Published online Jan 28, 2011. doi: 10.3748/wjg.v17.i4.514
Revised: September 18, 2010
Accepted: September 25, 2010
Published online: January 28, 2011
AIM: To investigate sensations to multimodal pain stimulation in the metaplastic and normal parts of the esophagus in patients with Barrett’s esophagus (BE).
METHODS: Fifteen patients with BE and 15 age-matched healthy volunteers were subjected to mechanical, thermal and electrical pain stimuli of the esophagus. Both the metaplastic part and the normal part (4 and 14 cm, respectively, above the esophago-gastric junction) were examined. At sensory thresholds the stimulation intensity, referred pain areas, and evoked brain potentials were recorded.
RESULTS: Patients were hyposensitive to heat stimulation both in the metaplastic part [median stimulation time to reach the pain detection threshold: 15 (12-34) s vs 14 (6-23) s in controls; F = 4.5, P = 0.04] and the normal part of the esophagus [median 17 (6-32) s vs 13 (8-20) s in controls; F = 6.2, P = 0.02]. Furthermore, patients were hyposensitive in the metaplastic part of the esophagus to mechanical distension [median volume at moderate pain: 50 (20-50) mL vs 33 (13-50) mL in controls; F = 5.7, P = 0.02]. No indication of central nervous system abnormalities was present, as responses were comparable between groups to electrical pain stimuli in the metaplastic part [median current evoking moderate pain: 13 (6-26) mA vs 12 (9-24) mA in controls; F = 0.1, P = 0.7], and in the normal part of the esophagus [median current evoking moderate pain: 9 (6-16) mA, vs 11 (5-11) mA in controls; F = 3.4, P = 0.07]. Furthermore, no differences were seen for the referred pain areas (P-values all > 0.3) or latencies and amplitudes for the evoked brain potentials (P-values all > 0.1).
CONCLUSION: Patients with BE are hyposensitive both in the metaplastic and normal part of esophagus likely as a result of abnormalities affecting peripheral nerve pathways.
- Citation: Krarup AL, Olesen SS, Funch-Jensen P, Gregersen H, Drewes AM. Proximal and distal esophageal sensitivity is decreased in patients with Barrett’s esophagus. World J Gastroenterol 2011; 17(4): 514-521
- URL: https://www.wjgnet.com/1007-9327/full/v17/i4/514.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i4.514
Barrett’s esophagus (BE) is characterized by intestinalized columnar epithelium in the esophageal mucosa, and patients have an elevated risk of developing esophageal adenocarcinoma[1]. The etiology is still not fully elucidated, but is thought to involve excessive acid exposure[2]. Abnormal acid exposure of the esophageal mucosa can be the result of increased reflux or slow esophageal clearing of acid. Several studies have confirmed that patients with BE have pathologic gastro-esophageal reflux and that the length of the Barrett’s segment is correlated to reflux duration[3,4]. However, the magnitude of pathological reflux in patients with BE and patients with erosive reflux disease without BE seems to be similar[5]. Thus the progression to BE has yet to be clarified. The missing link could be slow clearance of acid and non acidic reflux from the esophagus due to a defect in the afferent (sensory) or efferent (motor) part of the reflex arch. This will reduce clearance due to less saliva production and less secondary contractions. When studying the motility related to BE, conflicting results have been found[6-8]. More consistency is seen in studies of esophageal sensation, where most studies have reported a decreased sensitivity to esophageal acid in patients with BE[8-11]. It is not yet known whether this change in esophageal sensation is a result of the characteristics of the metaplastic mucosa or whether it is present before development of the metaplasia. If the first assumption is true only the metaplastic part of the esophagus will be hyposensitive. However, if the hyposensitivity is a predisposing pathogenetic factor it will be expected to be present both in the metaplastic distal part and the normal proximal part of the esophagus, but this has never been investigated.
Esophageal sensitivity can be examined on several levels and with many stimulation modalities using the multimodal pain model previously developed in our group[12]. The model has been used to study pain mechanisms in patients with erosive esophagitis, non-erosive esophagitis and non-cardiac chest pain, thereby proving its validity[13-15]. We hypothesized that patients with BE are hyposensitive both in the metaplastic part and in the normal proximal part of the esophagus. Using the multimodal pain model in patients and healthy volunteers, the aims were as follows: (1) to compare the sensory response to thermal and mechanical pain stimuli in the metaplastic and normal part of the esophagus; and (2) to determine from the response to electrical stimuli, referred pain areas and evoked brain potentials, whether the origin of symptoms in BE is suggestive of peripheral or central nervous system (CNS) abnormalities.
