INTRODUCTION
Modification of the N-terminal of chromosomal histone can alter chromatin structure by affecting the affinity between the histone and DNA[1,2]. Histone acetylation is a reversible dynamic process and can be regulated by histone acetyltransferase (HAT) and histone deacetylase (HDAC). Usually, HATs lead to the relaxation of chromatin structures and gene transcriptional activation, while HDACs are associated with chromatin condensation and transcriptional silence. Many studies have shown the close correlation of low histone acetylation or high expression of HDACs with the genesis and development of some tumors[3,4]. The activation of HATs and/or the suppression of HDACs were considered as a new approach to tumor therapy. The antitumor effect of histone deacetylase inhibitors (HDACIs) are attributed mainly to growth inhibition, apoptosis, or the induction of differentiation[5].
Gastric cancer is the second leading cause of cancer death in the world and will likely remain as one of the leading causes of all deaths in the near future[6,7]. In the past few years, evidence has accumulated showing that modification of acetylation status plays a central role in gastric carcinogenesis[8]. 4-phenyl butyric acid (PBA), a short-chain fatty acid, is a commonly used HDACI. However, the antitumor effect of PBA in gastric cancers has not yet been elucidated.
MATERIALS AND METHODS
Cell culture and reagents
Human gastric cancer cell lines, MGC-803 (lowly differentiated) and SGC-7901 (moderately differentiated), were obtained from the Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). MGC-803 and SGC-7901 cells were cultured in RPMI-1640 medium (Life Technologies, Grand Island, NY, United States) supplemented with 10% fetal bovine serum, glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin in a humidified, 5% CO2 in air atmosphere at 37 °C. Exponentially growing cells were used for the experiments.
MTT assay
To observe the effect of PBA on the growth of gastric carcinoma cells, the MTT assay was used. MGC-803 and SGC-7901 cells (1.5 × 104/mL) were added to 96-well plates. Twenty-four hours later, PBA was added at final concentrations of 5, 10, 20, 40 and 60 μmol/L, respectively. Four wells were used for each dose. After the cells were incubated for 24, 48, 72, and 96 h, 20 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution [2 g/L in phosphate buffered saline (PBS)] was added into each of the 96 wells. The cells were incubated at 37 °C for 4 h, the medium was removed, and 150 μL of dimethyl sulfoxide (DMSO) was added to solubilize the formazan. The microplate was shaken on a rotary platform for 10 min. Then, the optical density (OD) values were measured at 490 nm using a Wellscan reader (Labsystems, Santa Fe, NM, United States). The inhibition rate was determined to indicate the suppressive effect of PBA on gastric carcinoma cells. The relative inhibition rate was calculated as a percentage, as follows: (1-Aexperiment/Acontrol) × 100%. Three independent experiments were performed.
Cell cycle analysis
MGC-803 and SGC-7901 cells were treated with PBA at the above-mentioned concentrations for 24 h and 48 h. Then, the cells were collected and washed twice with PBS. Cold 70% ethanol was added and the cells were kept at 4 °C overnight. Subsequently, the cells were rinsed twice with PBS. Finally, the cells were incubated in propidium iodide/RNase Staining Buffer (BD, San Diego, CA, United States) according to the manufacturer’s manual, and the cell cycle was analyzed with a fluorescence-activated cell sorting Calibur Flow Cytometer (BD, San Diego, CA, United States). The percentage of cells in the different phases of the cell cycle (G0/G1, S or G2/M phase) was calculated by the BD FACStationTM Data Management System. Three wells of a 6-well plate were used for each dose. Three independent experiments were performed.
Statistical analysis
Statistical analysis was performed using the Statistical Program for Social Sciences (SPSS) software 13.0 (SPSS Inc, Chicago, IL, United States). Data were expressed as the mean ± SD. One-way analysis of variance (ANOVA) was used to analyze significant differences between control and treatment groups. A P value of less than 0.05 was considered statistically significant.
RESULTS
Effect of 4-phenyl butyric acid on the growth of gastric carcinoma cells
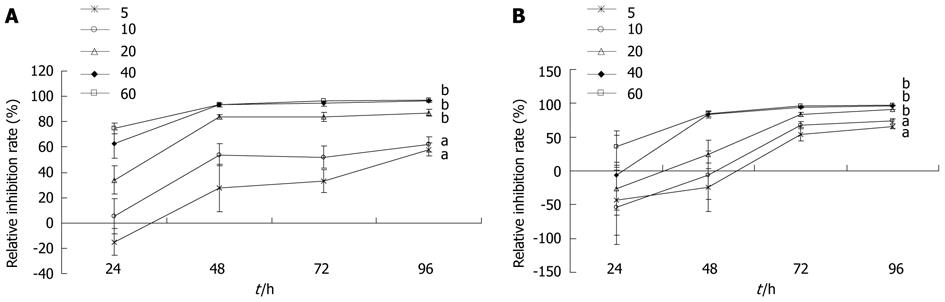
After exposure to PBA for 24, 48, 72, and 96 h, the growth of gastric carcinoma MGC-803 and SGC-7901 cells was inhibited significantly (5 and 10 μmol/L, P < 0.05; 20, 40, and 60 μmol/L, P < 0.01). The inhibitory effect was dose- and time-dependent (Figure 1).
