Published online Aug 14, 2011. doi: 10.3748/wjg.v17.i30.3510
Revised: May 19, 2011
Accepted: May 26, 2011
Published online: August 14, 2011
AIM: To evaluate the treatment options for nephrotoxicity due to cisplatin combination chemotherapy.
METHODS: We retrospectively reviewed patients who had received cisplatin combination chemotherapy for gastric cancer between January 2002 and December 2008. We investigated patients who had shown acute renal failure (ARF), and examined their clinical characteristics, laboratory data, use of preventive measures, treatment cycles, the amount of cisplatin administered, recovery period, subsequent treatments, and renal status between the recovered and unrecovered groups.
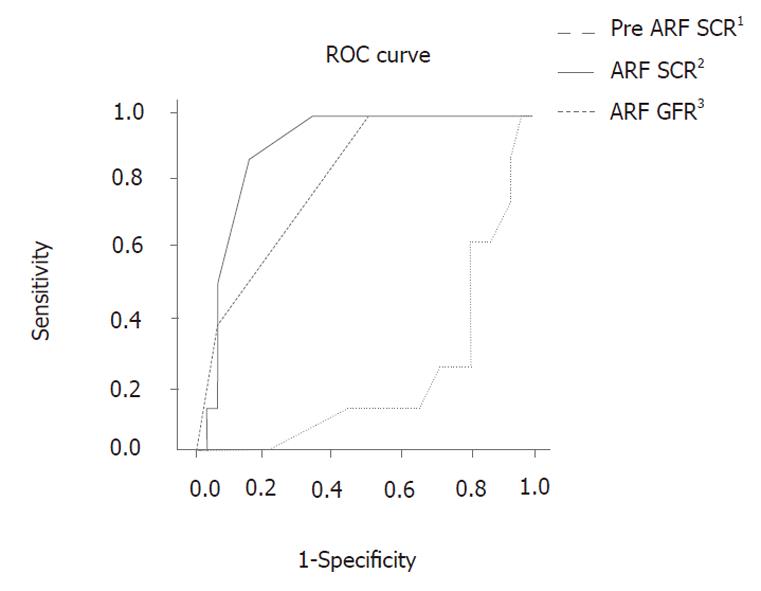
RESULTS: Forty-one of the 552 patients had serum creatinine (SCR) levels greater than 1.5 mg/dL. We found that pre-ARF SCR, ARF SCR, and ARF glomerular filtration rates were significantly associated with renal status post-ARF between the two groups (P = 0.008, 0.026, 0.026, respectively). On the receiver operating characteristic curve of these values, a 1.75 mg/dL ARF SCR value had 87.5% sensitivity and 84.8% specificity (P = 0.011).
CONCLUSION: Cessation or reduction of chemotherapy should be considered for patients who have an elevation of SCR levels during cisplatin combination chemotherapy.
- Citation: Moon HH, Seo KW, Yoon KY, Shin YM, Choi KH, Lee SH. Prediction of nephrotoxicity induced by cisplatin combination chemotherapy in gastric cancer patients. World J Gastroenterol 2011; 17(30): 3510-3517
- URL: https://www.wjgnet.com/1007-9327/full/v17/i30/3510.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i30.3510
Cisplatin is one of the most commonly used antineoplastic agents for the treatment of solid tumors[1,2]. It is generally used in combination with fluorouracil, docetaxel, paclitaxel, capecitabine or irinotecan for the treatment of gastric cancer[3]. However, cisplatin can induce severe side-effects such as bone-marrow suppression, gastrointestinal toxicity, nephrotoxicity, ototoxicity, and neuropathy. Of these, nephrotoxicity is the major side effect and main obstacle in the therapeutic use of cisplatin[1,4].
Many studies have attempted to determine the pathogenesis of nephrotoxicity caused by cisplatin in order to prevent and reduce patient symptoms. However, prevention cannot be achieved by the traditional manner of decreasing drug dosage, performing specific hydration procedures, and actively screening for renal abnormalities[5,6]. In fact, there are currently no unified recommendations for the treatment of nephrotoxicity. In this study, patients who displayed nephrotoxicity induced by a cisplatin combination regimen for gastric cancer were retrospectively reviewed. The aim of this study was to determine the appropriate therapeutic steps when nephrotoxicity occurs due to cisplatin combination chemotherapy.
We retrospectively examined 552 patients who were diagnosed with gastric cancer, and who received cisplatin combination chemotherapy between January 2002 and December 2008 at the Kosin University Gospel Hospital. Of these patients, 41 who developed nephrotoxicity induced by cisplatin combination chemotherapy were chosen for further analysis; a serum creatinine (SCR) level of 1.5 mg/dL was used as the threshold for nephrotoxicity. Patients were excluded if they had renal disease, hydronephrosis, severe dehydration, SCR > 1.5 mg/dL before the administration of cisplatin, or lack of follow-up care.