The study was conducted according to the Declaration of Helsinki and approved by the local Ethical Committee (VN 2003/120mch). Oral and written informed consent was obtained from all participants.
Fifteen patients with BE aged 18-70 years were selected from our outpatient unit. All patients had typical endoscopical features of BE confirmed by histology within the last 2 years. Patients with a hiatus hernia rated by endoscopists as moderate or large were excluded as were patients who previously had esophageal surgery. In addition, we excluded patients with concomitant painful visceral diseases or other critical medical, surgical, or psychiatric illness. Patients were compared to a control group of 15 age- and sex-matched healthy volunteers.
Before the study all subjects were instructed in the use of a 0-10 visual analogue scale, where 1 = sensation threshold; 3 = vague perception of moderate sensation; 5 = pain threshold; and 7 = moderate pain. This scale has previously been described in detail, and has been shown to be reliable in discriminating sensations in the esophagus[16]. The stimulation intensities at these defined sensory levels were measured for all stimulation modalities.
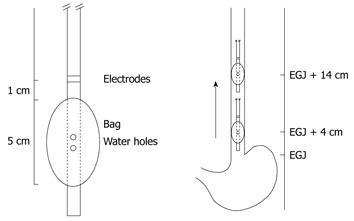
The multimodal probe is illustrated in Figure 1. A custom-made esophageal probe measuring 60 cm in length with a diameter of 6.2 mm was fitted with a bag near the tip (Ditens A/S, Aalborg, Denmark). The bag was made of 25 μm thick polyether urethanes had a maximum volume of 80 mL and was 5 cm long. An inlet and an outlet channel permitted recirculation of water in the bag during mechanical and thermal stimulation. A temperature wire with a tip sensor was placed in a separate channel for continuous temperature assessment during stimulation (TC Ltd., Uxbridge, England). Stainless steel electrodes for electrical stimuli were mounted on the probe 1 cm proximal to the bag. Detailed information on the probe and stimulation system has been described previously[12].
No analgesics or other medication potentially interfering with pain assessments were allowed in the 24 h prior to experimental testing. Treatment with proton pump inhibitors was stopped 4 d before the study day and the subjects fasted 4 h prior to probe intubation. The probe was inserted through the mouth. The bag close to the tip of the probe was positioned in the stomach and filled with 25 mL water. Retraction of the probe until resistance was met identified the position of the esophago-gastric junction (EGJ) corresponding to the middle of the bag. Finally, the bag was emptied and retracted 4 cm to ensure placement above the EGJ and the probe was taped to the chin.
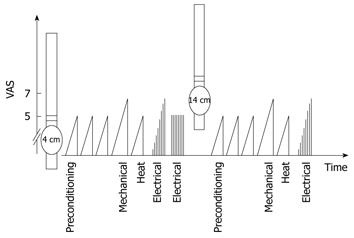
Figure 2 shows the stimulation protocol. Initially, three mechanical distensions were applied to precondition the tissue and to train the subjects in assessing sensations from the esophagus. These distensions were stopped at the pain threshold and have previously been shown to facilitate discrimination of the different sensory ratings[17]. Hereafter, pain tests were initiated: mechanical distension until moderate pain, heat stimulation to the pain threshold, and electrical stimulation until moderate pain. The participants outlined the area of referred pain after each stimulation type. Recordings of pain-specific evoked brain potentials were performed twice following 50 electrical stimulations at the pain threshold. The probe was then retracted 10 cm corresponding to 14 cm above the EGJ, and the mechanical, thermal and electrical stimulations were repeated after a break of 10 min.
Mechanical distension was done by inflating the bag with water (37°C) at a rate of 15 mL/min using a syringe pump (PHD 22/2000, Harvard Apparatus, Holliston, Massachusetts, USA). The maximum volume was set at 50 mL for safety reasons. The bag cross-sectional area, pressure and volume were continuously registered and stored for later analysis. If the maximum of 50 mL failed to evoke moderate pain, the remaining sensory levels were assigned a volume of 50 mL.