Figure 1 Effects of 4-phenyl butyric acid on the proliferation of gastric carcinoma MGC-803 cells (A) and SGC-7901 cells (B).
Cells were incubated with 4-phenyl butyric acid at various concentrations for 24, 48, 72, and 96 h. The proliferation of cells was determined by the MTT assay. aP < 0.05, bP < 0.01 vs control. The relative inhibition rate was calculated as a percentage, as follows: (1-Aexperiment/Acontrol) × 100%.
Effects of cell cycle distribution of 4-phenyl butyric acid on gastric carcinoma cells
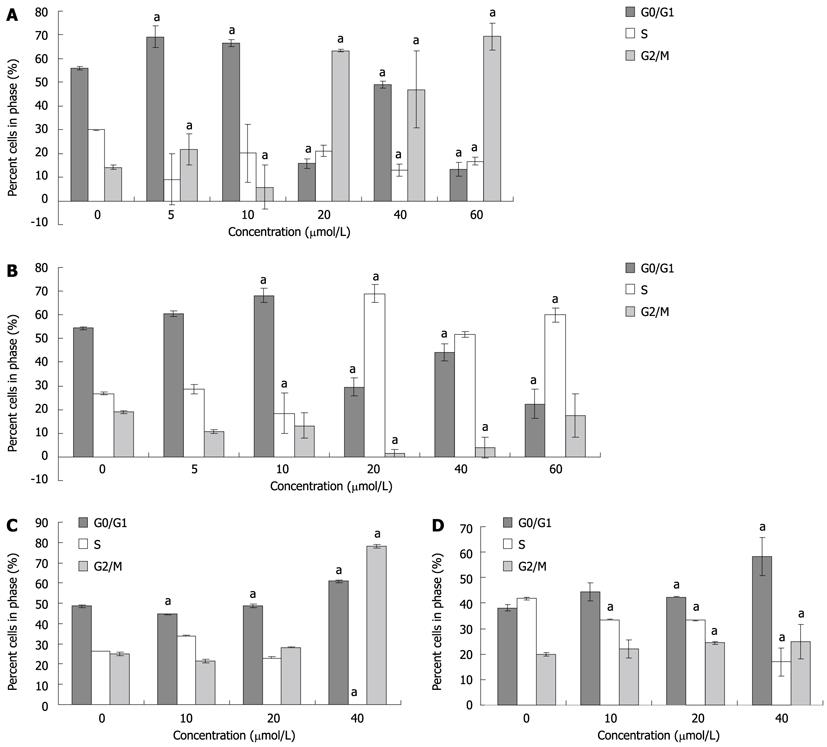
To decipher the suppressive mechanisms of PBA on gastric cancer cells, we monitored the changes in the cell cycle distribution by flow cytometry. The results of 48 h treatments showed that gastric carcinoma cells treated with various concentrations of PBA were arrested at different phases. MGC-803 cells treated with low concentrations (5 and 10 μmol/L) of PBA were arrested at the G0/G1 phase, while those treated with high concentrations (20-60 μmol/L) were arrested at G2/M (Figure 2A). There was a big difference in the cell cycle interruption between MGC-803 and SGC-7901. SGC-7901 cells were arrested at the G0/G1 phase at low concentrations (5 and 10 μmol/L) and at the S phase with high concentrations (Figure 2B). Based on these observations, we investigated another time-course effect. Three doses, 10, 20 and 40 μmol/L, which were among the significant concentrations for both cells at the 48 h treatment, were chosen to treat cells for 24 h. As indicated in Figures 2C and D, similar effects were found.
Figure 2 Effects of 4-phenyl butyric acid on cell cycle distribution of gastric carcinoma cells.
The cell cycle was measured by propidium iodide staining and fluorescence-activated cell sorting analysis. aP < 0.05 vs control. MGC-803 cells (A) and SGC-7901 cells (B) were treated for 48 h; and MGC-803 cells (C) and SGC-7901 cells (D) were treated for 24 h.
DISCUSSION
PBA is one of the HDACIs already tested in clinical trials in the treatment of recurrent malignant gliomas and myelodysplastic syndrome[9,10]. In addition, it is a FDA-approved, and well-tolerated drug for urea cycle disorders[11]. It is converted into phenylacetate (PA) by β-oxidation in liver and kidney mitochondria[12]. It has recently been demonstrated that PBA has various cellular effects, such as induction of differentiation and apoptosis[13]. PBA has also been proved to be an effective chemical compound in preventing gene mutations and in preventing the aggregation of denatured α-lactalbumin and bovine serum albumin[14,15]. Interestingly, PBA treatment results in the induction of apoptosis in prostate cancer cells, medulloblastoma cells and colon cancer cells[12,13-17]. Furthermore, PBA was found to cause the regression of tumors derived from hepatocarcinoma cells in a rat model system[18].
