Published online Jul 14, 2011. doi: 10.3748/wjg.v17.i26.3151
Revised: April 26, 2011
Accepted: May 3, 2011
Published online: July 14, 2011
AIM: To analyze the microbiota shift in the distal esophagus of Sprague-Dawley rats fed a high-fat diet.
METHODS: Twenty Sprague-Dawley rats were divided into high-fat diet and normal control groups of 10 rats each. The composition of microbiota in the mucosa from the distal esophagus was analyzed based on selective culture. A variety of Lactobacillus species were identified by molecular biological techniques. Bacterial DNA from Lactobacillus colonies was extracted, and 16S rDNA was amplified by PCR using bacterial universal primers. The amplified 16S rDNA products were separated by denaturing gradient gel electrophoresis (DGGE). Every single band was purified from the gel and sent to be sequenced.
RESULTS: Based on mucosal bacterial culturing in the distal esophagus, Staphylococcus aureus was absent, and total anaerobes and Lactobacillus species were decreased significantly in the high-fat diet group compared with the normal control group (P < 0.01). Detailed DGGE analysis on the composition of Lactobacillus species in the distal esophagus revealed that Lactobacillus crispatus, Lactobacillus gasseri (L. gasseri) and Lactobacillus reuteri (L. reuteri) comprised the Lactobacillus species in the high-fat diet group, while the composition of Lactobacillus species in the normal control group consisted of L. gasseri, Lactobacillus jensenii and L. reuteri.
CONCLUSION: High-fat diet led to a mucosal microflora shift in the distal esophagus in rats, especially the composition of Lactobacillus species.
-
Citation: Zhao X, Liu XW, Xie N, Wang XH, Cui Y, Yang JW, Chen LL, Lu FG.
Lactobacillus species shift in distal esophagus of high-fat-diet-fed rats. World J Gastroenterol 2011; 17(26): 3151-3157 - URL: https://www.wjgnet.com/1007-9327/full/v17/i26/3151.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i26.3151
Gastroesophageal reflux disease (GERD) is one of the most common clinical disorders, and its incidence has been steadily increasing around the world in recent years[1-4]. Numerous studies have demonstrated that obesity is a risk factor for GERD. The incidence of GERD and its complications in subjects who are overweight or obese is higher than in those with normal weight[1-4]. With obesity gradually increasing worldwide, obesity-associated GERD is becoming a more complicated clinical problem. Unfortunately, to date, the pathogenesis of obesity-associated GERD has not been fully understood. Thus, the usual management of GERD is merely to inhibit acid production and improve motility of the upper gastrointestinal tract to ameliorate symptoms, which can seriously affect quality of life[5-8].
It is well accepted that GERD is mainly due to abnormality of the gastroesophageal junction or a decrease in esophageal clearance capacity. Current data have demonstrated that the association of obesity with GERD is mainly related to changes in lower esophageal sphincter motility or a pressure imbalance between the distal esophagus and gastric fundus[1-8]. However, the detailed mechanism needs to be further elucidated.
Recent studies have found that the composition of intestinal flora in obese individuals differs from that in normal individuals. Bacterial diversity, either different composition or quantity of bacteria in the gastrointestinal tract, is associated with gastrointestinal smooth muscle motility[9-13]. In clinical practice, many bacterial agents have been used for treatment of diarrhea, irritable bowel syndrome and other gastrointestinal motility diseases, and have achieved a positive response. Based upon the published findings, it can be estimated that microflora in the distal esophagus, to some extent, might play a role in regulating smooth muscle motility. However, the features of colonization and functionality of the microflora in the distal esophagus remain obscure. Some evidence has shown that the esophageal bacteria are transitory, and other studies have demonstrated a complex, residential microflora in the distal esophagus. Several studies also have confirmed the presence of a residential bacterial population in the distal esophagus in patients with esophageal-reflux-related disorders[14,15]. More detailed research has shown a significant alteration in the number of lactobacilli in the intestine of obese rats[11,16-19]. Thus, it is reasonable that obesity, a passive risk factor for GERD, might alter the composition of microflora in the distal esophagus, and lead to the formation of GERD.
Therefore, we hypothesize that potential variations in microbial composition in the distal esophagus of obese individuals might influence the distal esophageal motility and lead to GERD. Thus, we focused on the variation in microflora, especially Lactobacillus species, in the distal esophagus in obese Sprague-Dawley rats induced by high-fat diet, compared with normal rats, to illustrate the role of obesity or high-fat diet in the microfloral composition shift.