Heat stimulation was done by recirculating heated water (60°C) using the above described pump at a rate of 180 mL/min. The stimulation was terminated at the pain threshold as a safety precaution and has been described in detail previously[18]. Time from stimulation onset until the patient reached the predefined sensory levels was measured.
The sensory response to (1) electrical stimulation; (2) the size of the referred pain area; and (3) the pain specific evoked brain potentials served as proxies for abnormalities in CNS pain processing. When the CNS is sensitized clinically or experimentally these measurements change with a decrease in pain threshold, increase in referred pain area and shortened latency of the evoked brain potential[18-20].
Electrical stimulation: Mucosal contact was secured by keeping the inter-electrode impedance below 3 kΩ during stimulations. A computer-controlled constant-current stimulator (University of Aalborg, Denmark) delivered the electrical stimulations to the esophagus[12]. A single stimulus consisted of five square-form constant-current pulses at 200 Hz with duration of 1 ms, and each stimulation in total had a duration of 25 ms. The current intensity was increased in 0.5 mA increments with random sham stimulations having the same or lower intensity until moderate pain was evoked. Calculations were based on current at the predefined sensory levels.
Referred pain area: After each stimulation the subjects outlined their referred pain area on transparent paper. The size of the referred pain area was later digitized (Trust, 1200 wireless tablet, Trust International BV, Dordrecht, The Netherlands).
Electroencephalographic recordings and evoked brain potentials: The electroencephalographic (EEG) was recorded with a 64 surface electrode cap (Quick-Cap, Neuroscan, El Paso, TX, USA) and an amplifier (Nuamp, Neuroscan, Compumedics, Hamburg, Germany). Four additional electrodes for eye movement detection were used. Electrode gel was applied to reduce the electrode impedance below 8.2 kΩ.
Fifty identical esophageal stimulations were applied at 0.2 Hz with a current intensity corresponding to the pain threshold. The subjects relaxed quietly with open eyes in light-dimmed room. The EEG signals were recorded in continuous mode with a sampling rate of 1000 Hz and the online notch filter switched off (SynAmp, Neuroscan, El Paso, TX, USA).
The evoked potentials from each session were analyzed offline (Neuroscan software v 4.3.1, Neuroscan, El Paso, TX, USA) and stimulations containing artefacts (from eye blinking or movements) were rejected. Amplitudes and latencies for the evoked potentials were obtained from the vertex electrode (Cz) with the electrodes above the ears (TP7 and TP8) as reference. Data was processed in the following steps: (1) zero-phase notch filtering (49-51 Hz) with a filter order of 24; (2) zero-phase band-pass filtering (1-70 Hz) with a filter order of 12; (3) epoching in the time window 50 ms prestimulus to 350 ms post-stimulus: (4) baseline correction; (5) linear detrending; (6) rejecting artefact sweeps manually, and (7) calculating average of accepted sweeps. Peak latencies to negative (N) and positive (P) deflections (N1, P1, N2, and P2) and peak-to-peak amplitudes were extracted for each subject.
Normality was checked by QQ-plots and the assumption of variance homogeneity by the Levene median test. Descriptive statistics for data that were normally distributed are reported as mean (standard deviation), not normally distributed data as median (range). The Student t-test was used for measurements with single endpoints and the Mann-Whitney Rank Sum test for non-parametric data. Two-way analysis of variance was used for measurements where stimulus intensities were measured at several sensory thresholds. The Holm-Sidak method was chosen for post hoc comparisons[21]. The chi-square test compared the dichotomous data. SigmaPlot 11.0 statistical software was used and P < 0.05 was considered statistically significant.
Thirty-three patients were invited to participate: 17 patients declined and 16 accepted. One of the 16 patients could not tolerate the probe and was excluded. The 15 remaining patients (12 male) had a mean age of 54 years (range, 25-68 years) and mean body mass index (BMI) of 30 kg/m2 (range, 23-56 kg/m2). Seven patients had long segment BE and the remaining patients had short segment BE, e.g. less than 3 cm. Three patients did not complete the protocol 14 cm above the EGJ due to nausea evoked by the mechanical distension. Mechanical and thermal data from one patient were discarded due to technical errors.