Forty-one patients were reviewed in terms of gender, age, body surface area (BSA), combined chemotherapy drugs, stage of gastric cancer, hemoglobin levels, hematocrit, total protein, albumin, electrolytes, blood urea nitrogen, SCR, glomerular filtration rate (GFR), magnesium, phosphate and calcium levels, use of mannitol, furosemide and amifostine, amount of hydration, dose of cisplatin/cycle × BSA, cumulative dose of cisplatin/BSA, recovery period, and course of acute renal failure (ARF). Laboratory data were checked immediately before the administration of chemotherapy drugs; SCR levels greater than 1.5 mg/dL were used as pre-ARF laboratory data. Laboratory data were also collected at peak SCR values after SCR levels increased to greater than 1.5 mg/dL at the time of ARF. Patients were divided into two groups (recovered and unrecovered) according to their post-ARF renal status. The recovered patients were those whose SCRs decreased to less than 1.5 mg/dL after ARF; the unrecovered patients were those whose SCRs were maintained at levels greater than 1.5 mg/dL after ARF. The two groups were compared in terms of the above-mentioned characteristics, before and after collection of the ARF laboratory data, use of protective measures, dose of cisplatin, recovery period, and the course of recovery. With these results, the predictive values for post-ARF renal status were examined. Also, in each group, the relationship between treatment and subsequent renal status in response to treatment was examined. The treatments were then divided into the categories of stop, reduce and continue. The subsequent renal status in response to these treatments was divided into the normal, recovered and unrecovered groups. Normal patients were those whose SCRs did not increase to levels greater than 1.5 mg/dL; definition of the recovered and the unrecovered groups is the same as previously noted. Data collection ceased in June, 2009.
Statistical analysis was performed using SPSS Statistics 17.0 for Windows. We collected the laboratory data, which were checked immediately before the SCR increased to > 1.5 mg/dL, for use as the pre-ARF laboratory data. Laboratory data were also checked at peak SCR values after levels increased to > 1.5 mg/dL at the time of ARF. The data on administration of the anticancer drug were reported in number and percentage with some overlap. Other data were reported as mean and standard deviation, and compared using the unpaired Student’s t test. The predictive value of the post-ARF renal status was examined by receiver operating characteristic (ROC) analysis. The χ2 test was used to examine the relationship between treatment and subsequent renal status in response to treatment. P values less than 0.05 were considered statistically significant.
Five hundred and fifty-two patients were diagnosed with gastric cancer and received cisplatin combination chemotherapy between January 2002 and December 2008. The patients received several different cisplatin combination drugs, including 5-flourouracil (5-FU) in 193 patients (34.96%), docetaxel in 113 (20.47%), TS-1 in 86 (15.58%), paclitaxel in 71 (12.86%), capecitabine in 30 (5.43%), irinotecan in 29 (5.25%), mitomycin in 23 (4.17%), and others in 4 patients (0.72%), with some overlap. In our investigation, 5-FU was the most frequently used anticancer drug in combination with cisplatin for gastric cancer chemotherapy. Table 1 lists the characteristics of the 41 patients who had an SCR > 1.5 mg/dL after receiving cisplatin combination chemotherapy for gastric cancer. There were 36 males and 5 females, with an average age of 58.36 years, and an average BSA of 1.677. 5-FU made up the largest proportion of the combined drug regimens (18 patients, 43.9%), and there were more stage IV patients than any other stage classification (19 patients, 46.3%).
