Published online Oct 7, 2011. doi: 10.3748/wjg.v17.i37.4235
Revised: August 1, 2011
Accepted: August 8, 2011
Published online: October 7, 2011
AIM: To compare non-liquid and clear-liquid diets, and to assess whether the latter is the optimal treatment for mild acute pancreatitis.
METHODS: The Cochrane Library, PUBMED, EMBASE, EBM review databases, Science Citation Index Expanded, and several Chinese databases were searched up to March 2011. Randomized controlled trials (RCTs) that compared non-liquid with clear-liquid diets in patients with mild acute pancreatitis were included. A meta-analysis was performed using available evidence from RCTs.
RESULTS: Three RCTs of adequate quality involving a total of 362 participants were included in the final analysis. Compared to liquid diet, non-liquid diet significantly decreased the length of hospitalization [mean difference (MD): 1.18, 95% CI: 0.82-1.55; P﹤0.00001] and total length of hospitalization (MD: 1.31, 95% CI: 0.45-2.17; P = 0.003). The subgroup analysis showed solid diet was more favorable than clear liquid diet in the length of hospitalization, with a pooled MD being -1.05 (95% CI: -1.43 to -0.66; P﹤0.00001). However, compared with clear liquid diet, both soft and solid diets did not show any significant differences for recurrence of pain after re-feeding, either alone [relative risk (RR): 0.95; 95% CI: 0.51-1.87; P = 0.88] and (RR: 1.22; 95% CI: 0.69-2.16; P = 0.49), respectively, or analyzed together as non-liquid diet (RR: 0.80; 95% CI: 0.47-1.36; P = 0.41).
CONCLUSION: The non-liquid soft or solid diet did not increase pain recurrence after re-feeding, compared with the clear-liquid diet. The non-liquid diet reduced hospitalization.
- Citation: Meng WB, Li X, Li YM, Zhou WC, Zhu XL. Three initial diets for management of mild acute pancreatitis: A meta-analysis. World J Gastroenterol 2011; 17(37): 4235-4241
- URL: https://www.wjgnet.com/1007-9327/full/v17/i37/4235.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i37.4235
Early enteral nutrition therapy is important for management of severe as well as mild acute pancreatitis[1]. The initial part of the necessity is to prevent bacterial infection, as well as energy supplementation[2]. This kind of nutrition is always preferable via the nasojejunal route with a nasal bowel nutrition tube[3]. However, it is not necessary for mild acute pancreatitis patients because of longer length of hospitalization and discomfort[4,5].
In daily clinical care, 80%-90% of patients with acute pancreatitis demonstrate a mild clinical course of the disease[6]. The traditional initial treatment in mild acute pancreatitis has included: (1) fasting for the first few days; and (2) administration of parenteral fluids followed by clear-liquid-diet intake, orally until the abdominal pain has resolved and the levels of pancreatic enzymes have decreased[7-9]. It sounds reasonable that clear-liquid diet intake will shorten the presence of food in the duodenum, which reduces cholecystokinin release that stimulates pancreatic enzyme secretion[10]. Hospital discharge is usually planned on the basis of the patient’s tolerance to solid diet[11].
Oral re-feeding has been recommended to start with small amounts of clear-liquid diet, rich in carbohydrates and proteins and low in fat, gradually increasing or shifting the intake to soft or solid diet during 3-7 d, to avoid abdominal pain and pancreatitis relapse[12,13]. Unfortunately, to date, evidence is sparse concerning when one kind of diet should be shifted to another, and what kind of diet is definitely optimal re-feeding[14].
Some recent studies have suggested that oral re-feeding with soft or solid diet instead of clear liquids can be considered safe for pain recurrence, and shorten the length of hospitalization. Some randomized trials in patients with mild acute pancreatitis have shown that non-liquid diets are feasible and safe[15-17]. However, the results of these studies were inconclusive. The aim of the present study was to perform a meta-analysis of current randomized controlled trials (RCTs) to evaluate non-liquid diet (including soft and solid diets) as an initial treatment in mild acute pancreatitis.
The titles and abstracts of all citations identified by the literature search were reviewed. Selection criteria were then applied to all potentially relevant studies. Editorials and expert opinions, reviews without original data, case reports and studies lacking control groups were excluded. The selection criteria for inclusion in the meta-analysis were as follows: (1) only RCTs that compared non-liquid diet, including soft and solid diet, with clear-liquid diet were included; (2) diagnosis of mild acute pancreatitis was confirmed according to computed tomography scores, APACHE II scores, and basic laboratory examination; (3) outcomes of length of hospitalization (LOH), total length of hospitalization (TLOH), and recurrence of pain after re-feeding were reported; and (4) no other nutritious supplement treatment was given to patients.
Trials were identified by searching the Cochrane Library (Issue 1 2011), PubMed (March 2011), EMBASE (March 2011), Science Citation Index Expanded, and CBM (Chinese Biomedical Literature Database). The query was constructed by using the combination of the following keywords: (mild pancreatitis or acute pancreatitis) and (diet or nutritious supplement or nutrition). Articles published in any language were considered. Reference lists from the trials selected by electronic searching were hand-searched to identify further relevant trials. Abstracts of the articles selected from each of these multiple searches were reviewed and those meeting the criteria were recorded. In the case of duplicate reports, or studies obviously reporting results from the same study population, only the latest published results were used.
The quality of included studies was assessed independently by two authors (Meng WB and Li YM) without blinding to authorship or journal. Discrepancies were resolved by involving the third author, Xun Li. The quality of the studies was assessed using the scores proposed by Cochrane handbook 5 standards: randomization, allocation, concealment, blinding (participants, investigators, outcomes assessors, and data analysis), and completeness of follow-up.
Two investigators (Meng WB and Li YM) extracted the data from the studies that met the selection criteria (Tables 1, 2 and 3). The outcomes were totalled from the three studies. There was > 98% agreement for data extraction between the two investigators.
| Study comparisons | Moraes et al[15] | Jacobson et al[16] | Sathlaraj et al[17] | ||||
| Type of diet | Solid | Soft | Liquid | Soft | Liquid | Solid | Liquid |
| No. of patients included | 70 | 70 | 70 | 66 | 55 | 49 | 52 |
| Mean age (yr) | 53 | 49 | 51 | 51 | 47 | 37 | 39 |
| Male/female | 42/28 | 43/27 | 33/37 | 23/43 | 34/21 | 39/10 | 44/8 |
| Mean body mass index (%) | ND | ND | ND | 29 | 29 | 21.3 | 20.9 |
| Cause | |||||||
| Biliary system (n) | 33 | 35 | 32 | 15 | 15 | 7 | 9 |
| Alcohol (n) | 17 | 14 | 16 | 14 | 19 | 26 | 25 |
| Unknown and others (n) | 20 | 21 | 22 | 26 | 32 | 16 | 18 |
| Type of pain | |||||||
| Acute (n) | ND | ND | ND | 52 | 53 | 40 | 41 |
| Acute or chronic (n) | ND | ND | ND | 3 | 3 | 9 | 11 |
| Time between admission and first meal (d) | 3.4 ± 0.8 | 3.6 ± 1.0 | 3.5 ± 1.5 | 2 | 1 | 1.6 | 1.4 |
| Total number of meals on study day 1 (n) | 2 | 2 | 2 | 2 | 2 | 3 | 4 |
| Calories in first meal on day 1 (kcal) | 620 | 120 | 124 | 350 | 157 | 262 | 137 |
| Fat in first meal on day 1 (g) | 14 | 2 | 1 | 5 | 1 | 3 | 4 |
| Total calories on first day (kcal) | 1240 | 241 | 248 | 622 | 301 | 921 | 370 |
| Total fat on first day (g) | 28 | 4 | 2 | 13 | 2 | 15 | 8 |
| Study comparisons | Moraes et al[15] | Jacobson et al[16] | Sathlaraj et al[17] | ||||
| Type of diet | Solid | Soft | Liquid | Solid | Liquid | Soft | Liquid |
| LOH (d) | 5.8 ± 1.1 | 7.4 ± 1.5 | 7.3 ± 1.6 | 1.71 ± 2.04 | 1.68 ± 1.85 | 4.18 ± 2.86 | 6.75 ± 3.37 |
| TLOH (d) | 7.5 ± 3.5 | 8.2 ± 2.4 | 8.2 ± 2.6 | 4 (3-6) | 4 (3-5) | 5.92 ± 2.978 | 8.71 ± 4.995 |
| Recurrence of pain (n) | 15 | 12 | 14 | 6 | 4 | 4 | 3 |
We analyzed the data using Review Manager (version 5.0)[18] and pooled data for summary estimates. We expressed results for dichotomous outcomes as relative risk (RR), and mean difference (MD) with 95% CIs for continuous outcomes. We used the χ2 test to assess heterogeneity between trials and the I2 statistic to assess the extent of inconsistency. Statistical significance cut-off for the test of heterogeneity was set at 0.10. We used a fixed-effect model for calculations of summary estimates unless there was significant heterogeneity, in which case, results were confirmed using a random-effects statistical model.
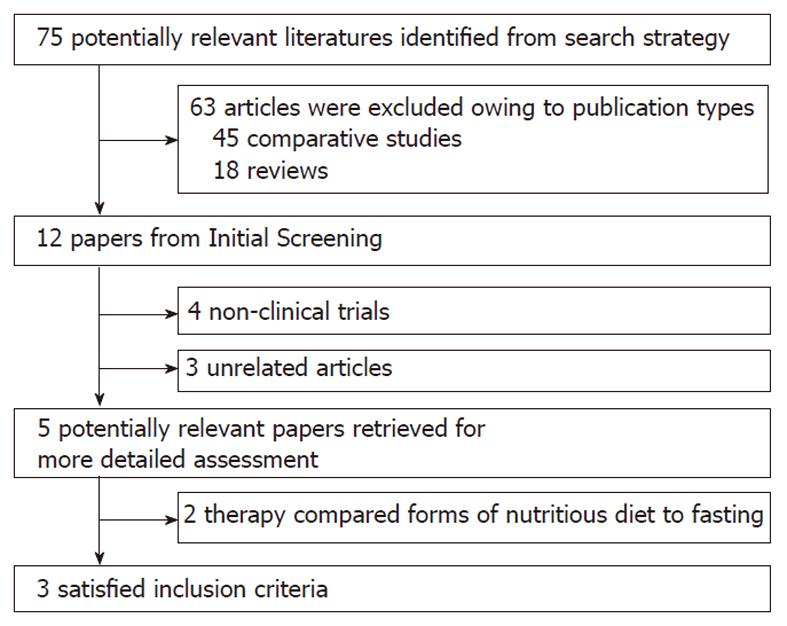
The flowchart of reviews shows the detailed process of study selection (Figure 1). The comparison was made between non-liquid and clear-liquid diets[15-17]. Three trials fulfilled the inclusion criteria.
Data regarding characteristics of the studies, including patients, baseline characteristics and quality assessment of the studies are summarized in Tables 1, 2 and 3, respectively.
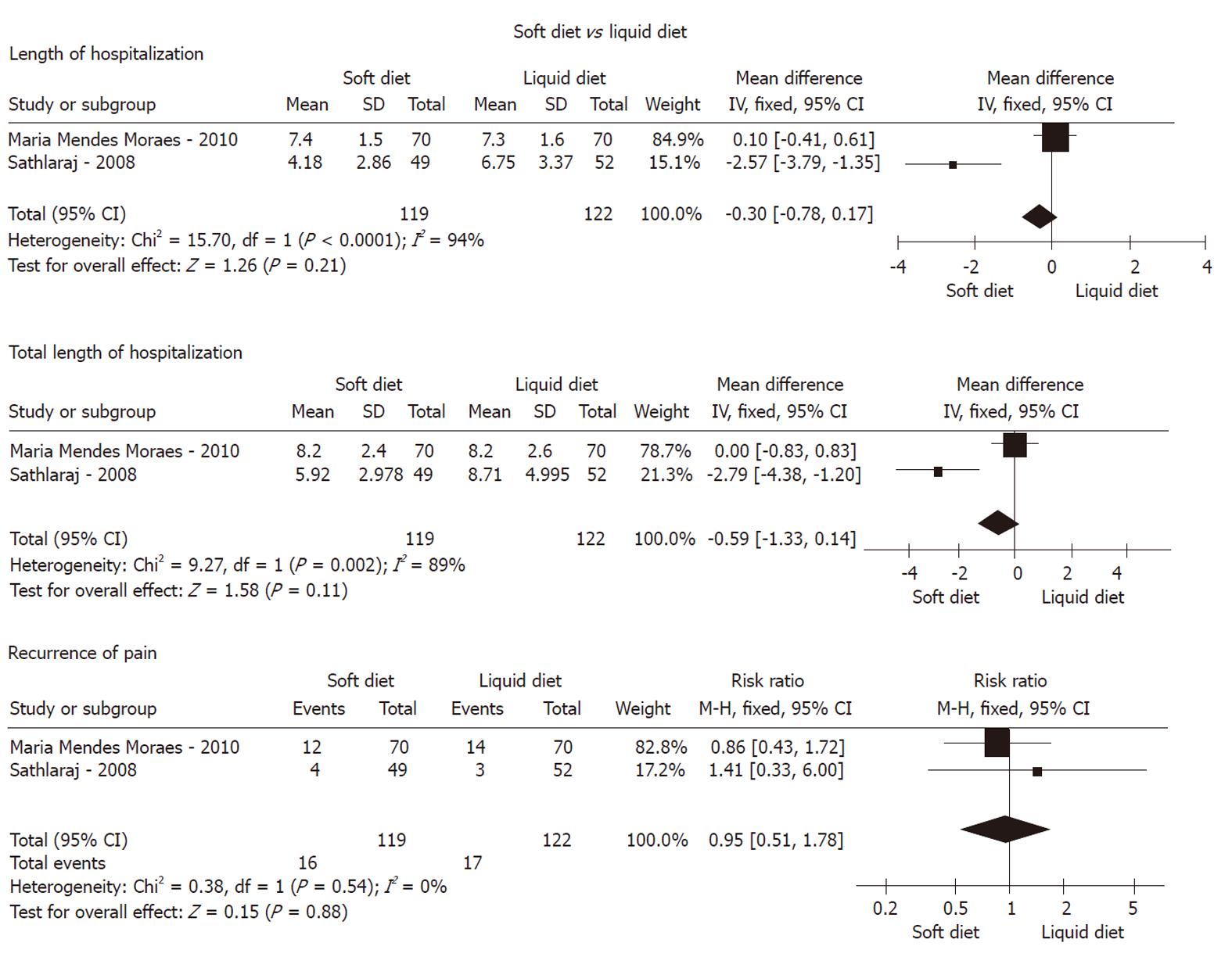
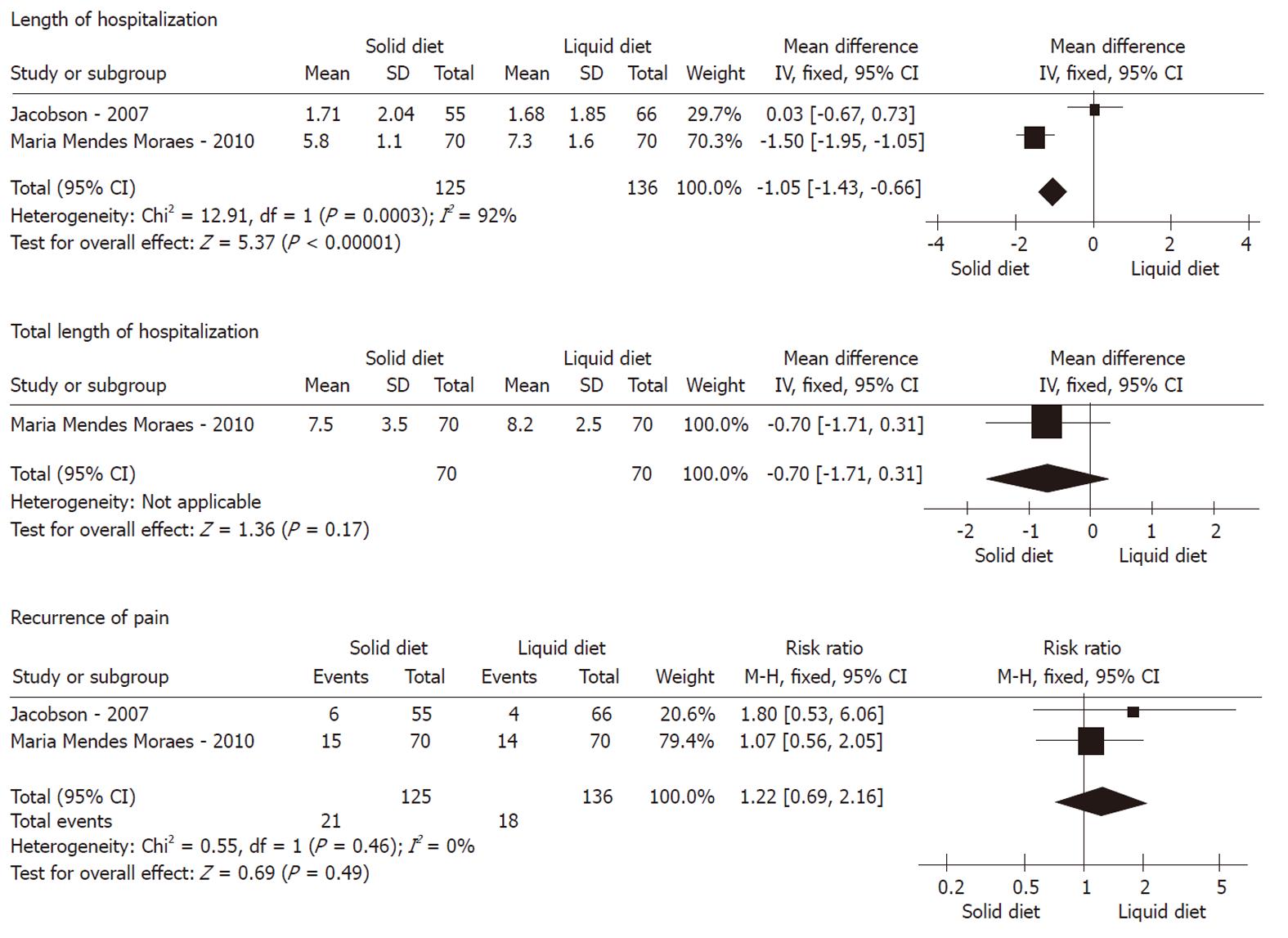
Groups for non-liquid diet vs clear-liquid diet were established first. We deemed both solid and soft diets as non-liquid diets to perform the analysis. In the study of Moraes et al[15] in Table 1, there were three arms with solid diet, soft diet, and clear-liquid diet, which were compared with each other simultaneously. We extracted the solid arm and placed it into the non-liquid diet group, and excluded the soft diet arm for good balance of the statistics. According to the type of control group compared, all the included studies were divided into two subgroups: subgroup A, soft diet vs liquid diet; and subgroup B, solid diet vs liquid diet.
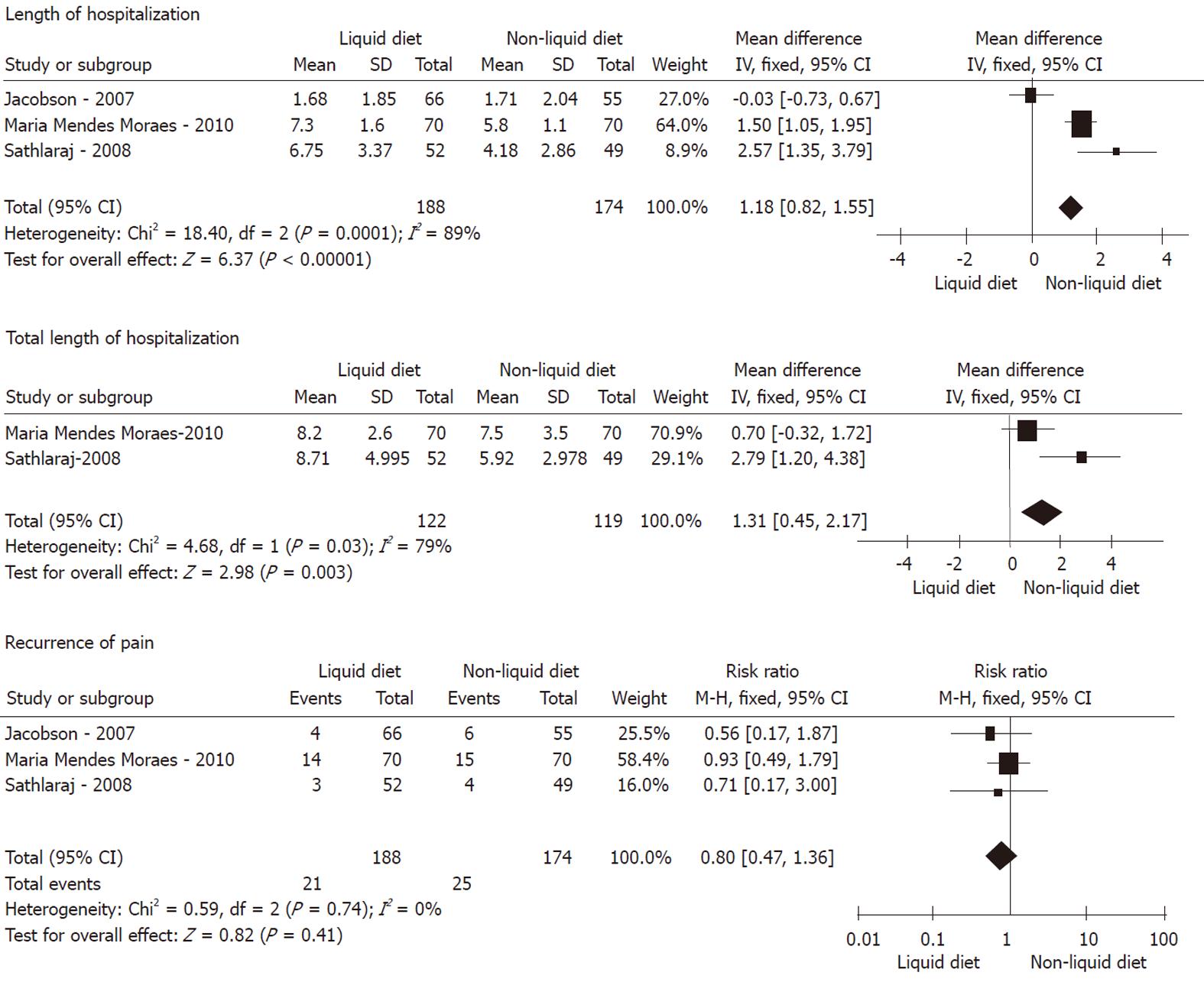
Recurrence of pain: As shown in Figures 2, 3 and 4, meta-analysis did not show any statistically significant difference between 174 patients in the non-liquid diet group and 188 in the clear-liquid diet group with regard to pain recurrence (RR: 0.80, 95% CI: 0.47-1.36; P = 0.41). There was no significant heterogeneity between them (P = 0.74, I2 = 0%). In the subcategory analysis, there was no difference between soft diet and clear-liquid diet (RR: 0.95, 95% CI: 0.51-1.87; P = 0.88), nor was heterogeneity (P = 0.54, I2 = 0%). Similarly, in subgroup B, RR was 1.22 (95% CI: 0.69-2.16, P = 0.49), and no significant heterogeneity was observed (P = 0.46, I2 = 0%).
LOH: Three trials comprising a total of 174 patients in the non-liquid diet group and 188 in the clear-liquid diet group reported LOH. There was a significant difference between the non-liquid and clear-liquid diet groups (Figure 2), with a pooled MD of 1.18 (95% CI: 0.82-1.55; P﹤0.00001). There was significant heterogeneity between them (P = 0.0001, I2 = 89%). In subgroup A, there was heterogeneity between two trials (P﹤0.0001, I2 = 94%), although a pooled MD was -0.30 (95% CI: -0.78 to 0.17, P = 0.21). In subgroup B, MD was -1.05 (95% CI: -1.43 to -0.66; P﹤0.00001); However, significant heterogeneity was observed (P = 0.0003, I2 = 92%).
TLOH: TLOH was reported by three trials comprising a total of 119 patients on non-liquid diet and 122 on clear-liquid diet. There was a significant difference between the two groups (MD: 1.31, 95% CI: 0.45-2.17; P = 0.003) (Figure 2). The subgroup analyses showed no significant difference for subgroup A (MD: -0.59, 95% CI: -1.33 to 0.14; P = 0.11) and subgroup B (MD: -0.70, 95% CI: -1.71 to 0.31; P = 0.17). No significant heterogeneity was found between the non-liquid and clear-liquid diet groups (P = 0.03, I2 = 79%). However, significant heterogeneity was seen in subgroup A (P = 0.002, I2 = 89%).
The current meta-analysis demonstrated that, compared with clear-liquid diet, non-liquid diet did not increase the recurrence of pain after re-feeding in mild acute pancreatitis, and this finding was supported by the subgroup analyses. These outcomes totally challenged our belief that solid diet, even soft diet, would definitely induce the recurrence of abdominal pain and increase pancreatic enzyme secretion[19-21]. Physicians had previously hypothesized that oral re-feeding could promote inflammatory processes in the pancreas and increase production of enteral hormones (such as cholecystokinin, motilin and serotonin), which have a negative trophic effect on the pancreatic tissue, thus decreasing pancreatic blood flow and gastrointestinal motility[22-25]. However, the meta-analysis did not show any significant difference between non-liquid and clear-liquid diets (RR: 1.22, 95% CI: 0.69-2.16; P = 0.49), as well as the two subgroups. There have only been a few studies on diet in acute pancreatitis thus far, therefore, it is possible to speculate that most of the patients could tolerate non-liquid diet successfully. Another key point was that our analysis only selected data from mild acute pancreatitis, with potentially severe types being excluded. The inclusion criteria of the three RCTs were similar. There was no significant heterogeneity between any of the groups.
Non-liquid diet, especially solid diet, showed superiority over clear-liquid diet on the LOH in the meta-analysis. Consequently, as compared to soft diet, solid diet supplement showed significant beneficial effects on both LOH and TLOH in patients with mild acute pancreatitis. Significant heterogeneity was found in the non-liquid diet vs the clear-liquid diet groups (P = 0.0001, I2 = 89%) and in the comparison of solid diet vs clear-liquid diet (P = 0.0003, I2 = 92%). This heterogeneity could have originated from discrepancies in the criteria for hospital discharge and the limited number of RCTs. Although practice management guidelines have presented detailed information concerning the appropriate timing and form of nutrition in severe acute pancreatitis, little attention has been paid to optimizing the dietary management of mild pancreatitis[4,14,26,27]. Earlier studies were not able to explain the benefits of soft or solid diet with fat re-feeding, or the patients’ tolerance. There is a viewpoint that the pancreas may be less responsive to stimulation by nutrients in normal digestive tract than when patients are suffering from pancreatitis[20,27].
With the respect to TLOH, a significant difference was only seen in the non-liquid diet vs the clear-liquid diet group. In the subgroup study, although outcomes favored the soft and solid diet, there was no significant difference. Significant heterogeneity was found in the non-liquid diet vs clear-liquid diet groups (P = 0.03, I2 = 79%) and soft diet vs clear-liquid diet groups (P = 0.002, I2 = 89%). Due to the lack of exact data on TLOH, the heterogeneity analysis could not be performed. The heterogeneity would also have derived from discrepancies in the criteria of discharge and the time zone difference from being hospitalized to re-feeding.
One of the disadvantages of this meta-analysis was that only three RCTs were included. All three studies had high methodological quality and generalizability, nonetheless, there may still have been bias in the final results. There was one study from Brazil[15] for which we could not obtain accurate data for TLOH. Additionally, another study[16] that showed shorter LOH may have been related to the fast discharge protocol, which could have led to heterogeneity. Therefore, more multicenter cooperative studies with prospective design are needed.
To the best of our knowledge, many diseases can cause mild acute pancreatitis. The tolerant form of the different diets should be projected separately by disease. That is probably why there are always some patients who cannot tolerate re-feeding, hence prolonging LOH in patients with mild pancreatitis[28]. For example, if patients suffer from bile duct obstruction or infection, high pressure in the bile duct can cause deterioration of pancreatitis by increasing inflammation if the pressure is not released immediately[29-31]. In that situation, even a small load with any kind of diet could lead to serious consequences[32-34]. Therefore, treatment directed against the causes of pancreatitis is still an essential step. It is advisable to try and cure pancreatitis as soon as possible after the cause has been established, therefore, we should focus therapeutic options on the pathogenesis, in addition to the necessary supporting treatments; not only to ameliorate abdominal pain, but also to recover the whole function of the gastrointestinal tract. This will in turn improve the tolerance of the patients to earlier application of enteral nutritional therapy, thus reducing LOH. According to our meta-analysis, we obtained novel results that will encourage us to promote further new protocols with regard to dietary management of pancreatitis secondary to different protopathies. Also, more multicenter cooperative studies with prospective design are needed for ultimate conclusions about this issue.
In conclusion, the encouraging outcomes in this analysis may demonstrate a different notion from our previous experience in nutritional supplementation of the patients who are diagnosed with mild acute pancreatitis. None of the soft or solid non-liquid diets showed greater recurrence of pain after re-feeding, compared to the clear-liquid diet. Non-liquid diet nutritional supplementation, especially with solid diet, could reduce LOH and TLOH. At this point, we cannot explain our findings with previous pathophysiological experiments performed on acute pancreatitis. One possibility is that the upper digestive tract is less responsive to stimulation by nutrients than we assumed. However, new dietary experiments with animal models of acute pancreatitis and more RCTs comparing roles of different diet forms in the recovery of mild acute pancreatitis are expected to resolve these issues.
Early enteral nutrition therapy is important for management of mild acute pancreatitis. Initial treatment includes fasting in the first few days and administration of parenteral fluids, followed by gradual clear-liquid diet intake until abdominal pain has resolved and levels of pancreatic enzymes have decreased. Hospital discharge is usually planned on the basis of the patients’ tolerance to solid diet.
Some recent studies have suggested that oral re-feeding with soft or solid diet instead of clear liquids can be considered safe for recurrence of pain, but it inconsistently shortens length of hospitalization (LOH). Some randomized trials in mild acute pancreatitis have shown that non-liquid diets are both feasible and safe.
To review systematically the outcomes of non-liquid diet including soft and solid diet compared with clear-liquid diet in mild acute pancreatitis. The meta-analysis demonstrated that none of the non-liquid soft and solid diets increased recurrence of pain after re-feeding, compared with clear-liquid diet. Surprisingly, non-liquid diet nutritious supplement re-feeding reduced LOH and total length of hospitalization (TLOH). Furthermore, solid diet decreased LOH.
With the encouraging outcomes, non-liquid diet nutritional supplementation, especially solid diet, could reduce LOH and TLOH. It might potentially improve the management of mild acute pancreatitis. However more randomized controlled studies on dietary experiments comparing roles of different diet forms in the recovery of mild acute pancreatitis are expected.
LOH means the minimum number of days that patients stay in hospital. TLOH is the total length of hospitalization.
Nutrition management of mild acute pancreatitis was the focus of the study. In the literature, comparison between total parenteral and enteral nutrition have been performed but not among different types of oral feeding. This study was unique in describing a carefully conducted meta-analysis comparing liquid, soft and solid diets. The conclusions are intriguing but sound. It potentially can improve the management of mild acute pancreatitis as well as further understanding of gastrointestinal physiology.
Peer reviewer: Shiu-Ming Kuo, MD, University at Buffalo, 15 Farber Hall, 3435 Main Street, Buffalo, New York, NY 14214, United States
S- Editor Tian L L- Editor Kerr C E- Editor Li JY
| 1. | Choi NW, Shettigara PT, Abu-Zeid HA, Nelson NA. Herpesvirus infection and cervical anaplasia: a seroepidemiological study. Int J Cancer. 1977;19:167-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Ammori BJ. Role of the gut in the course of severe acute pancreatitis. Pancreas. 2003;26:122-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 127] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 3. | Spanier BW, Mathus-Vliegen EM, Tuynman HA, Van der Hulst RW, Dijkgraaf MG, Bruno MJ. Nutritional management of patients with acute pancreatitis: a Dutch observational multicentre study. Aliment Pharmacol Ther. 2008;28:1159-1165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Eckerwall GE, Tingstedt BB, Bergenzaun PE, Andersson RG. Immediate oral feeding in patients with mild acute pancreatitis is safe and may accelerate recovery--a randomized clinical study. Clin Nutr. 2007;26:758-763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 136] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 5. | McClave SA, Greene LM, Snider HL, Makk LJ, Cheadle WG, Owens NA, Dukes LG, Goldsmith LJ. Comparison of the safety of early enteral vs parenteral nutrition in mild acute pancreatitis. JPEN J Parenter Enteral Nutr. 1997;21:14-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 313] [Article Influence: 11.6] [Reference Citation Analysis (1)] |
| 6. | Mitchell RM, Byrne MF, Baillie J. Pancreatitis. Lancet. 2003;361:1447-1455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 240] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 7. | Meier R, Beglinger C, Layer P, Gullo L, Keim V, Laugier R, Friess H, Schweitzer M, Macfie J. ESPEN guidelines on nutrition in acute pancreatitis. European Society of Parenteral and Enteral Nutrition. Clin Nutr. 2002;21:173-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501-523; quiz 524. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Pupelis G, Snippe K, Plaudis H, Rudakovska M. Early oral feeding in acute pancreatitis: an alternative approach to tube feeding. Preliminary report. Acta Chir Belg. 2006;106:181-186. [PubMed] [Cited in This Article: ] |
| 10. | McClave SA, Dryden GW. Issues of nutritional support for the patient with acute pancreatitis. Semin Gastrointest Dis. 2002;13:154-160. [PubMed] [Cited in This Article: ] |
| 11. | Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006;354:2142-2150. [PubMed] [Cited in This Article: ] |
| 12. | Kim YT. [Medical management of acute pancreatitis and complications]. Korean J Gastroenterol. 2005;46:339-344. [PubMed] [Cited in This Article: ] |
| 13. | Petrov MS, van Santvoort HC, Besselink MG, Cirkel GA, Brink MA, Gooszen HG. Oral refeeding after onset of acute pancreatitis: a review of literature. Am J Gastroenterol. 2007;102:2079-284; quiz 2085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Dervenis C. Enteral nutrition in severe acute pancreatitis: future development. JOP. 2004;5:60-63. [PubMed] [Cited in This Article: ] |
| 15. | Moraes JM, Felga GE, Chebli LA, Franco MB, Gomes CA, Gaburri PD, Zanini A, Chebli JM. A full solid diet as the initial meal in mild acute pancreatitis is safe and result in a shorter length of hospitalization: results from a prospective, randomized, controlled, double-blind clinical trial. J Clin Gastroenterol. 2010;44:517-522. [PubMed] [Cited in This Article: ] |
| 16. | Jacobson BC, Vander Vliet MB, Hughes MD, Maurer R, McManus K, Banks PA. A prospective, randomized trial of clear liquids versus low-fat solid diet as the initial meal in mild acute pancreatitis. Clin Gastroenterol Hepatol. 2007;5:946-951; quiz 886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Sathiaraj E, Murthy S, Mansard MJ, Rao GV, Mahukar S, Reddy DN. Clinical trial: oral feeding with a soft diet compared with clear liquid diet as initial meal in mild acute pancreatitis. Aliment Pharmacol Ther. 2008;28:777-781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Cochrane Handbook for Systematic Reviews of Interventions. Higgins JPT, Green S ,editors. Available from: http: //www.mrc-bsu.cam.ac.uk/cochrane/handbook502/whnjs.htm. [Cited in This Article: ] |
| 19. | Chebli JM, Gaburri PD, De Souza AF, Junior EV, Gaburri AK, Felga GE, De Paula EA, Forn CG, De Almeida GV, De Castro Nehme F. Oral refeeding in patients with mild acute pancreatitis: prevalence and risk factors of relapsing abdominal pain. J Gastroenterol Hepatol. 2005;20:1385-1389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | O'Keefe SJ, Lee RB, Li J, Stevens S, Abou-Assi S, Zhou W. Trypsin secretion and turnover in patients with acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2005;289:G181-G187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Qader SS, Ekelund M, Andersson R, Obermuller S, Salehi A. Acute pancreatitis, expression of inducible nitric oxide synthase and defective insulin secretion. Cell Tissue Res. 2003;313:271-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Zhou ZG, Chen YD, Sun W, Chen Z. Pancreatic microcirculatory impairment in experimental acute pancreatitis in rats. World J Gastroenterol. 2002;8:933-936. [PubMed] [Cited in This Article: ] |
| 23. | Piri M, Alhan E, Küçüktülü U, Erçin C, Deger O, Yücel K, Cicek R. The effects of somatostatin on the microperfusion of the pancreas during acute necrotizing pancreatitis in rats. Hepatogastroenterology. 2002;49:833-837. [PubMed] [Cited in This Article: ] |
| 24. | Zhou Z, Zhang Z, Yan L, Shu Y, Cheng Z, Zhao J, Lan P, Feng X, Wang R. The feature of pancreatic microcirculatory impairment in caerulein induced acute pancreatitis. Zhonghua Waike Zazhi. 1999;37:138-140, 9. [PubMed] [Cited in This Article: ] |
| 25. | Wang X, Gong Z, Wu K, Wang B, Yuang Y. Gastrointestinal dysmotility in patients with acute pancreatitis. J Gastroenterol Hepatol. 2003;18:57-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Abou-Assi S, O'Keefe SJ. Nutrition in acute pancreatitis. J Clin Gastroenterol. 2001;32:203-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (1)] |
| 27. | Boreham B, Ammori BJ. A prospective evaluation of pancreatic exocrine function in patients with acute pancreatitis: correlation with extent of necrosis and pancreatic endocrine insufficiency. Pancreatology. 2003;3:303-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | De La Mano A, Sevillano S, De Dios I, Vicente S, Manso MA. Low enzyme content in the pancreas does not reduce the severity of acute pancreatitis induced by bile-pancreatic duct obstruction. Mol Cell Biochem. 2002;240:75-81. [PubMed] [Cited in This Article: ] |
| 29. | Siqin D, Wang C, Zhou Z, Li Y. The key event of acute pancreatitis: pancreatic duct obstruction and bile reflux, not a single one can be omitted. Med Hypotheses. 2009;72:589-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Teoh AY, Poon MC, Leong HT. Role of prophylactic endoscopic sphincterotomy in patients with acute biliary pancreatitis due to transient common bile duct obstruction. J Gastroenterol Hepatol. 2007;22:1415-1418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | van Erpecum KJ. Gallstone disease. Complications of bile-duct stones: Acute cholangitis and pancreatitis. Best Pract Res Clin Gastroenterol. 2006;20:1139-1152. [PubMed] [Cited in This Article: ] |
| 32. | Karne S, Gorelick FS. Etiopathogenesis of acute pancreatitis. Surg Clin North Am. 1999;79:699-710. [PubMed] [Cited in This Article: ] |
| 33. | Steer ML. Pathogenesis of acute pancreatitis. Digestion. 1997;58 Suppl 1:46-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 34. | Schmidt J, Klar E. Etiology and pathophysiology of acute pancreatitis. Ther Umsch. 1996;53:322-332. [PubMed] [Cited in This Article: ] |