Twenty-five healthy male Sprague-Dawley rats (purchased from the Experimental Animal Co., Hayes Lake, Hunan, China) aged 3 wk, weighing 50-70 g, were used. The animal experiments were approved by the Animal Experiment Ethics Committee of Central South University, Changsha, China.
Sprague-Dawley rats were housed individually in cages at constant room temperature of 18-22°C, 50% humidity, in a 12-h light/dark cycle, and had free access to common diet and water. After 1 wk of adaptive feeding, five rats were killed and the other 20 were randomly divided into two groups of 10. One group was fed a high-fat diet (Dongchuang Nursery, Hunan, China; Table 1) for 6 wk, and the normal control group was fed a common diet. The daily diet was sterilized by Co60 irradiation and water by autoclave before feeding to the rats. Rats were killed at the end of 7 wk. Body weight, body length and feed consumption were recorded weekly and daily[20-22].
| Diet | Protein (g) | Fat (g) | Carbohydrate (g) | Energy (KJ) |
| Common diet | 17.53 | 6.08 | 59.98 | 1250 |
| High-fat diet | 16.52 | 25.17 | 56.66 | 1810 |
The esophagus of each rat was removed and dissected longitudinally. Esophageal specimens were obtained from 1.5 cm above the gastroesophageal junction. Then 0.5 cm of each longitudinal strip was fixed in 10% formalin-buffered saline, embedded in paraffin, and processed for histopathological analysis. Two sections were cut from each paraffin block and stained with hematoxylin-eosin (HE) for evaluation of inflammation.
After fasting for 10 h, venous blood was obtained at the end of 7 wk. Three hundred microliters of serum for each sample was extracted and stored at -20°C. Serum triglyceride and total cholesterol were detected by GPO-PAP and CHOD-PAP methods, respectively.
The esophagus of each rat was removed and dissected longitudinally. Esophageal mucosa samples were obtained from 1 cm above the gastroesophageal junction. After being weighed on an electronic balance, the samples were homogenized immediately, and the homogenates were diluted at 10-7 in sterile normal saline. Ten microliters was spread thoroughly over the surfaces of specific medium plates. The media utilized were as follows: Mannitol Salt Agar for Staphylococcus aureus (S. aureus); Actinomyces Agar for Actinomyces; TTC Agar for Enterococcus; KF Streptococcus Agar for Streptococcus; EMB Agar for Enterobacter; Anaerobic Agar for total anaerobes; Clostridium Agar for Clostridium; PY Agar for Clostridium perfringens; LBS Agar for Lactobacillus and Lactobacillus crispatus (L. crispatus); and BL Agar for Bifidobacterium. All aerobic plates were incubated at 37°C for 24 h, followed by incubation for 12 h at room temperature. The LBS plates were incubated in a candle-jar atmosphere of 10% CO2. Anaerobes were grown in an anaerobic jar at 37°C for 72 h. The number of colonies from each plate was recorded.
DNA from Lactobacillus cultures was extracted according to the protocol of BioTeke Corporation (Beijing, China) and stored at -20°C. Universal bacterial primers (Invitrogen Biotechnology, Shanghai, China) HAD1-GC(CGCCCGGGGCGCGCCCCGGGCGGGGCGGGGGCACGGGGGGACTCCTACGGGAGGCAGCAGT-3′) and HAD2(5′-GTATTACCGCGGCTGCTGGCAC-3′)[23] were used to amplify V2 to V3 regions of the bacterial 16S rDNA. PCR reactions were run at 95°C for 5 min, followed by 38 cycles of amplification at 94°C for 40 s, 52°C for 40 s, and 72°C for 50 s, and a 7-min extension at 72°C.
Denaturing gradient gel electrophoresis (DGGE) was performed with the DCode™ Universal Detection System (Bio-Rad) that utilized 16 cm × 16 cm × 1 cm gels. Eight percent polyacrylamide gels were prepared and run with 1 × TAE buffer (2 mol/mL Tris base, 1 mol/mL glacial acetic acid, and 50 mmol/mL EDTA) diluted from 50 × TAE buffer (Sigma, Beijing, China). The denaturing gradient was formed with two 8% acrylamide (acrylamide/bis, 37.5:1) stock solutions (Bio-Rad). The gels contained a 30%-70% gradient of urea and formamide that increased in the direction of electrophoresis. A 100% denaturing solution contained 40% (v/v) formamide and 7.0 mol/mL urea. The electrophoresis was conducted with a constant voltage of 110 V at 60°C for 12 h. Gels were stained with ethidium bromide solution (5 μg/mL) for 30 min, washed with deionized water, and viewed by UV transillumination (Bio-Rad).
Single predominant bands of the DGGE gel were selected by cutting with a sterile scalpel, and added to 50 μL deionized sterile water (Fermentas Life Sciences, Shenzhen, China). The gel pieces were placed at 4°C for 24 h, centrifuged at 12 000 ×g for 10 min, and the supernatants were used as templates for PCR amplification. Universal bacterial primers (Invitrogen Biotechnology) HAD1(5′-TCCTACGGGAGGCAGCAGT-3′) and HAD2(5′-GTATTACCGCGGCTGCTGGCAC-3′)[23] were used for amplification. For each PCR amplification, 5 μL template was added to 45 μL PCR reaction mixture (Fermentas Life Sciences) that contained 5 μL 10 × PCR buffer, 5 μL 25 mmol/L MgCl2, 1.5 μL 10 mmol/L dNTPs, 1.5 μL 100 ng/μL each primer, and 2.5 U Taq DNA polymerase. Reactions were run at 95°C for 5 min, followed by 36 cycles of amplification at 94°C for 30 s, 56°C for 40 s, and 72°C for 40 s, and a 10-min extension at 72°C. Wizard PCR Preps DNA Purification System (Promega, Beijing, China) were used to purify the PCR products. The purified products were sent to Beijing Genomics Institute of Technology for sequencing. Blast sequences were obtained through GenBank BLAST searches (http://www.ncbi.nlm.nih.gov/blast).
Statistical analysis was performed using SPSS for Windows version 15.0. Results were expressed as measurement and enumeration data. Data are presented as means and SDs. For statistical comparisons, the t test and χ2 test were performed and P < 0.05 was considered statistically significant.
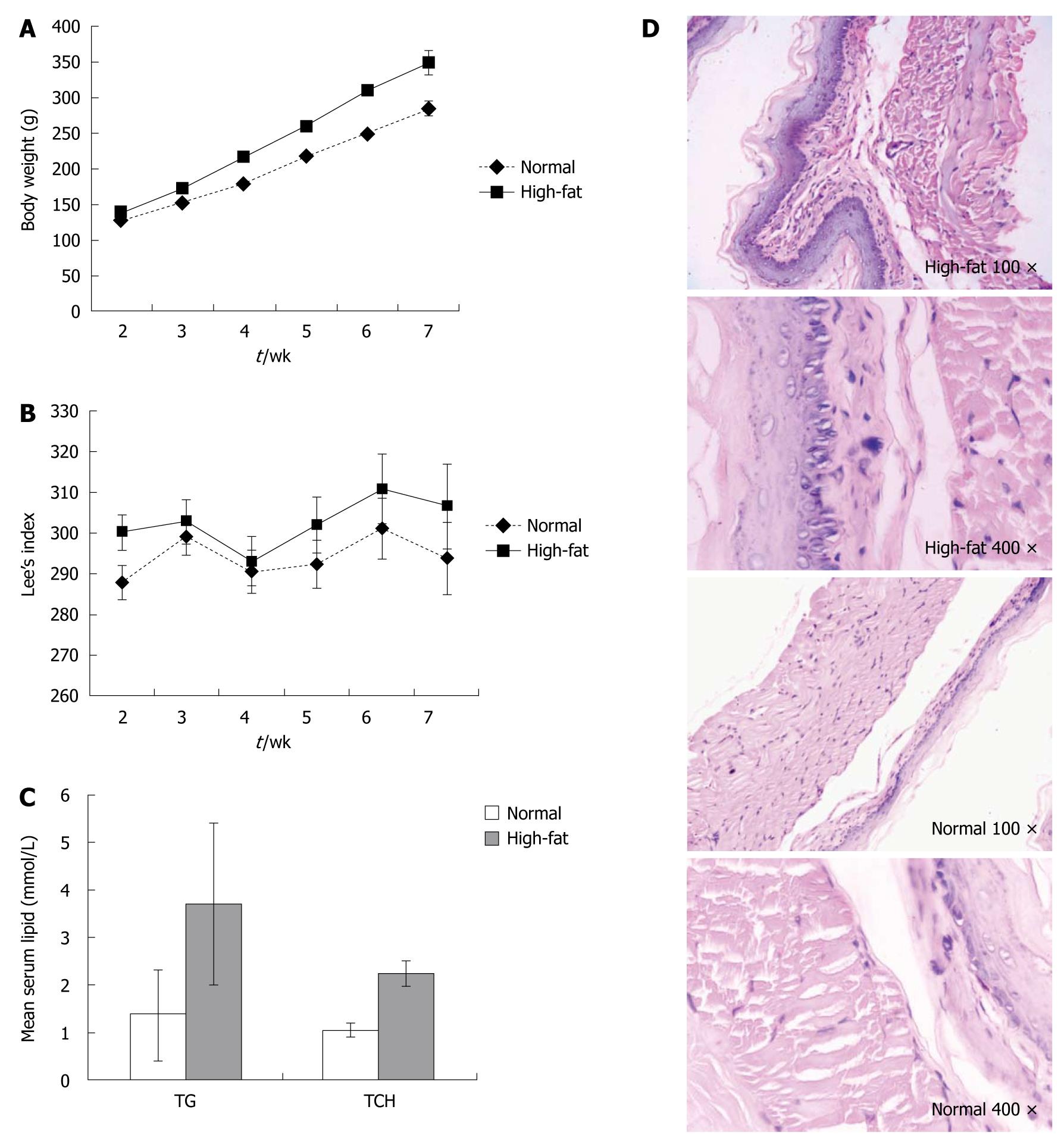
There was no significant change in body weight in the high-fat diet and normal control groups at the end of the first week. Since the second week, the body weight and Lee’s index increased weekly (Figure 1A and B). Accordingly, serum triglyceride and total cholesterol in the high-fat diet group were significantly increased compared with the normal control group (Figure 1C). HE staining of esophageal mucosa showed no musculature damage or mucosal injury in the distal esophagus in the high-fat diet or normal control group (Figure 1D).
Bacteria in the distal esophagus were cultured on selective media. In the high-fat diet group, S. aureus was absent, and the numbers of total anaerobes and lactobacilli were reduced significantly in the distal esophagus compared with the normal control group. There was no obvious difference between the common and adaptive feeding groups for composition of cultivable bacteria in the distal esophagus of Sprague-Dawley rats (Table 2).
