 232] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 5. | Anthony LB, Strosberg JR, Klimstra DS, Maples WJ, O'Dorisio TM, Warner RR, Wiseman GA, Benson AB, Pommier RF. The NANETS consensus guidelines for the diagnosis and management of gastrointestinal neuroendocrine tumors (nets): well-differentiated nets of the distal colon and rectum. Pancreas. 2010;39:767-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 6. | Boudreaux JP, Klimstra DS, Hassan MM, Woltering EA, Jensen RT, Goldsmith SJ, Nutting C, Bushnell DL, Caplin ME, Yao JC. The NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the Jejunum, Ileum, Appendix, and Cecum. Pancreas. 2010;39:753-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 350] [Cited by in F6Publishing: 338] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 7. | Phan AT, Oberg K, Choi J, Harrison LH, Hassan MM, Strosberg JR, Krenning EP, Kocha W, Woltering EA, Maples WJ. NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the thorax (includes lung and thymus). Pancreas. 2010;39:784-798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Strosberg J, Gardner N, Kvols L. Survival and prognostic factor analysis in patients with metastatic pancreatic endocrine carcinomas. Pancreas. 2009;38:255-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 776] [Cited by in F6Publishing: 711] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 10. | Ellis L, Shale MJ, Coleman MP. Carcinoid tumors of the gastrointestinal tract: trends in incidence in England since 1971. Am J Gastroenterol. 2010;105:2563-2569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 11. | Hlatky R, Suki D, Sawaya R. Carcinoid metastasis to the brain. Cancer. 2004;101:2605-2613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Scigliano S, Lebtahi R, Maire F, Stievenart JL, Kianmanesh R, Sauvanet A, Vullierme MP, Couvelard A, Belghiti J, Ruszniewski P. Clinical and imaging follow-up after exhaustive liver resection of endocrine metastases: a 15-year monocentric experience. Endocr Relat Cancer. 2009;16:977-990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Stoeltzing O, Loss M, Huber E, Gross V, Eilles C, Mueller-Brand J, Schlitt HJ. Staged surgery with neoadjuvant 90Y-DOTATOC therapy for down-sizing synchronous bilobular hepatic metastases from a neuroendocrine pancreatic tumor. Langenbecks Arch Surg. 2010;395:185-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Fendrich V, Michl P, Habbe N, Bartsch DK. Liver-specific therapies for metastases of neuroendocrine pancreatic tumors. World J Hepatol. 2010;2:367-373. [PubMed] [Cited in This Article: ] |
| 15. | Fendrich V, Langer P, Celik I, Bartsch DK, Zielke A, Ramaswamy A, Rothmund M. An aggressive surgical approach leads to long-term survival in patients with pancreatic endocrine tumors. Ann Surg. 2006;244:845-851; discussion 852-853. [PubMed] [Cited in This Article: ] |
| 16. | van Tuyl SA, van Noorden JT, Timmer R, Stolk MF, Kuipers EJ, Taal BG. Detection of small-bowel neuroendocrine tumors by video capsule endoscopy. Gastrointest Endosc. 2006;64:66-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Boudreaux JP, Putty B, Frey DJ, Woltering E, Anthony L, Daly I, Ramcharan T, Lopera J, Castaneda W. Surgical treatment of advanced-stage carcinoid tumors: lessons learned. Ann Surg. 2005;241:839-845; discussion 845-846. [PubMed] [Cited in This Article: ] |