The patients were compared to 15 healthy volunteers (10 male) with a mean age of 47 years (range, 28-63 years) (P = 0.07), and mean BMI of 25 kg/m2 (range, 19-30 kg/m2) (P = 0.1). Volunteers were recruited among hospital staff and had no gastrointestinal symptoms. All volunteers completed the protocol. No adverse events were observed.
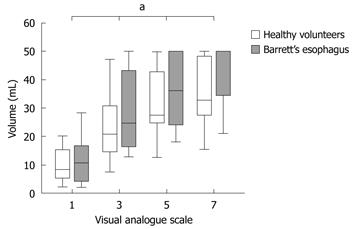
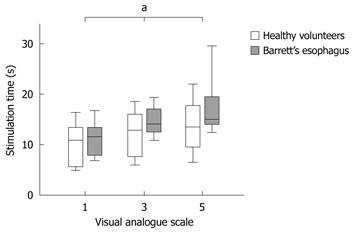
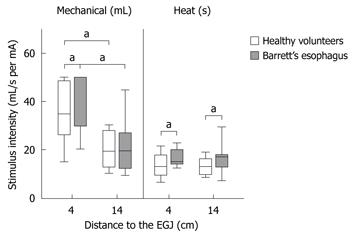
It was possible to evoke moderate pain in 13 of 15 healthy volunteers compared to only 5 of 14 patients when stimulating mechanically in the metaplastic part 4 cm above the EGJ (P = 0.01). Four of these hyposensitive patients, but none of the controls, responded with nausea or vomiting rather than pain (P = 0.03). To achieve moderate pain, patients with BE required higher mechanical stimulus intensity compared to healthy volunteers (F = 5.7, P = 0.02; Table 1, Figure 3). Furthermore, patients required heat stimulation for a longer time to reach the pain threshold (F = 4.5, P = 0.04; Table 1, Figure 4).
| VAS level | Patients | Controls | ||||||
| 1 | 3 | 5 | 7 | 1 | 3 | 5 | 7 | |
| Heat (s) | ||||||||
| 4 cm1 | 12 (7-18) | 14 (11-20) | 15 (12-34) | NA | 11 (5-18) | 13 (6-20) | 14 (6-23) | NA |
| 14 cm1 | 12 (5-14) | 15 (6-24) | 17 (6-32) | NA | 9 (4-17) | 11 (7-18) | 13 (8-20) | NA |
| Mechanical (mL) | ||||||||
| 4 cm1 | 11 (1-38) | 25 (12-50) | 36 (18-50) | 50 (20-50) | 8 (2-24) | 21 (6-50) | 28 (10-50) | 33 (13-50) |
| 14 cm | 5 (3-17) | 14 (9-26) | 18 (10-34) | 25 (11-46) | 5 (1-27) | 14 (4-40) | 18 (8-44) | 19 (10-46) |
| Electrical (mA) | ||||||||
| 4 cm | 7 (4-17) | 9 (5-21) | 12 (6-25) | 13 (6-26) | 7 (4-15) | 8 (5-20) | 10 (8-21) | 12 (9-24) |
| 14 cm | 4 (2-6) | 5 (3-10) | 7 (5-13) | 9 (6-16) | 4 (2-9) | 6 (4-12) | 8 (4-13) | 11 (5-15) |
Patients with BE were also hyposensitive to heat in the normal part of esophagus 14 cm above the EGJ (F = 6.2, P = 0.02), but not to mechanical distension (F = 0.2, P = 0.6) (Table 1, Figure 5).
Both patients and controls were less sensitive to mechanical pain stimulation 4 cm above compared to 14 cm above the EGJ (patients with BE: F = 26.9, P < 0.001; controls: F = 25.2, P < 0.001) (Table 1, Figure 5). In contrast, no differences in sensitivity were found for thermal pain stimuli comparing stimulations 4 cm and 14 cm above the EGJ (patients with BE: F = 0.3 P = 0.6; controls: F = 1.1, P = 0.3) (Table 1, Figure 5).
Electrical stimulation: No differences between patients and controls were found for the response to electrical pain stimuli in the metaplastic part 4 cm above the EGJ (F = 0.1, P = 0.7) (Table 1). Furthermore, no differences were found for the normal area 14 cm above the EGJ (F = 3.4, P = 0.07) (Table 1). Within groups, the area 4 cm above the EGJ was less sensitive to current compared to the area 14 cm above (patients with BE: F = 32.5, P < 0.001; controls: F = 21.7, P < 0.001) (Table 1).