Histone acetylation and DNA methylation represent epigenetic modifications that are essential for chromatin organization and the regulation of gene expression. Histone acetylation leads to an open chromatin structure favoring gene transcription, whereas deacetylation induces transcriptional repression through chromatin condensation[19]. In addition, the function of nonhistone proteins can be modified by acetylation and deacetylation[20]. Aberrant gene expression resulting from epigenetic alterations is critical for tumor development in many tumors, including gastric cancer, and it is also implicated in response to chemotherapy[21,22]. Modulation of chromatin structure has been suggested to influence the accessibility of DNA-targeting drugs such as short-chain fatty acid, and thus to affect the extent of the DNA damage[23-25]. Enzymes involved in these chromatin modifications with opposing activities are the histone acetyltransferases and HDACs. According to the structure, there are four types of HDACIs: hydroxamic acid and analogs, short-chain fatty acids, circum tetrapeptide, and benzamides[26]. PBA, a short-chain fatty acid, has not been used widely against gastric cancer, and its antineoplastic function has not been studied.
The present study has shown that PBA has a time- and dose-dependent effect on the proliferation of gastric carcinoma cells (Figure 1). These effects are similar to those of PBA on prostate cancer[27]. It is known that chemotherapeutic drugs act on the cell cycle of tumor cells. To decipher the growth suppressive mechanisms of PBA on gastric cancer cells, cell cycle distribution was measured by flow cytometry. The results indicated that the cell cycle distributions of two types of gastric carcinoma cells were interrupted by PBA (Figure 2). It was interesting to find that the modes of cell cycle arrests were different when high concentrations of PBA were used. The state of differentiation of MGC-803 and SGC-7901 cells is very different. MGC-803 cells are lowly differentiated, and SGC-7901 cells are moderately differentiated. These effects are similar to those of lycium barbarum polysaccharide (LBP) on MGC-803 and SGC-7901 cells. Miao et al[28] found that LBP treatment inhibited the growth of MGC-803 and SGC-7901 cells, with cell-cycle arrest at the G0/G1 and S phase, respectively. The cell cycles are regulated by the cell cycle-associated proteins, cyclin and cyclin-dependent kinase (CDK). Cyclin A binds and activates CDK2, and thus promotes both G1/S and G2/M transitions in the cell cycle[29]. For SGC-7901 cells, LBP increased the expression of cyclin A and decreased the expression of CDK2, and thus arrested cells at the S phase[28]. At the G0/G1 phase, the main cell cycle regulators are cyclin D, cyclin E, and CDK2[30]. For MGC-803 cells, LBP decreased the expressions of cyclin D, cyclin E, and CDK2[28]. These results are consistent with the effect of LBP on the arrest of the MGC-803 cell cycle at the G0/G1 phase.
In summary, our study identified PBA effects on gastric cancer cell growth. These observations may be correlated with the suppression of the cell cycle, and they suggest that an HDAC inhibitor such as PBA may be a potential anti-cancer drug.
COMMENTS
Background
Gastric cancer is the second leading cause of cancer death in the world and will likely remain as one of the leading causes of all deaths in the near future. Evidence has accumulated showing that modification of acetylation status plays a central role in gastric carcinogenesis.
Research frontiers
4-phenyl butyric acid (PBA), a short-chain fatty acid, is a commonly used histone deacetylase inhibitor (HDACI). However, the antitumor effect of PBA in gastric cancer has not yet been elucidated. In this study, the authors demonstrate that the proliferation of gastric carcinoma cells was inhibited by PBA in a dose- and time-dependent fashion.
Innovations and breakthroughs
Recent reports have highlighted the importance of PBA in the growth inhibitory effects on prostate cancer cells. This is the first study to report that PBA also suppresses the growth of gastric cancer cells. Furthermore, our studies show that the cell cycle arrest of PBA in gastric cancer cells is different between differentiated level of cancer cells.
Applications
By understanding how PBA inhibits the growth of gastric cancer cells, this study may represent a future strategy for therapeutic intervention in the treatment of patients with gastric adenocarcinoma.
Terminology
Histone acetylation is a reversible dynamic process which can be regulated by histone acetyltransferase and histone deacetylase (HDAC). Many studies have shown the close correlation of low histone acetylation or high expression of HDACs with the genesis and development of some tumors. Unsurprisingly, the use of PBA, a HDACI, will suppress the growth of gastric cancer cells.
Peer review
The authors assessed the inhibitory effect of PBA on human gastric cancer cells. They showed that PBA had growth inhibitory effects in a dose- and time-dependent manner. They also found cell cycle arrest was different between differentiated levels of gastric cancer cells. Advanced gastric cancer is a high mortality tumor as the result of repeated anti-cancer therapy. A further therapeutic strategy is needed to raise the remission rate of gastric cancer. The approach of the authors in this study is one of the next generation therapies for gastric cancer. Therefore, the concept of this study is reasonable and important. It is interesting that the inhibitory effect was different between differentiated levels of gastric cancer cells. Also, methodology is interesting to investigate the mechanism of inhibitory effect of HDACIs.