| 18. | Spigel DR, Hainsworth JD, Greco FA. Neuroendocrine carcinoma of unknown primary site. Semin Oncol. 2009;36:52-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Sarmiento JM, Que FG, Grant CS, Thompson GB, Farnell MB, Nagorney DM. Concurrent resections of pancreatic islet cell cancers with synchronous hepatic metastases: outcomes of an aggressive approach. Surgery. 2002;132:976-982; discussion 982-983. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Ito T, Sasano H, Tanaka M, Osamura RY, Sasaki I, Kimura W, Takano K, Obara T, Ishibashi M, Nakao K. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol. 2010;45:234-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 243] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 21. | Ito T, Tanaka M, Sasano H, Osamura YR, Sasaki I, Kimura W, Takano K, Obara T, Ishibashi M, Nakao K. Preliminary results of a Japanese nationwide survey of neuroendocrine gastrointestinal tumors. J Gastroenterol. 2007;42:497-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Hauso O, Gustafsson BI, Kidd M, Waldum HL, Drozdov I, Chan AK, Modlin IM. Neuroendocrine tumor epidemiology: contrasting Norway and North America. Cancer. 2008;113:2655-2664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in F6Publishing: 376] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 23. | Elsayes KM, Menias CO, Bowerson M, Osman OM, Alkharouby AM, Hillen TJ. Imaging of carcinoid tumors: spectrum of findings with pathologic and clinical correlation. J Comput Assist Tomogr. 2011;35:72-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Plöckinger U, Rindi G, Arnold R, Eriksson B, Krenning EP, de Herder WW, Goede A, Caplin M, Oberg K, Reubi JC. Guidelines for the diagnosis and treatment of neuroendocrine gastrointestinal tumours. A consensus statement on behalf of the European Neuroendocrine Tumour Society (ENETS). Neuroendocrinology. 2004;80:394-424. [PubMed] [Cited in This Article: ] |
| 25. | Oberg K, Astrup L, Eriksson B, Falkmer SE, Falkmer UG, Gustafsen J, Haglund C, Knigge U, Vatn MH, Välimäki M. Guidelines for the management of gastroenteropancreatic neuroendocrine tumours (including bronchopulmonary and thymic neoplasms). Part II-specific NE tumour types. Acta Oncol. 2004;43:626-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Huang YQ, Xu F, Yang JM, Huang B. Primary hepatic neuroendocrine carcinoma: clinical analysis of 11 cases. Hepatobiliary Pancreat Dis Int. 2010;9:44-48. [PubMed] [Cited in This Article: ] |
| 27. | Fenoglio LM, Severini S, Ferrigno D, Gollè G, Serraino C, Bracco C, Castagna E, Brignone C, Pomero F, Migliore E. Primary hepatic carcinoid: a case report and literature review. World J Gastroenterol. 2009;15:2418-2422. [PubMed] [Cited in This Article: ] |
| 28. | Schwartz G, Colanta A, Gaetz H, Olichney J, Attiyeh F. Primary carcinoid tumors of the liver. World J Surg Oncol. 2008;6:91. [PubMed] [Cited in This Article: ] |
| 29. | Dörffel Y, Wermke W. Neuroendocrine tumors: characterization with contrast-enhanced ultrasonography. Ultraschall Med. 2008;29:506-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Bader TR, Semelka RC, Chiu VC, Armao DM, Woosley JT. MRI of carcinoid tumors: spectrum of appearances in the gastrointestinal tract and liver. J Magn Reson Imaging. 2001;14:261-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Johanssen S, Boivin M, Lochs H, Voderholzer W. The yield of wireless capsule endoscopy in the detection of neuroendocrine tumors in comparison with CT enteroclysis. Gastrointest Endosc. 2006;63:660-665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | de Jong M, Breeman WA, Kwekkeboom DJ, Valkema R, Krenning EP. Tumor imaging and therapy using radiolabeled somatostatin analogues. Acc Chem Res. 2009;42:873-880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 33. | Frilling A, Sotiropoulos GC, Radtke A, Malago M, Bockisch A, Kuehl H, Li J, Broelsch CE. The impact of 68Ga-DOTATOC positron emission tomography/computed tomography on the multimodal management of patients with neuroendocrine tumors. Ann Surg. 2010;252:850-856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 34. | Kayani I, Conry BG, Groves AM, Win T, Dickson J, Caplin M, Bomanji JB. A comparison of 68Ga-DOTATATE and 18F-FDG PET/CT in pulmonary neuroendocrine tumors. J Nucl Med. 2009;50:1927-1932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 35. | Prasad V, Ambrosini V, Hommann M, Hoersch D, Fanti S, Baum RP. Detection of unknown primary neuroendocrine tumours (CUP-NET) using (68)Ga-DOTA-NOC receptor PET/CT. Eur J Nucl Med Mol Imaging. 