| Adaptive group (n = 5) | High-fat diet (n = 10) | Common diet (n = 10) | |
| Total aerobic bacteria | 4.92 ± 0.83 | 3.81 ± 1.83 | 4.45 ± 1.46 |
| S. aureus | 4.80 ± 1.42 | 0 | 3.80 ± 2.83a |
| Actinomycetes | 4.52 ± 1.61 | 4.58 ± 0.47 | 4.12 ± 1.51 |
| Enterococcus | 3.21 ± 1.64 | 2.02 ± 1.82 | 2.89 ± 2.47 |
| Streptococcus | 4.14 ± 1.91 | 2.82 ± 3.55 | 3.74 ± 2.19 |
| Enterobacter | 3.22 ± 1.47 | 1.45 ± 2.91 | 3.05 ± 1.32 |
| Total anaerobes | 4.61 ± 0.42 | 2.11 ± 0.80a | 4.48 ± 0.38a |
| Clostridium | 4.47 ± 1.67 | 5.28 ± 1.60 | 4.77 ± 1.86 |
| Clostridium perfringens | 4.97 ± 1.49 | 3.59 ± 2.49 | 5.11 ± 1.58 |
| Lactobacillus | 5.31 ± 1.62 | 2.44 ± 0.97 | 5.44 ± 1.54a |
| Bifidobacterium | 4.24 ± 2.97 | 2.53 ± 2.68 | 4.32 ± 3.21 |
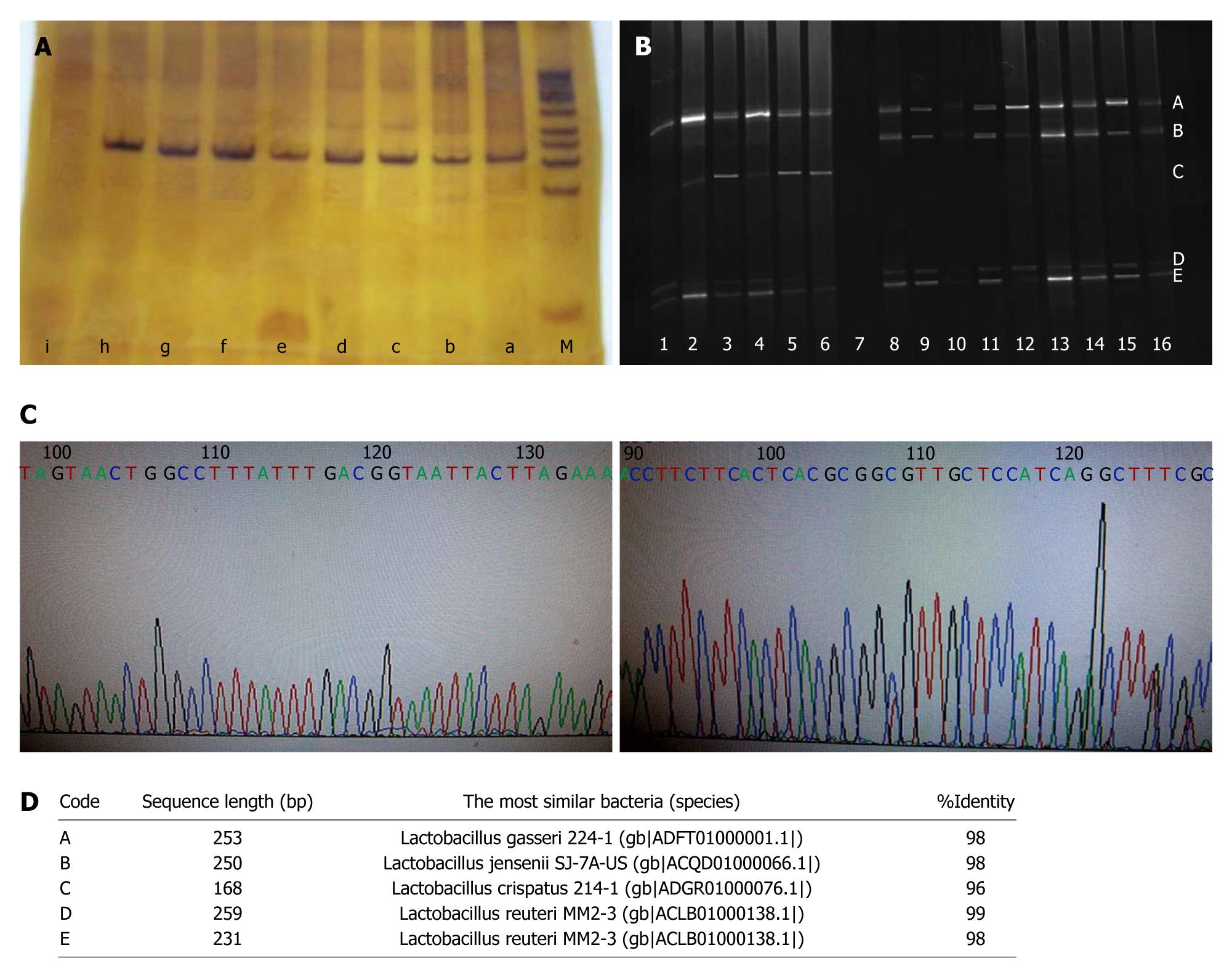
16S rDNA of cultivable Lactobacillus from the high-fat and common diet groups was amplified by PCR using universal bacteria primers HAD1-GC and HAD2 (Figure 2A). The amplified products of 16S rDNA were separated by DGGE. The two groups shared dramatically different bands, with bands A, C, D and E in the high-fat diet group, and bands B, C, D and E in the common diet group (Figure 2B). The purified bands were sequenced and BLASTed online with the V2-V3 region. The composition of Lactobacillus species in the distal esophagus in the common diet group was Lactobacillus gasseri (L. gasseri), Lactobacillus jensenii (L. jensenii) and Lactobacillus reuteri (L. reuteri), whereas the composition shifted to L. crispatus, L. gasseri and L. reuteri in the high-fat diet group (Figure 2C and D).
Accumulating evidence has shown that obesity is a risk factor in the development of GERD[1-8,24,25]. However, is not well established how obesity affects the incidence of GERD. According to the published experimental data, no musculature damage has been reported in the distal esophagus of obese rats or mice. However, different components of the microbiota have been identified between obese and lean animals/humans. Based on this evidence, the purpose of this study was to analyze the microbiota composition and focus on how the shift in microflora occurred in the distal esophagus of rats fed a high-fat diet.
To date, the role of microbiota in the distal esophagus under conditions of weight gain remains obscure. Some studies have shown that high-fat/high-sugar chow diet-fed, germ-free mice gain significantly less weight than their control littermates. The absence of microbiota has a protective role against weight gain in mice that consume a Western-style diet. Thus, body weight gain could be associated with a shift in the microbiota in mice fed a western-style diet. On the other hand, current research has demonstrated that there is a complex residential microbiota shift in the distal esophagus of patients with esophageal-reflux-related disorders, such as GERD, reflux esophagitis and Barrett’s esophagus[14,15,26]. The possible explanations are that the reflux content might contain gastric bacteria or damage the esophageal mucosa and lead to an abnormal inhabitation niche for the distal esophageal microbiota. Thus, the abnormal interactions among distal esophageal residential microbiota, gastric bacteria, and distal esophageal mucosa could lead to a composition shift in the microbiota in the distal esophagus.
Based on analysis of cultivable microbiota of the distal esophagus, we found a certain microbiota compositional shift in the distal esophagus of obese rats. The major microbiota shift in the distal esophagus involved the loss of S. aureus and decrease in anaerobes and Lactobacillus species. The focus in this research was diet-associated obesity, with integrated esophageal mucosal changes, and no obvious histological changes. This means that the microbiota shift in the distal esophagus was not due to the reflux of gastric content or alteration of the inhabitable niche of microbiota in the distal esophagus. Thus, a possible explanation for this microbiota shift in mice fed a western-style diet, compared with their control littermates, could be the components of high-energy materials in the distal esophageal mucosa. These high-energy components might alter the mucosal microenvironment and help the local bacteria to colonize the distal esophageal mucosa.
GERD is an obvious motility disorder of the distal esophagus. Recent research has shown that several Lactobacillus or other bacterial species can alter the motility of smooth muscle in the distal esophagus[9-12]. It has been shown that different composition of Lactobacillus species can lead to different influences on smooth muscle motility in the distal esophagus[9-12]. However, the details are still unclear. Some evidence has shown increased smooth muscle contraction in certain GERD cases, whereas other studies have shown opposite results. The probable reason is that analysis of the shift in Lactobacillus was restricted to the level of the Lactobacillus genus. Fortunately, with the development of modern molecular techniques, analysis of Lactobacillus at the species level has become possible.
In our study, the combination of bacterial culture and DGGE was used to analyze the composition of Lactobacillus in the distal esophagus. It was clearly demonstrated that three cultivable Lactobacillus species, L. gasseri, L. jensenii and L. reuteri, colonized the distal esophagus in normal control rats. However, the composition of Lactobacillus species was shifted to L. crispatus, L. gasseri and L. reuteri in the distal esophagus in high-fat diet-fed rats. Four Lactobacillus species constituted a specific group in the distal esophagus of normal control rats as well as in obese rats. To our surprise, L. jensenii disappeared from the mucosa of the distal esophagus, which was replaced by L. crispatus in obese rats. It is possible that the disappearance of L. jensenii and re-colonization with L. crispatus results in alteration of smooth muscle motility in the distal esophagus. Therefore, this could probably be involved in the etiology of GERD, because of the variations in cultivable Lactobacillus species in the distal esophagus in obese rats. Another reasonable explanation is that different Lactobacillus species could have different metabolic characteristics, and influence the inhabitation niche of the microbiota in the distal esophagus. Yet another possibility is that the composition of cultivable Lactobacillus species in the distal esophagus of obese rats could be beneficial in preventing esophageal reflux.
Clearly, many unresolved questions remain to be elucidated. Our research will next focus on the effects of the composition shift in Lactobacillus on smooth muscle motility in the distal esophagus. Then, we will distinguish between the promoting or inhibitory properties of ingredients or metabolites of different Lactobacillus species. Therefore, the data could result in potential therapeutic targets in GERD.
Obesity is a risk factor for gastroesophageal reflux disease (GERD), but how obesity affects the incidence of GERD is not well established. Current research has demonstrated that there is a complex residential microbiota shift in distal esophagus of esophageal-reflux-related disorders in humans. The different components of the microbiota are identified between obese animals/humans and lean animals/humans. Thus, it is reasonable that obesity, a passive risk factor of GERD, might alter the composition of microflora in the distal esophagus, and lead to development of GERD.
Bacterial diversity is associated with gastrointestinal smooth muscle motility, and is also identified between obese animals/humans and lean animals/humans. However, the microbiota shift in the distal esophagus in obesity has not been fully addressed. In this study, we demonstrated the composition of cultivable Lactobacillus species shift in the distal esophagus of obese rats.
Published data show that different constitution of Lactobacillus species may exert different influences on smooth muscle motility in the distal esophagus. However, the details are still unclear. Analysis on the shift in Lactobacillus is restrained at the genus level. This study analyzed the composition of Lactobacillus in the distal esophagus, and found that the composition of cultivable Lactobacillus species shifted in the distal esophagus in obese rats. Therefore, potential variations in microbial composition in the distal esophagus in obesity may influence the distal esophageal motility and lead to GERD.
This study proved that the composition of cultivable Lactobacillus species shifted in the distal esophagus in obese rats, therefore, it may represent a further study between the microbial composition shift in the distal esophagus and GERD. This could lead to the development of a potential therapeutic target in GERD.
GERD is a more serious form of gastroesophageal reflux, which is common. Persistent reflux that occurs more than twice weekly is considered GERD, and it can affect people of all ages. The main symptom of GERD in adults is frequent heartburn, also called acid indigestion - burning-type pain in the lower part of the mid-chest, behind the breast bone, and in the mid-abdomen.
This study considers the investigation of microbiota composition in the distal esophagus of high-fat-diet-fed rats. The authors hypothesize that potential variations in microbial composition in the distal esophagus of obese individuals may influence distal esophageal motility and lead to GERD. In this study, the composition of Lactobacillus spp. was analyzed using the combination of bacterial culturing, denaturing gradient gel electrophoresis and sequencing in a rat model. The interesting and important finding of this study was the fact that high-fat diet led to a shift in mucosal microflora (especially Lactobacillus species) in the distal esophagus in rats, which resulted in alteration of smooth muscle motility. This study makes an additional contribution to studies of the etiology of GERD.
Peer reviewer: Dr. Tamara Vorobjova, MD, PhD, Scimed. Senior Researcher in Immunology, Department of Immunology, Institute of General and Molecular Pathology, University of Tartu, Ravila, 19, Tartu, 51014, Estonia
S- Editor Sun H L- Editor Kerr C E- Editor Zheng XM
| 1. | El-Serag H. The association between obesity and GERD: a review of the epidemiological evidence. Dig Dis Sci. 2008;53:2307-2312. [Cited in This Article: ] |
| 2. | Sakaguchi M, Oka H, Hashimoto T, Asakuma Y, Takao M, Gon G, Yamamoto M, Tsuji Y, Yamamoto N, Shimada M. Obesity as a risk factor for GERD in Japan. J Gastroenterol. 2008;43:57-62. [Cited in This Article: ] |
| 3. | Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005;143:199-211. [Cited in This Article: ] |
| 4. | El-Serag HB, Ergun GA, Pandolfino J, Fitzgerald S, Tran T, Kramer JR. Obesity increases oesophageal acid exposure. Gut. 2007;56:749-755. [Cited in This Article: ] |
| 5. | Eslick GD, Talley NJ. Gastroesophageal reflux disease (GERD): risk factors, and impact on quality of life-a population-based study. J Clin Gastroenterol. 2009;43:111-117. [Cited in This Article: ] |
| 6. | Ali T, Miner PB. New developments in gastroesophageal reflux disease diagnosis and therapy. Curr Opin Gastroenterol. 2008;24:502-508. [Cited in This Article: ] |
| 7. | Herbella FA, Sweet MP, Tedesco P, Nipomnick I, Patti MG. Gastroesophageal reflux disease and obesity. Pathophysiology and implications for treatment. J Gastrointest Surg. 2007;11:286-290. [Cited in This Article: ] |
| 8. | Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54:710-717. [Cited in This Article: ] |
| 9. | Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299:853-855. [Cited in This Article: ] |
| 10. | Bajzer M, Seeley RJ. Physiology: obesity and gut flora. Nature. 2006;444:1009-1010. [Cited in This Article: ] |
| 11. | DiBaise JK, Zhang H, Crowell MD, Krajmalnik-Brown R, Decker GA, Rittmann BE. Gut microbiota and its possible relationship with obesity. Mayo Clin Proc. 2008;83:460-469. [Cited in This Article: ] |
| 12. | Massi M, Ioan P, Budriesi R, Chiarini A, Vitali B, Lammers KM, Gionchetti P, Campieri M, Lembo A, Brigidi P. Effects of probiotic bacteria on gastrointestinal motility in guinea-pig isolated tissue. World J Gastroenterol. 2006;12:5987-5994. [Cited in This Article: ] |
| 13. | Wu WC, Zhao W, Li S. Small intestinal bacteria overgrowth decreases small intestinal motility in the NASH rats. World J Gastroenterol. 2008;14:313-317. [Cited in This Article: ] |
| 14. | Pei Z, Bini EJ, Yang L, Zhou M, Francois F, Blaser MJ. Bacterial biota in the human distal esophagus. Proc Natl Acad Sci USA. 2004;101:4250-4255. [Cited in This Article: ] |
| 15. | Pei Z, Yang L, Peek RM, Jr Levine SM, Pride DT, Blaser MJ. Bacterial biota in reflux esophagitis and Barrett’s esophagus. World J Gastroenterol. 2005;11:7277-7283. [Cited in This Article: ] |
| 16. | Mozes S, Bujnáková D, Sefcíková Z, Kmet V. Developmental changes of gut microflora and enzyme activity in rat pups exposed to fat-rich diet. Obesity (Silver Spring). 2008;16:2610-2615. [Cited in This Article: ] |
| 17. | Tennyson CA, Friedman G. Microecology, obesity, and probiotics. Curr Opin Endocrinol Diabetes Obes. 2008;15:422-427. [Cited in This Article: ] |
| 18. | Sefcíková Z, Kmet V, Bujnáková D, Racek L, Mozes S. Development of gut microflora in obese and lean rats. Folia Microbiol (Praha). 2010;55:373-375. [Cited in This Article: ] |
| 19. | Mozes S, Bujnáková D, Sefcíková Z, Kmet V. Intestinal microflora and obesity in rats. Folia Microbiol (Praha). 2008;53:225-228. [Cited in This Article: ] |
| 20. | West DB, York B. Dietary fat, genetic predisposition, and obesity: lessons from animal models. Am J Clin Nutr. 1998;67:505S-512S. [Cited in This Article: ] |
| 21. | Xu ZJ, Fan JG, Ding XD, Qiao L, Wang GL. Characterization of high-fat, diet-induced, non-alcoholic steatohepatitis with fibrosis in rats. Dig Dis Sci. 2010;55:931-940. [Cited in This Article: ] |
| 22. | Bernardis LL, Patterson BD. Correlation between ‘Lee index’ and carcass fat content in weanling and adult female rats with hypothalamic lesions. J Endocrinol. 1968;40:527-528. [Cited in This Article: ] |
| 23. | Walter J, Tannock GW, Tilsala-Timisjarvi A, Rodtong S, Loach DM, Munro K, Alatossava T. Detection and identification of gastrointestinal Lactobacillus species by using denaturing gradient gel electrophoresis and species-specific PCR primers. Appl Environ Microbiol. 2000;66:297-303. [Cited in This Article: ] |
| 24. | El-Serag HB, Satia JA, Rabeneck L. Dietary intake and the risk of gastro-oesophageal reflux disease: a cross sectional study in volunteers. Gut. 2005;54:11-17. [Cited in This Article: ] |
| 25. | Küper MA, Kramer KM, Kirschniak A, Zdichavsky M, Schneider JH, Stüker D, Kratt T, Königsrainer A, Granderath FA. Dysfunction of the lower esophageal sphincter and dysmotility of the tubular esophagus in morbidly obese patients. Obes Surg. 2009;19:1143-1149. [Cited in This Article: ] |
| 26. | Yang L, Lu X, Nossa CW, Francois F, Peek RM, Pei Z. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology. 2009;137:588-597. [Cited in This Article: ] |