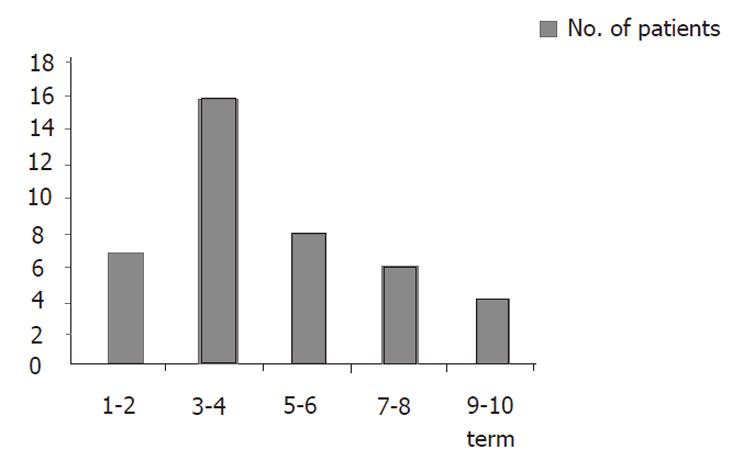
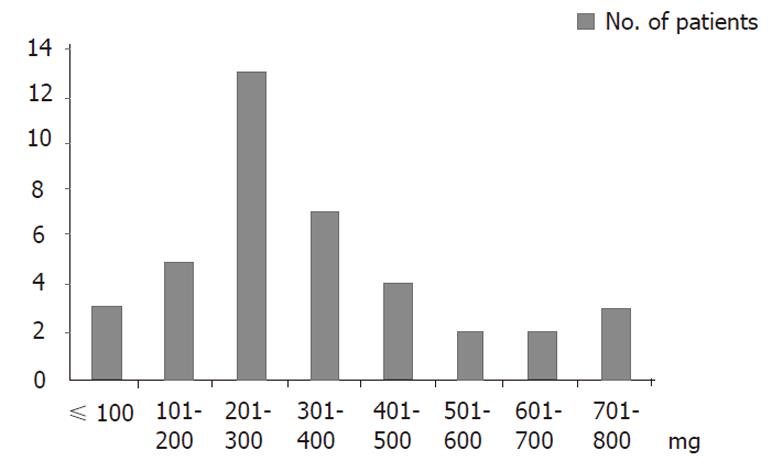
Of 41 patients, nephrotoxicity occurred more frequently during the 3rd-4th cycle (16 patients), and 7 patients experienced nephrotoxicity during the 1st-2nd cycle. The most common cumulative dose of cisplatin/BSA at which nephrotoxicity occurred was 200-300 mg, while the second most common cumulative dose was 300-400 mg, and these were correlated with the greatest number of cycles and the dose of cisplatin/cycle × BSA (Figures 1, 2).
The average length of recovery time among the patients was 15 d, and was less than 7 d for 27 patients, 8-14 d for 1 patient, 15-30 d for 2 patients, and more than 30 d for 3 patients. These results showed that approximately 70% of recovered patients reached this state within 2 wk.
Patients were divided into two groups based on their post-ARF renal status: the recovered patients and the unrecovered patients. The average age of patients in the unrecovered group (51.88 ± 6.01) was lower than that of the recovered group (59.94 ± 10.86), with a P value (P = 0.051) near 0.05. In the analysis of the laboratory data (Tables 2 and 3), the pre-ARF SCR, ARF SCR, and ARF GFR were significantly associated with the renal status post-ARF (P = 0.008, 0.026, 0.026, respectively). The ROC curve was constructed using these values (Figure 3). On the ROC curve, an ARF SCR value of 1.75 mg/dL showed 87.5% sensitivity and 84.8% specificity. The use of amifostine, mannitol, and furosemide was not significantly different between the two groups (P = 0.203, P = 0.587, P = 0.542, respectively), as nearly all the patients who were followed-up, received a routine formula of hydration and diuretics. The time during which nephrotoxicity occurred and the cumulative dose of cisplatin in each group was assessed and compared (Table 4). The time during which nephrotoxicity occurred was greater in the unrecovered group than in the recovered group (6.63 cycles ± 2.62 cycles vs 4.24 cycles ± 2.09 cycles, respectively, P = 0.009), and the cumulative dose of cisplatin/BSA was also significantly greater in the unrecovered group compared to the recovered group (497.75 ± 222.61 vs 302.85 ± 152.73, respectively, P = 0.005).
| Renal status of post-ARF | P value3 | ||||
| Variable | Normal range | Unit | Recovered (n = 33) | Unrecovered (n = 8) | |
| Hb | 14.0–16.7 | g/dL | 10.33 ± 1.28 (n = 33) | 9.75 ± 0.95 (n = 8) | 0.238 |
| HT | 14.7–50.7 | % | 29.88 ± 3.33 (n = 33) | 28.56 ± 2.78 (n = 8) | 0.307 |
| Protein | 6.3–8.3 | g/dL | 6.792 ± 0.69 (n = 26) | 6.429 ± 0.39 (n = 7) | 0.191 |
| Albumin | 3.5–5.0 | g/dL | 3.97 ± 0.54 (n = 26) | 3.87 ± 0.38 (n = 7) | 0.657 |
| BUN | 5–23 | mg/dL | 18.07 ± 5.35 (n = 33) | 15.63 ± 4.03 (n = 8) | 0.236 |
| SCR | 0.3-1.5 | mg/dL | 1.17 ± 0.20 (n = 33) | 1.38 ± 0.13 (n = 8) | 0.008 |
| GFR | 120-130 | mL/min | 68.03 ± 13.31 (n = 33) | 59.13 ± 6.64 (n = 8) | 0.076 |
| Na | 136–150 | meg/L | 139.24 ± 3.19 (n = 33) | 140.13 ± 4.09 (n = 8) | 0.510 |
| Cl | 98–110 | meg/L | 105.31 ± 4.42 (n = 24) | 105.57 ± 3.65 (n = 7) | 0.887 |
| K | 3.5–5.3 | meg/L | 4.49 ± 0.46 (n = 8) | 4.66 ± 0.65 (n = 8) | 0.284 |
| P | 3.0–4.5 | mg/dL | 3.97 ± 0.81 (n = 20) | 3.93 ± 1.16 (n = 6) | 0.940 |
| Mg | 1.6–2.6 | mg/dL | 2.08 ± 0.26 (n = 24) | 2.03 ± 0.29 (n = 7 ) | 0.635 |
| Ca | 8.0-10.0 | mg/dL | 9.15 ± 0.53 (n = 24) | 8.86 ± 0.30 (n = 7) | 0.170 |
| Renal status post-ARF | P value3 | ||||
| Variable | Normal range | Unit | Recovered (n = 33) | Unrecovered (n = 8) | |
| Hb | 14.0–16.7 | g/dL | 10.28 ± 1.62 (n = 31) | 10.238 ± 1.30 (n = 8) | 0.941 |
| HT | 14.7–50.7 | % | 29.52 ± 4.70 (n = 32) | 29.93 ± 3.41 (n = 8) | 0.820 |
| Protein | 6.3–8.3 | g/dL | 6.72 ± 0.78 (n = 24) | 7.00 ± 0.95 (n = 3) | 0.564 |
| Albumin | 3.5–5.0 | g/dL | 3.95 ± 0.61 (n = 24) | 4.03 ± 0.65 (n = 3) | 0.818 |
| BUN | 5–23 | mg/dL | 23.78 ± 13.60 (n = 33) | 23.0 ± 4.87 (n = 8) | 0.876 |
| SCR | 0.3-1.5 | mg/dL | 1.75 ± 0.48 (n = 33) | 2.21 ± 0.61 (n = 8) | 0.026 |
| GFR | 120-130 | mL/min | 43.30 ± 7.81 (n = 33) | 36.0 ± 8.98 (n = 8) | 0.026 |
| Na | 136–150 | meg/L | 136.60 ± 4.36 (n = 32) | 137.63 ± 2.72 (n = 8) | 0.529 |
| Cl | 98–110 | meg/L | 101.49 ± 6.59 (n = 24) | 99.50 ± 2.65 (n = 4) | 0.564 |
| K | 3.5–5.3 | meg/L | 4.22 ± 0.93 (n = 32) | 4.46 ± 0.60 (n = 8) | 0.491 |
| P | 3.0–4.5 | mg/dL | 4.13 ± 0.87 (n = 18) | 3.93 ± 0.43 (n = 4) | 0.659 |
| Mg | 1.6–2.6 | mg/dL | 1.83 ± 0.42 (n = 22) | 1.833 ± 0.47 (n = 3) | 0.995 |
| Ca | 8.0-10.0 | mg/dL | 9.06 ± 0.51 (n = 23) | 9.40 ± 0.80 (n = 4) | 0.269 |
In the recovered group, the relationship between treatment and renal status following ARF was examined. Table 5 shows that more recovered patients were present in the group that stopped therapy; their SCRs returned to normal. Meanwhile, there were more unrecovered patients in the group that continued treatment; their SCRs remained above 1.5 mg/dL. The relationship between treatment and renal status was significant (P = 0.011). Seven normal and recovered patients stopped treatment, including two patients who changed their chemotherapy regimens, two patients who ceased chemotherapy due to metastasis to other organs, two patients who ceased chemotherapy due to poor quality of life (weight loss, anorexia), and one patient who terminated cisplatin in their combination regimen.
The relationship between subsequent treatment and renal status was also examined in unrecovered patients, but it was not statistically significant (Table 6). Two patients stopped receiving cisplatin combination chemotherapy and were switched to another regimen. As a result, their SCRs returned to values less than 1.5 mg/dL after 150 and 181 d, respectively.
Cisplatin is the single most active antitumor agent in the treatment of solid tumors, including gastric cancer. Nevertheless, the use of cisplatin has been restricted because of its side effects, especially nephrotoxicity[1,2]. It has been reported that approximately 25% of patients who received a single dose of cisplatin developed reversible azotemia[7]. In addition, irreversible renal failure can occur when large doses are administered, or with repeated cycles of treatment[8]. In this study, the incidence of nephrotoxicity due to cisplatin combination chemotherapy was 7.43% (41/552). Since patients who had an SCR > 1.5 mg/dL as a measure of nephrotoxicity were selected, these results probably underestimated the incidence of nephrotoxicity. In this study, 5-FU was the most frequently used anticancer drug combined with cisplatin for gastric cancer chemotherapy; the 5-FU/cisplatin regimen is also the most traditional adjuvant chemotherapy for gastric cancer in South Korea.
Nephrotoxicity is evaluated by GFR and creatinine clearance values using the Modification of Diet in Renal Disease (MDRD) formula or the Cockcroft and Gault formula, as well as SCR values[9-11]. Only SCR was used for the selection of patients with nephrotoxicity, although the use of a single cutoff to define an elevated SCR is not appropriate[12,13]. The National Kidney Foundation (NKF) recommended that clinicians should not use serum creatinine concentration as the sole means of assessing the level of kidney function[14]. The Renal Insufficiency and Cancer Medications study group suggested that renal function should be evaluated in all cancer patients, including those with normal SCR levels, using either the Cockcroft-Gault formula or the MDRD formula[15]. In this context, the definition of nephrotoxicity in this study as > 1.5 mg/dL is a limitation. In 41 patients, the averages of the pre-nephrotoxic ARF SCR and GFR using MDRD were 1.21 mg/dL ± 0.20 mg/dL and 66.29 mg/dL ± 12.74 mL/min, respectively. Thus, their renal status prior to ARF was already stage 2 according to the clinical guidelines published by the Working Group of the NKF. However, in the case of ARF or acute renal injury (AKI), SCR can be used as a criterion for the definition of ARF or AKI[13,14]. RIFLE and AKIN defined an increase in SCR > 1.5 fold from baseline as a risk or stage 1[16,17]. In this methodology, the SCR can be used as one of the predictive values for renal status after a nephrotoxic event.
The purpose of this study was not to detect and eva-luate renal toxicity due to cisplatin. Rather, this study was focused on choosing the appropriate next step after nephrotoxicity occurs due to cisplatin combination chemotherapy. Our data showed that the pre-ARF SCR, ARF SCR, and ARF GFR values were significantly associated with renal status post-ARF (P = 0.008, 0.026, 0.026, respectively). When the ROC curves of these values were assessed, an ARF SCR of 1.75 mg/dL showed 87.5% sensitivity and 84.8% specificity (Figure 3). This indicated that if a patient with nephrotoxicity experiences an SCR > 1.75 mg/dL, then that patient’s renal status can progress to severe renal failure. Thus, an ARF SCR value of 1.75 mg/dL can be considered as a predictive measure for renal status post-ARF.
Cisplatin accumulates in the kidneys, and the nephrotoxic effect of cisplatin is proportional to the amount of drug accumulated[3,5,18]. It is known that cisplatin accumulates in the mitochondrial DNA more than in the nucleus or other organelles[2,6]. In a rodent study, the mitochondrial DNA decreased by up to 63% 3–4 d after cisplatin injection[19,20]. Thus, repetitive cisplatin administration lowers the GFR in a dose-related manner[2,21]. In this respect, the dose-related toxicity of cisplatin correlated with the results from this study in terms of the number of cycles before nephrotoxicity occurred. Moreover, the cumulative dose of cisplatin/BSA was greater in the unrecovered group compared to the recovered group (Table 4), suggesting that the earlier nephrotoxicity occurs and the lower the cumulative dose of cisplatin combination chemotherapy, the more quickly the patient will recover (Table 4).
Figures 1 and 2 show that most of the nephrotoxicity occurred in the 3rd-4th cycles of treatment, and the most common cumulative dose of cisplatin/BSA was 200-300 mg. However, in seven patients, nephrotoxicity occurred in the 1st-2nd cycle. Thus, it appears that the threshold of nephrotoxicity varies according to the individual. Furthermore, renal function should be evaluated, and chemotherapy must be carefully considered before administering cisplatin combination chemotherapy[15,22].
Upon analysis of the relationship between chemotherapy and renal status in the recovery group, it was found that continuing chemotherapy imparts an increased risk of severe renal failure, compared to ceasing treatment or decreasing the dosage of cisplatin combination chemotherapy (Table 5, P = 0.011). In the unrecovered group, all of the cases in which chemotherapy was not stopped remained unrecovered according to their renal status. There were only two patients who stopped receiving cisplatin combination chemotherapy and began another regimen. Their SCRs returned to values less than 1.5 mg/dL, although their recovery took a long time; 150 and 181 d, respectively. Therefore, if a nephrotoxic patient’s SCR is > 1.5 mg/dL, chemotherapy should be stopped, the drug dosage should be reduced, or the regimen should be changed.
A common complication resulting from cisplatin treatment is electrolyte wasting, or hypomagnesemia[23,24]. The laboratory data of all the patients in this study were not checked routinely as this was not a prospective study. However, hyponatremia, hypokalemia, hypocalcemia, and hypomagnesemia were found in some patients (Tables 2, 3). Electrolyte imbalances are common in these types of patients, but are not severe[18]. Severe electrolyte imbalance can induce ototoxicity and neurotoxicity, or it can aggravate nephrotoxicity. Such conditions should be corrected by supplementation[18,25,26].
The most commonly used protective measure against renal toxicity is to establish solute diuresis[18,27]. Nearly all of the patients received a routine formula of hydration and diuretics which included hydrations of 1-2 L before and after administration of chemotherapy, diuretics after hydration, and sometimes amifostine. Despite the many recent physiopathological advances in the understanding of the mechanism of anticancer drug nephrotoxicity, especially that of cisplatin, prevention still relies on decreases in drug dosage, hydration measures, and active screening for renal abnormalities as part of the usual pre-therapeutic biological work-up in patients treated with anticancer drugs[6,18]. The European Society of Clinical Pharmacy Special Interest Group on Cancer Care suggested that hydration should be maintained for at least 3 d after the chemotherapy course, and by IV or oral route when feasible[6]. However, there are no specific recommendations or convincing data on the renal protective effect of cisplatin administration in fractionated doses[28].
In this study, the nephrotoxicity of combined anticancer drugs was not considered. Mitomycin is known to have renal toxicity. In fact, it has been reported that the onset of renal insufficiency induced by mitomycin administration occurs after an average time of 10-11 mo[29]. However, since the kidney is not a major route of mitomycin excretion, it is not suggested that the dose be adjusted in patients with renal insufficiency[29]. Paclitaxel and irinotecan are also known to cause potential nephrotoxicity, but the need for dosage adjustment has not been confirmed. A comparative prospective study of renal toxicity induced by combined drugs is needed[29,30]. This study has several limitations. Nevertheless, we believe that this issue is important and worthy of further prospective studies.
The author reviewed patients who were diagnosed with gastric cancer, who received cisplatin combination chemotherapy, and who displayed nephrotoxicity. The results show that the patients who experienced a SCR > 1.75 mg/dL after receiving cisplatin combination chemotherapy had a greater risk of chronic renal failure than did patients with a SCR < 1.75 mg/dL. Secondly, in subsequent chemotherapy regimens in patients who experienced SCR > 1.5 mg/dL, the patients who continued cisplatin combination chemotherapy had a greater tendency to experience severe chronic renal disease. Therefore, these results suggest that when a patient experiences a SCR > 1.5 mg/dL after receiving cisplatin combination chemotherapy, the chemotherapy should be stopped, reduced, or the regimen should be changed, and when a patient experiences a SCR > 1.75 mg/dL after receiving cisplatin combination chemotherapy, the chemotherapy should be stopped or changed. More prospective and comparative studies are needed on this subject.
Cisplatin is one of the most commonly used drugs in the chemotherapy of solid tumors. The major adverse effect of cisplatin is nephrotoxicity, with an incidence of up to 25%. Cisplatin accumulates in the kidneys, and the nephrotoxic effect of cisplatin is proportional to the accumulated drug dose. It is known that cisplatin accumulates in the mitochondrial DNA more than it does in the nucleus or other organelles. Thus, repeated cisplatin administration lowers the glomerular filtration rate (GFR) in a dose-related manner. The aim of this study was to determine the appropriate therapeutic steps when nephrotoxicity occurs due to cisplatin combination chemotherapy in gastric cancer.
Nephrotoxicity is evaluated by the GFR and creatinine clearance (CrCl) using the Modification of Diet in Renal Disease formula or the Cockcroft and Gault formula, and not only by serum creatinine (SCR). However, in the case of acute renal failure (ARF) or acute renal injury (AKI), SCR can be used as a criterion for the definition of ARF or AKI. The authors suggest that the SCR can be used as one of the predictive values for renal status after a nephrotoxic event. The purpose of this study was not to detect and evaluate the renal toxicity of cisplatin. This study focused on choosing the next step after nephrotoxicity due to cisplatin combination chemotherapy.
Forty-one out of 552 patients, who received cisplatin combination chemotherapy, had SCR levels greater than 1.5 mg/dL. These patients were divided into two groups according to post-ARF renal status, the recovered patients and unrecovered patients. The two groups were compared in terms of the above-mentioned characteristics, before and after ARF laboratory data, use of protective measures, dose of cisplatin, recovery period, and the course of recovery. With these results, the predictive values for the post-ARF renal status were examined. The authors found that pre-AFR SCR, ARF SCR, and ARF GFR were significantly associated with renal status post-ARF in the two groups (P = 0.008, 0.026, 0.026, respectively). In the receiver operating characteristic curve of these values, a 1.75 mg/dL ARF SCR value showed 87.5% sensitivity and 84.8% specificity. This indicated that if a patient with nephrotoxicity experienced an SCR > 1.75 mg/dL, then the patient’s renal status can progress to severe renal failure. Thus, an ARF SCR value of 1.75 mg/dL can be considered as a predictive value for renal status post-ARF. In addition, in each group, the relationship between subsequent treatment and renal status in response to treatment was examined. In the recovered group, the relationship between subsequent treatment and renal status following ARF was determined. The results showed that more recovered patients were present in the group who stopped therapy; their SCRs had returned to normal. Meanwhile, in patients who continued treatment, more unrecovered patients whose SCRs were maintained above 1.5 mg/dL were present. The relationship showed a significant difference (P = 0.011). Therefore, if a nephrotoxic patient’s SCR is > 1.5 mg/dL, chemotherapy should be stopped, the drug dosage should be reduced, or the regimen should be changed.
In cisplatin combination chemotherapy in gastric cancer patients, when a patient has experienced a SCR level greater than 1.5 mg/dL, cessation or reduction of chemotherapy should be considered. Furthermore, when a patient experiences a SCR greater than 1.75 mg/dL, chemotherapy should be stopped or changed.
The recovered patients consisted of those whose SCRs had decreased to less than 1.5 mg/dL after ARF. The unrecovered patients consisted of those whose SCRs were maintained at greater than 1.5 mg/dL. Subsequent treatment is the next chemotherapy regimen after cisplatin-induced nephrotoxicity, which was divided into stop, reduce and continue. Subsequent renal status is the renal status (recovered or unrecovered) corresponding to subsequent treatment.
Despite the many recent physiopathological advances in the understanding of the mechanism of anticancer drug nephrotoxicity, especially that of cisplatin, prevention still relies on a drug dosage decrease, hydration measures, and active screening for renal abnormalities as part of the usual pre-therapeutic biological work-up in patients treated with anticancer drugs. In addition, there are no specific recommendations or convincing data about the renal protective effect of the administration of cisplatin and the subsequent step of nephrotoxicity.
Peer reviewer: Aldo Torre Delgadillo, Professor, MD, MSc, Department of Gastroenterology, Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán”, 14000 México City, México
S- Editor Sun H L- Editor Webster JR E- Editor Zhang L
| 1. | Safirstein R, Winston J, Goldstein M, Moel D, Dikman S, Guttenplan J. Cisplatin nephrotoxicity. Am J Kidney Dis. 1986;8:356-367. [PubMed] [Cited in This Article: ] |
| 2. | Cornelison TL, Reed E. Nephrotoxicity and hydration management for cisplatin, carboplatin, and ormaplatin. Gynecol Oncol. 1993;50:147-158. [PubMed] [Cited in This Article: ] |
| 3. | Taguchi T, Nazneen A, Abid MR, Razzaque MS. Cisplatin-associated nephrotoxicity and pathological events. Contrib Nephrol. 2005;148:107-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 187] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 4. | Srivastava RC, Farookh A, Ahmad N, Misra M, Hasan SK, Husain MM. Reduction of cis-platinum induced nephrotoxicity by zinc histidine complex : the possible implication of nitric oxide. Biochem Mol Biol Int. 1995;36:855-862. [PubMed] [Cited in This Article: ] |
| 5. | Finley RS, Fortner CL, Grove WR. Cisplatin nephrotoxicity: a summary of preventative interventions. Drug Intell Clin Pharm. 1985;19:362-367. [PubMed] [Cited in This Article: ] |
| 6. | Launay-Vacher V, Rey JB, Isnard-Bagnis C, Deray G, Daouphars M. Prevention of cisplatin nephrotoxicity: state of the art and recommendations from the European Society of Clinical Pharmacy Special Interest Group on Cancer Care. Cancer Chemother Pharmacol. 2008;61:903-909. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 182] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 7. | Kovach JS, Moertel CG, Schutt AJ, Reitemeier RG, Hahn RG. Phase II study of cis-diamminedichloroplatinum (NSC-119875) in advanced carcinoma of the large bowel. Cancer Chemother Rep. 1973;57:357-359. [PubMed] [Cited in This Article: ] |
| 8. | Higby DJ, Wallace HJ, Holland JF. Cis-diamminedichloroplatinum (NSC-119875): a phase I study. Cancer Chemother Rep. 1973;57:459-463. [PubMed] [Cited in This Article: ] |
| 9. | Kuan Y, Hossain M, Surman J, El Nahas AM, Haylor J. GFR prediction using the MDRD and Cockcroft and Gault equations in patients with end-stage renal disease. Nephrol Dial Transplant. 2005;20:2394-2401. [PubMed] [Cited in This Article: ] |
| 10. | Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-470. [PubMed] [Cited in This Article: ] |
| 11. | Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31-41. [PubMed] [Cited in This Article: ] |
| 12. | Jones CA, McQuillan GM, Kusek JW, Eberhardt MS, Herman WH, Coresh J, Salive M, Jones CP, Agodoa LY. Serum creatinine levels in the US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis. 1998;32:992-999. [PubMed] [Cited in This Article: ] |
| 13. | Buitrago F, Calvo JI, Gómez-Jiménez C, Cañón L, Robles NR, Angulo E. [Comparison and agreement of the Cockcroft-Gault and MDRD equations to estimate glomerular filtration rate in diagnosis of occult chronic kidney disease]. Nefrologia. 2008;28:301-310. [PubMed] [Cited in This Article: ] |
| 14. | Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137-147. [PubMed] [Cited in This Article: ] |
| 15. | Launay-Vacher V, Oudard S, Janus N, Gligorov J, Pourrat X, Rixe O, Morere JF, Beuzeboc P, Deray G. Prevalence of Renal Insufficiency in cancer patients and implications for anticancer drug management: the renal insufficiency and anticancer medications (IRMA) study. Cancer. 2007;110:1376-1384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 301] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 16. | Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int. 2008;73:538-546. [PubMed] [Cited in This Article: ] |
| 17. | Bagshaw SM, George C, Bellomo R. A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1569-1574. [PubMed] [Cited in This Article: ] |
| 18. | Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol. 2003;23:460-464. [PubMed] [Cited in This Article: ] |
| 19. | Maniccia-Bozzo E, Espiritu MB, Singh G. Differential effects of cisplatin on mouse hepatic and renal mitochondrial DNA. Mol Cell Biochem. 1990;94:83-88. [PubMed] [Cited in This Article: ] |
| 20. | Salazar I, Tarrago-Litvak L, Gil L, Litvak S. The effect of benzo[a]pyrene on DNA synthesis and DNA polymerase activity of rat liver mitochondria. FEBS Lett. 1982;138:45-49. [PubMed] [Cited in This Article: ] |
| 21. | Aass N, Fosså SD, Aas M, Lindegaard MW. Renal function related to different treatment modalities for malignant germ cell tumours. Br J Cancer. 1990;62:842-846. [PubMed] [Cited in This Article: ] |
| 22. | Schetz M, Dasta J, Goldstein S, Golper T. Drug-induced acute kidney injury. Curr Opin Crit Care. 2005;11:555-565. [PubMed] [Cited in This Article: ] |
| 23. | Grau JJ, Estapé J, Cuchi MA, Fírvida JL, Blanch JL, Ascaso C. Calcium supplementation and ototoxicity in patients receiving cisplatin. Br J Clin Pharmacol. 1996;42:233-235. [PubMed] [Cited in This Article: ] |
| 24. | Blachley JD, Hill JB. Renal and electrolyte disturbances associated with cisplatin. Ann Intern Med. 1981;95:628-632. [PubMed] [Cited in This Article: ] |
| 25. | Uozumi J, Koikawa Y, Yasumasu T, Tokuda N, Kumazawa J. The protective effect of methylprednisolone against cisplatin-induced nephrotoxicity in patients with urothelial tumors. Int J Urol. 1996;3:343-347. [PubMed] [Cited in This Article: ] |
| 26. | Laurell G, Jungnelius U. High-dose cisplatin treatment: hearing loss and plasma concentrations. Laryngoscope. 1990;100:724-734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 102] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Heidemann HT, Gerkens JF, Jackson EK, Branch RA. Attenuation of cisplatinum-induced nephrotoxicity in the rat by high salt diet, furosemide and acetazolamide. Naunyn Schmiedebergs Arch Pharmacol. 1985;329:201-205. [PubMed] [Cited in This Article: ] |
| 28. | Litterst CL, LeRoy AF, Guarino AM. Disposition and distribution of platinum following parenteral administration of cis-dichlorodiammineplatinum(II) to animals. Cancer Treat Rep. 1979;63:1485-1492. [PubMed] [Cited in This Article: ] |
| 29. | Lichtman SM, Wildiers H, Launay-Vacher V, Steer C, Chatelut E, Aapro M. International Society of Geriatric Oncology (SIOG) recommendations for the adjustment of dosing in elderly cancer patients with renal insufficiency. Eur J Cancer. 2007;43:14-34. [PubMed] [Cited in This Article: ] |
| 30. | Merouani A, Davidson SA, Schrier RW. Increased nephrotoxicity of combination taxol and cisplatin chemotherapy in gynecologic cancers as compared to cisplatin alone. Am J Nephrol. 1997;17:53-58. [PubMed] [Cited in This Article: ] |