2010;37:67-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 36. | Ruf J, Heuck F, Schiefer J, Denecke T, Elgeti F, Pascher A, Pavel M, Stelter L, Kropf S, Wiedenmann B. Impact of Multiphase 68Ga-DOTATOC-PET/CT on therapy management in patients with neuroendocrine tumors. Neuroendocrinology. 2010;91:101-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 37. | Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1848] [Cited by in F6Publishing: 1766] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 38. | Scherübl H, Cadiot G, Jensen RT, Rösch T, Stölzel U, Klöppel G. Neuroendocrine tumors of the stomach (gastric carcinoids) are on the rise: small tumors, small problems? Endoscopy. 2010;42:664-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 39. | Kongkam P, Al-Haddad M, Attasaranya S, O'Neil J, Pais S, Sherman S, DeWitt J. EUS and clinical characteristics of cystic pancreatic neuroendocrine tumors. Endoscopy. 2008;40:602-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Pozzato P, Brancaccio M, Tomassetti P, Casetti T, Ventrucci M. Capsule endoscopy for the diagnosis of midgut neuroendocrine carcinoma. Dig Liver Dis. 2008;40:966-967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 41. | Baichi MM, Arifuddin RM, Mantry PS. Small-bowel masses found and missed on capsule endoscopy for obscure bleeding. Scand J Gastroenterol. 2007;42:1127-1132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Yamamoto H, Sekine Y, Sato Y, Higashizawa T, Miyata T, Iino S, Ido K, Sugano K. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest Endosc. 2001;53:216-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 896] [Cited by in F6Publishing: 828] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 43. | Yamamoto H, Yano T, Kita H, Sunada K, Ido K, Sugano K. New system of double-balloon enteroscopy for diagnosis and treatment of small intestinal disorders. Gastroenterology. 2003;125:1556; author reply 1556-1557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 173] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 44. | Pasha SF, Hara AK, Leighton JA. Diagnostic evaluation and management of obscure gastrointestinal bleeding: a changing paradigm. Gastroenterol Hepatol (NY). 2009;5:839-850. [PubMed] [Cited in This Article: ] |
| 45. | Riccioni ME, Urgesi R, Cianci R, Spada C, Nista EC, Costamagna G. Single-balloon push-and-pull enteroscopy system: does it work? A single-center, 3-year experience. Surg Endosc. 2011;25:3050-3056. [PubMed] [Cited in This Article: ] |
| 46. | Bellutti M, Fry LC, Schmitt J, Seemann M, Klose S, Malfertheiner P, Mönkemüller K. Detection of neuroendocrine tumors of the small bowel by double balloon enteroscopy. Dig Dis Sci. 2009;54:1050-1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 47. | Keum B, Chun HJ. Capsule endoscopy and double balloon enteroscopy for obscure gastrointestinal bleeding: which is better? J Gastroenterol Hepatol. 2011;26:794-795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Teshima CW, Kuipers EJ, van Zanten SV, Mensink PB. Double balloon enteroscopy and capsule endoscopy for obscure gastrointestinal bleeding: an updated meta-analysis. J Gastroenterol Hepatol. 2011;26:796-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 189] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 49. | Wang SC, Parekh JR, Zuraek MB, Venook AP, Bergsland EK, Warren RS, Nakakura EK. Identification of unknown primary tumors in patients with neuroendocrine liver metastases. Arch Surg. 2010;145:276-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 50. | Imamura M. Recent standardization of treatment strategy for pancreatic neuroendocrine tumors. World J Gastroenterol. 2010;16:4519-4525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 36] [Cited by in F6Publishing: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |