Published online Jun 7, 2011. doi: 10.3748/wjg.v17.i21.2674
Revised: April 19, 2011
Accepted: April 26, 2011
Published online: June 7, 2011
AIM: To investigate the contribution of periostin in nicotine-promoted gastric cancer cell proliferation, survival, invasion, drug resistance, and epithelial-mesenchymal transition (EMT).
METHODS: Gastric cancer cells were treated with nicotine and periostin protein expression was determined by immunoblotting. Periostin mRNA in gastric cancer cells was silenced using small interfering RNA (siRNA) techniques and periostin gene expression was evaluated by quantitative reverse transcription-polymerase chain reaction. Gastric cancer cells transfected with control or periostin siRNA plasmid were compared in terms of cell proliferation using the methylthiazolyldiphenyl-tetrazolium bromide assay. Cell apoptosis was compared using annexin V-fluoresceine isothiocyanate and propidium iodine double staining. Tumor invasion was determined using the Boyden chamber invasion assay, and the EMT marker Snail expression was evaluated by immunoblotting.
RESULTS: Nicotine upregulated periostin in gastric cancer cells through a COX-2 dependent pathway, which was blocked by the COX-2-specific inhibitor NS398. Periostin mRNA expression was decreased by ~87.2% by siRNA in gastric cancer cells, and stable periostin-silenced cells were obtained by G418 screening. Periostin-silenced gastric cancer cells exhibited reduced cell proliferation, elevated sensitivity to chemotherapy with 5-fluorouracil, and decreased cell invasion and Snail expression (P < 0.05).
CONCLUSION: Periostin is a nicotine target gene in gastric cancer and plays a role in gastric cancer cell growth, invasion, drug resistance, and EMT facilitated by nicotine.
- Citation: Liu Y, Liu BA. Enhanced proliferation, invasion, and epithelial-mesenchymal transition of nicotine-promoted gastric cancer by periostin. World J Gastroenterol 2011; 17(21): 2674-2680
- URL: https://www.wjgnet.com/1007-9327/full/v17/i21/2674.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i21.2674
Gastric cancer is malignant and has a high occurrence. It originates from normal gastric epithelial cells that undergo various precancerous diseases, such as chronic atrophic gastritis and intestinal metaplasia, and then eventually cancer[1].
The relationship between smoking and gastric cancer has received increasing interest. A large population epidemiological investigation revealed that previous and current smokers had a 43% and 57% higher incidence of gastric cancer than non-smokers, respectively[2]. Therefore, smoking is the most important behavioral factor leading to gastric cancer[3]. About 60% of tobacco components are carcinogens, including conjugated aromatic chemicals, nitric amines, aromatic amines, trace metals, and nicotine[4]. Nicotine is the most active carcinogen in cigars, and has been shown to promote gastric cancer cell proliferation and neoangiogenesis in vivo and in vitro[5]. Nicotine activates β-adrenoceptors and the downstream protein kinase C-β, extracellular signal-regulated kinases 1/2, and cyclooxgenase-2 (COX-2) signaling pathways in gastric cancer cells, thus promoting gastric cell mitosis and proliferation[6]. In addition, nicotine contributes to the invasion and metastasis of gastric cancer through the activation of COX-2 and vascular endothelial growth factor receptors in gastric cancer cells[7]. Therefore, COX-2 is an important nicotine-regulated molecule in gastric cancer cells and an important oncogene in the pathogenesis of gastric cancer. Tumor invasion means that malignant cells penetrate the basal membrane, migrate away from primary tumor sites and invade surrounding tissues. Tumor invasion results from the interaction between tumor cells and surrounding mesenchymal cells, leading to further spreading and the malignant advancement of tumors. During the invasion of epithelial-originated malignant tumors, the polarity of the epithelial cells disappears and their migration and mobility increase. Subsequently, epithelial cells are transformed into mesenchymal cells, which is known as the epithelial-mesenchymal transition (EMT).
Periostin, or osteoblast-specific factor 2 (OSF-2), is a newly discovered adhesion molecule that is over-expressed in various tumors, including oral cancer, thyroid cancer, breast cancer, and gastric cancer, and is correlated with tumor invasion, distant metastasis, and angiogenesis[8-11]. Periostin is a mesenchyme-specific protein expressed and secreted by tumor mesenchymal cells. Transfection and over-expression of the periostin gene in 293T cells has been shown to cause EMT as well as cell migration, invasion, and adhesion[12]. Moreover, metastatic loci were formed when recombinant cells over-expressing periostin were transplanted into immune-deficient mice[12].
Although facilitation by nicotine of the invasion and EMT of tumor cells has been confirmed by numerous investigators[5-7], to the best of our knowledge, the relationship between nicotine and the EMT-marker periostin has not been reported. We studied the expression of periostin induced by nicotine treatment in gastric cancer cells in vitro, silenced the periostin gene using small interfering RNA techniques and investigated the effects of nicotine on the growth, apoptosis, and invasion of gastric cancer cells. Our study suggested that periostin might be a downstream target gene regulated by nicotine in gastric cancer cells and may significantly contribute to nicotine-induced proliferation, metastasis, and EMT in gastric cancer cells.
The 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), annexin V-fluoresceine isothiocyanate (V-FITC), propidium iodine (PI), nicotine, and the COX-2-specific inhibitor NS 398 were from Sigma (St. Louis, MO, USA). The fetal bovine serum was from Sijichun Bioengineering Materials, Inc. (Hangzhou, Zhejiang, China). The DMEM culture media was from Invitrogen-GIBCO (Carlsbad, CA, USA). Vectors for periostin siRNA were constructed by lab co-workers. LipofectAMINE-2000 and TRIzol were from Invitrogen. The reverse transcription-polymerase chain reaction (RT-PCR) kits were from Treasure Biological, Inc. (Dalian, Liaoning, China). The forward and reverse primers were from Shanghai Bioengineering Inc. (Shanghai, China). The flow cytometer (FACS-440) was from BD Biosciences (Franklin Lakes, NJ, USA). The inverted phase contrast microscope (CK-40) was from Olympus (Center Valley, PA, USA). The gastric cancer cell line SGC-7901 was from the cell bank of the Shanghai Biological Institute of the Chinese Institute of Science. The T4 DNA Ligase was from Takara Bio Inc. (Otsu, Shiga, Japan) and the Boyden chamber was from Corning Costar Corp. (Cambridge, MA, USA).
Gastric cancer cell line SGC-7901 was cultured in DMEM media (high glucose) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were maintained at 37°C and 5% CO2 in an incubator, and the culture medium was changed every 2-3 d. SGC-7901 cells were treated with nicotine at 200 ng/mL for 24 h before lysing for immunoblotting experiments. To determine if periostin upregulation by nicotine was related to the COX-2 pathway, the COX-2-specific inhibitor NS 398 was applied to SGC-7901 cells 6 h before nicotine treatment.
Targeting sequences (complimentary oligonucleotides) for siRNA of periostin were designed from the human periostin sequence (GenBank accession number NM_006475.2) based on general design principles of siRNA. Forward primers for U6 promoter amplification and reverse primers (at bp 136, 246, and 268) for periostin amplification were used for 2-step PCR to amplify siRNA expression cassettes at 94°C for 30 s and 72°C for 90 s over 40 cycles. PCR end products were ligated with precut plasmid pRNAT-U6.1 and T4 DNA ligase at 16°C overnight. Ligation products were transformed into competent Escherichia coli DH5α, followed by blue-white screening, enzyme digestion confirmation, and glycerol storage for gene sequencing at Shen You, Inc. The pRNAT-U6.1-periostin plasmid with the correct sequence was used to prepare and purify a large amount of plasmid DNA.
The gastric cancer cell line SGC-7901 was recovered, subcultured, and plated in 6-well plates at a density of 4 × 105/mL 24 h before transfection, such that the cells were 90% confluent at transfection. SGC-7901 cells were transfected with either pRNAT-U6.1-periostin siRNA plasmid or pRNAT-U6.1 control plasmid. Cells were observed separately for the periostin gene and protein expression by RT-PCR and immunoblots 48 h after transfection. SGC-7901 cells with successful transient pRNAT-U6.1-periostin siRNA transfection and optimal periostin siRNA were selected, and culture medium with G418 at 400 and 800 μg/mL was used for concentration increase screening.
Total RNA was isolated from SGC-7901 cells with different treatments 48 h after transfection, and RT-PCR was performed to quantify periostin mRNA expression normalized against GAPDH. Five micrograms of RNA was used to synthesize cDNA, followed by PCR.