Referred pain areas: Referred pain areas to the following stimulations did not differ between patient and control groups in the metaplastic part: mechanical [median 8.9 cm2 (0.8-66.6) vs controls 14.9 cm2 (2.3-38.6), P = 0.5], thermal [mean 23.7 cm2 (20.7) vs controls 16.0 cm2 (14.8), P = 0.3], and electrical [median 2.3 cm2 (0.1-33.4) vs controls 3.2 cm2 (0.2-40.7), P = 0.7]. Also in the upper part of the esophagus the results from both patients and controls were comparable: referred pain area in response to mechanical [median 14.5 cm2 (2.1-72.9) vs controls 10.0 cm2 (1.0-53.5), P = 0.4], thermal [mean 23.2 cm2 (22.8) vs controls 19.5 cm2 (23.2), P = 0.5], and electrical stimulation [median 20.6 cm2 (2.9-70.0) vs controls 15.1 cm2 (1.2-92.2), P = 1.0].
Recordings of evoked brain potential: No differences were found between patients and controls for the evoked brain potentials (F = 2.7, P = 0.1) (Table 2).
| Patients | Controls | |
| N1 latency (ms) | 76 (20) | 69 (14) |
| P1 latency (ms) | 105 (19) | 106 (15) |
| N2 latency (ms) | 177 (15) | 172 (25) |
| P2 latency (ms) | 255 (35) | 266 (39) |
| N1P1 amplitude (mV) | 2.5 (2.5) | 3.0 (1.7) |
| P1N2 amplitude (mV) | 6.8 (5.1) | 9.3 (5.5) |
| N2P2 amplitude (mV) | 7.6 (5.9) | 12.6 (7.6) |
In this study the main findings were that patients with BE were hyposensitive to heat stimulation both in the metaplastic and normal parts of the esophagus. Furthermore, they were hyposensitive to noxious mechanical distension in the metaplastic part of the esophagus and many reported nausea rather than pain in response to noxious stimulations. The hyposensitivity is most likely a result of abnormalities affecting peripheral nerves, since there were no differences between groups for the response to the stimuli reflecting central pain processing.
In clinical assessment, characterization of symptoms may be confounded by psychological, cognitive, and social aspects of the illnesses. To encompass these problems, human experimental pain models have been developed. In this study, we used one of these models: the multimodal pain model that has proven reliable as an esophageal research tool[18,22]. An abnormal response to one or more of the stimulation modalities indicate pathology in different receptors or visceral nerve pathways[23,24]. Validity has been proven in studies of pain mechanisms in erosive esophagitis, non-erosive esophagitis and non-cardiac chest pain proving its validity[13-15]. Patients with both short and long segment BE were included. When stimulating the “metaplastic area”, the balloon reached from 1.5-6.5 cm above the EGJ, therefore patients with short segment BE were stimulated partly in an area with normal mucosa and partly in the metaplastic area. However, as the patients were hyposensitive both low and high in the esophagus, bias was not a problem in the present study. The controls were slightly but non significantly leaner than the patients, but this is not thought to have influenced the results.
To our knowledge, studies examining thermal sensation in BE have not previously been conducted. In the current study, patients were hyposensitive both in the metaplastic part of the esophagus as well as in the normal control area 14 cm above the EGJ. The hyposensitivity in BE patients is interesting as these patients often have pathological reflux and could be expected to have sensory thresholds comparable to patients with gastro-esophageal reflux disease. However, both patients with erosive and non-erosive reflux disease have previously been shown to be hypersensitive to heat and have signs of CNS abnormalities underlining the differences between them and patients with BE[13,15]. The hyposensitivity to heat in the normal part of the esophagus in BE patients suggests that heat hyposensitivity may be generalized in the esophagus and therefore independent of the metaplastic mucosa. Hence, it can be hypothesized that dysfunction of the afferent pathways may be one of several pathogenetic factors in BE. This fits well with previous studies that have demonstrated hyposensitivity to acid in patients with BE, and several acid sensitive receptors could potentially be involved[5,9,10,25]. One of them - the transient receptor potential vanilloid 1 (TRPV1) - is of special interest as it is activated by heat and acid[26]. Although speculative, the hyposensitivity to both heat and acid found in patients with BE could therefore be due to a defect or downregulation of TRPV1 receptors in the esophagus. A study involving assessment of heat and acid sensitivity as well as TRPV1 quantification in biopsies could elucidate this further.
Trimble and coworkers found that patients with BE were mechanically hyposensitive, but their data may have been biased by the large age difference between the patients and the control group[27,28]. Compared with an age-matched control group we also found patients with BE to be hyposensitive to mechanical stimulation in the metaplastic area 4 cm above the EGJ. Some patients developed nausea rather than pain in response to noxious esophageal distension. It should be kept in mind that nausea rather than pain for some BE patients may be their only “warning response” to noxious distension, and further studies are needed to establish if nausea is a predictor of serious disease.
We found no differences for the response to mechanical distension between patients and controls in the area with normal mucosa 14 cm above the EGJ. There were four dropouts and a statistical type 2 error could be responsible. Hence, it is not possible to conclude from this study whether mechanical hyposensitivity is present in the normal part of the esophagus.
Changes in the sensory response to electrical stimulation, referred pain areas, and evoked brain potentials has been shown to reflect changes in the CNS, such as seen experimentally after esophageal acid perfusion[18-20]. Electrical stimulation bypasses the receptors stimulating the nerve fibers directly, and the sensitivity increases after sensitization. Referred pain areas increase, possibly as a result of stimulation of sensitized neurons receiving converging neural information from the esophageal nerves and the somatic nerves in the chest. Pain specific EEG recordings evoked brain potentials are believed to change due to faster spinal conduction and altered brain processing. Such changes have been reported in erosive reflux disease, non-erosive reflux disease and non-cardiac chest pain, where central sensitization is thought to play a role in symptom generation[24,29]. We found comparable results in patients with BE and controls to electrical stimulation, referred pain areas, and waveforms of evoked brain potentials. Consequently, the cause of sensory changes in patients with BE is probably not found in the CNS, but rather at receptor level in the peripheral nervous tissue.
We measured differences between the sensory responses evoked 4 and 14 cm above the EGJ and found that both the patients and the controls were more sensitive to mechanical and electrical stimuli 14 cm above the EGJ. Patel et al[30] previously found the same in healthy volunteers for mechanical stimulation and an explanation could be a greater number of nociceptors and mechanoreceptors proximal in the esophagus as seen in animal studies[31,32]. However, other nociceptors seem to be responsible for heat sensation as no differences in heat sensitivity was observed at the two esophageal levels.
In conclusion, patients with BE are hyposensitive both in the metaplastic and normal part of the esophagus likely as a result of abnormalities affecting peripheral nerves. Hence, hyposensitivity is likely not to be a consequence of sensory changes due to the metaplastic epithelium, but may be a pathogenetic factor in the development of the disease.
Barrett’s esophagus (BE) is a complication of long-term severe acid reflux to the esophagus and patients are at higher risk of developing esophageal cancer. In several studies it has been demonstrated that patients with BE are less sensitive to acid than healthy controls. It is not known if this decreased sensitivity precedes the development of the condition or if it is a result of the mucosa changes of the disease. This study investigated the sensitivity in the esophagus of patients with BE in comparison to healthy controls. The esophagus was examined both in the part where the disease was present and in the normal part. Furthermore, the pain processing of the spinal cord and brain was examined.
In the patients, a decreased sensitivity to heat both in the normal part and the diseased part of esophagus was demonstrated. Distension was also felt later in the diseased part of esophagus. The spinal cord and brain was not responsible for the decreased sensitivity as they had a normal reaction to esophageal pain. The decreased sensitivity in the normal part of the esophagus could indicate that sensory changes are present before development of BE.
Results of this study has led to a better understanding of the disease and may, in the future, help to identify subjects with a higher risk of developing this disease. When identified, a more aggressive treatment of their acid reflux can be chosen and perhaps thereby prevent development of BE.
BE is a metaplastic mucosal change in the distal esophagus resulting from long term acid exposure.
This is an excellent work, carefully done and very well interpreted. The data are extremely original and represent a major breakthrough in the understanding of hyposensitivity in BE.
Peer reviewer: Jean Paul Galmiche, MD, Professor, Department of Gastroenterology and Hepatology, Hôpital Hôtel Dieu, 44093 Nantes cedex, France
S- Editor Sun H L- Editor Cant MR E- Editor Zheng XM
| 1. | Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241-2252. [Cited in This Article: ] |
| 2. | Shaheen NJ, Richter JE. Barrett's oesophagus. Lancet. 2009;373:850-861. [Cited in This Article: ] |
| 3. | Fass R, Hell RW, Garewal HS, Martinez P, Pulliam G, Wendel C, Sampliner RE. Correlation of oesophageal acid exposure with Barrett's oesophagus length. Gut. 2001;48:310-313. [Cited in This Article: ] |
| 4. | Oberg S, DeMeester TR, Peters JH, Hagen JA, Nigro JJ, DeMeester SR, Theisen J, Campos GM, Crookes PF. The extent of Barrett's esophagus depends on the status of the lower esophageal sphincter and the degree of esophageal acid exposure. J Thorac Cardiovasc Surg. 1999;117:572-580. [Cited in This Article: ] |
| 5. | Schlesinger PK. Diminished esophageal acid sensitivity, in patients with advanced esophagitis. Gastroenterology. 1987;92:1622. [Cited in This Article: ] |
| 6. | Gutschow CA, Bludau M, Vallböhmer D, Schröder W, Bollschweiler E, Hölscher AH. NERD, GERD, and Barrett's esophagus: role of acid and non-acid reflux revisited with combined pH-impedance monitoring. Dig Dis Sci. 2008;53:3076-3081. [Cited in This Article: ] |
| 7. | Király A, Süto G, Czimmer J, Horváth OP, Mózsik G. Failure of capsaicin-containing red pepper sauce suspension to induce esophageal motility response in patients with Barrett's esophagus. J Physiol Paris. 2001;95:197-200. [Cited in This Article: ] |
| 8. | Orr WC, Lackey C, Robinson MG, Johnson LF, Welsh JD. Esophageal acid clearance during sleep in patients with Barrett's esophagus. Dig Dis Sci. 1988;33:654-659. [Cited in This Article: ] |
| 9. | Ortiz A, Martínez de Haro LF, Parrilla P, Molina J, Bermejo J, Munitiz V. 24-h pH monitoring is necessary to assess acid reflux suppression in patients with Barrett's oesophagus undergoing treatment with proton pump inhibitors. Br J Surg. 1999;86:1472-1474. [Cited in This Article: ] |
| 10. | Ronkainen J, Aro P, Storskrubb T, Johansson SE, Lind T, Bolling-Sternevald E, Vieth M, Stolte M, Talley NJ, Agréus L. Prevalence of Barrett's esophagus in the general population: an endoscopic study. Gastroenterology. 2005;129:1825-1831. [Cited in This Article: ] |
| 11. | Stein HJ, Hoeft S, DeMeester TR. Functional foregut abnormalities in Barrett's esophagus. J Thorac Cardiovasc Surg. 1993;105:107-111. [Cited in This Article: ] |
| 12. | Drewes AM, Schipper KP, Dimcevski G, Petersen P, Andersen OK, Gregersen H, Arendt-Nielsen L. Multimodal assessment of pain in the esophagus: a new experimental model. Am J Physiol Gastrointest Liver Physiol. 2002;283:G95-G103. [Cited in This Article: ] |
| 13. | Drewes AM, Reddy H, Pedersen J, Funch-Jensen P, Gregersen H, Arendt-Nielsen L. Multimodal pain stimulations in patients with grade B oesophagitis. Gut. 2006;55:926-932. [Cited in This Article: ] |
| 14. | Drewes AM, Pedersen J, Reddy H, Rasmussen K, Funch-Jensen P, Arendt-Nielsen L, Gregersen H. Central sensitization in patients with non-cardiac chest pain: a clinical experimental study. Scand J Gastroenterol. 2006;41:640-649. [Cited in This Article: ] |
| 15. | Reddy H, Staahl C, Arendt-Nielsen L, Gregersen H, Drewes AM, Funch-Jensen P. Sensory and biomechanical properties of the esophagus in non-erosive reflux disease. Scand J Gastroenterol. 2007;42:432-440. [Cited in This Article: ] |
| 16. | Drewes AM, Gregersen H, Arendt-Nielsen L. Experimental pain in gastroenterology: a reappraisal of human studies. Scand J Gastroenterol. 2003;38:1115-1130. [Cited in This Article: ] |
| 17. | Drewes AM, Pedersen J, Liu W, Arendt-Nielsen L, Gregersen H. Controlled mechanical distension of the human oesophagus: sensory and biomechanical findings. Scand J Gastroenterol. 2003;38:27-35. [Cited in This Article: ] |
| 18. | Olesen AE, Staahl C, Brock C, Arendt-Nielsen L, Drewes AM. Evoked human oesophageal hyperalgesia: a potential tool for analgesic evaluation? Basic Clin Pharmacol Toxicol. 2009;105:126-136. [Cited in This Article: ] |
| 19. | Sami SA, Rössel P, Dimcevski G, Nielsen KD, Funch-Jensen P, Valeriani M, Arendt-Nielsen L, Drewes AM. Cortical changes to experimental sensitization of the human esophagus. Neuroscience. 2006;140:269-279. [Cited in This Article: ] |
| 20. | Sarkar S, Hobson AR, Furlong PL, Woolf CJ, Thompson DG, Aziz Q. Central neural mechanisms mediating human visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1196-G1202. [Cited in This Article: ] |
| 21. | Drewes AM, Reddy H, Staahl C, Funch-Jensen P, Arendt-Nielsen L, Gregersen H, Lundbye-Christensen S. Statistical modeling of the response characteristics of mechanosensitive stimuli in the human esophagus. J Pain. 2005;6:455-462. [Cited in This Article: ] |
| 22. | Staahl C, Reddy H, Andersen SD, Arendt-Nielsen L, Drewes AM. Multi-modal and tissue-differentiated experimental pain assessment: reproducibility of a new concept for assessment of analgesics. Basic Clin Pharmacol Toxicol. 2006;98:201-211. [Cited in This Article: ] |
| 23. | Drewes AM, Reddy H, Staahl C, Pedersen J, Funch-Jensen P, Arendt-Nielsen L, Gregersen H. Sensory-motor responses to mechanical stimulation of the esophagus after sensitization with acid. World J Gastroenterol. 2005;11:4367-4374. [Cited in This Article: ] |
| 24. | Hollerbach S, Bulat R, May A, Kamath MV, Upton AR, Fallen EL, Tougas G. Abnormal cerebral processing of oesophageal stimuli in patients with noncardiac chest pain (NCCP). Neurogastroenterol Motil. 2000;12:555-565. [Cited in This Article: ] |
| 25. | Holzer P. Taste receptors in the gastrointestinal tract. V. Acid sensing in the gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2007;292:G699-G705. [Cited in This Article: ] |
| 26. | Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816-824. [Cited in This Article: ] |
| 27. | Lasch H, Castell DO, Castell JA. Evidence for diminished visceral pain with aging: studies using graded intraesophageal balloon distension. Am J Physiol. 1997;272:G1-G3. [Cited in This Article: ] |
| 28. | Trimble KC, Pryde A, Heading RC. Lowered oesophageal sensory thresholds in patients with symptomatic but not excess gastro-oesophageal reflux: evidence for a spectrum of visceral sensitivity in GORD. Gut. 1995;37:7-12. [Cited in This Article: ] |
| 29. | Sarkar S, Aziz Q, Woolf CJ, Hobson AR, Thompson DG. Contribution of central sensitisation to the development of non-cardiac chest pain. Lancet. 2000;356:1154-1159. [Cited in This Article: ] |
| 30. | Patel RS, Rao SS. Biomechanical and sensory parameters of the human esophagus at four levels. Am J Physiol. 1998;275:G187-G191. [Cited in This Article: ] |
| 31. | Rodrigo J, Hernández J, Vidal MA, Pedrosa JA. Vegetative innervation of the esophagus. II. Intraganglionic laminar endings. Acta Anat (Basel). 1975;92:79-100. [Cited in This Article: ] |
| 32. | Sengupta JN, Kauvar D, Goyal RK. Characteristics of vagal esophageal tension-sensitive afferent fibers in the opossum. J Neurophysiol. 1989;61:1001-1010. [Cited in This Article: ] |