The periostin forward primer was 5'-GCACTCTGGGCATCGTGGGA-3' and the periostin reverse primer was 5’-AATCCAAGTTGTCCCAAGCC-3'. The GAPDH forward primer was 5'-CTGCACCACCAACTGCTTAG-3' and the GAPDH reverse primer was 5'-TGAAGTCAGAGGAGACCACC-3'.
The amplicons for periostin and GAPDH were 132 and 407 bp, respectively.
The thermal profile of PCR for periostin and GAPDH mRNA detection was 94°C for 4 min over 1 cycle, 94°C for 30 s, 57°C for 30 s, and 72°C for 1 min over 33 cycles, followed by 72°C for 7 min over 1 cycle.
The PCR products were electrophorized on 1.5% agarose gel in Tris-acetate-EDTA (TAE) buffer. The bands of the PCR products were quantified by grayscale readings using a gel imaging system. The ratios of the grayscale readings of the band for periostin vs those for GAPDH using the same samples were calculated as the relative mRNA expression of periostin. Periostin suppression rate (%) = (1 - periostin mRNA relative expression in the pRNAT-U6.1-periostin siRNA group/periostin mRNA relative expression in pRNAT-U6.1 control group) × 100%.
Proteins were isolated from SGC-7901 cells and the protein concentrations were determined. The proteins were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels (polyacrylamide concentration 100 g/L) and electrophoretically transferred to PVDF membranes. The PVDF membranes were blocked with 3% BSA at 37°C for 1 h and probed with the primary antibody mouse anti-human periostin, Snail (1:100), or β-actin (1:1000) monoclonal antibody for 2 h. The bound antibody was detected by horseradish peroxidase-conjugated goat anti-mouse IgG (1:5000) and enhanced chemiluminescence. The blots were washed with 1 × Tris-buffered saline with Tween buffer for 10 min, 3 times between each step. The density of the targeted bands was quantified using the Developer 100 Plus Imaging Analysis System.
The cell growth rate was determined using the methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay and growth curve depictions. Cells with pRNAT-U6.1-periostin siRNA or control plasmid (2 × 105 cell/mL) were seeded onto 96-well plates with 100 μL in each well, cultured with DMEM media supplemented with 10% fetal bovine serum, and observed for cell proliferation at 24, 48, and 72 h after seeding. For the cell viability and growth assay, 10 μL of MTT solution (5 mg/mL) was added into each well and incubated at 37°C for 4 h, and the reaction terminated with a detergent solution to lyse the cells and solubilize the colored formazan crystals. The supernatant was centrifuged at 3000 r/min for 10 min to obtain a formazan pellet. The supernatant was removed, and the pellet was dissolved completely with 100 μL DMSO and observed at a wavelength of 570 nm using an ELISA plate reader.
Cell apoptosis was analyzed by annexin V-FITC and PI double staining. Cells with pRNAT-U6.1-periostin siRNA or control plasmid were evaluated for apoptosis after 24, 48, and 72 h of culture. From each well, 2 × 105 cells were harvested, and the supernatant was collected by centrifugation at 2000 r/min for 5 min. The pellet was washed with PBS buffer once, resuspended in 100 μL 1 × binding buffer, supplied with 2.5 μL annexin V and 5 μL PI (final concentration of 10 μg/mL), incubated for 15 min in the dark, and assayed for cell apoptosis by flow cytometry. The results were analyzed using Lysis software.
The Boyden chamber invasion assay was used to evaluate the ability of tumor cells to invade. Tissue culture plates with 24 wells and Transwell filter membranes were used for the experiments. Cells with pRNAT-U6.1-periostin siRNA or control plasmid were cultured in the presence or absence of nicotine for 4 days, harvested at 3 × 106/mL, and seeded onto the upper part of the Transwell chamber at 100 μL/well. The basement membrane matrix preparation, Matrigel, served as the matrix barrier and the lower part of the Transwell chamber was filled with 500 μL of culture medium as a chemoattractant. The chamber was kept at 37°C in a 5% CO2 incubator for 24 h before regular fixation and Trypan blue staining. The cells on the upper surface of the filter membrane were removed with a cotton swab, and the number of cells migrating through the membrane was determined. Three parallel membranes were counted for each condition. The number of cells migrating through the Matrigel was normalized to those through the non-Matrigel to obtain an index of relative percent migration, indicating the ability of tumor cells to invade in vitro.
Statistical analysis was performed using commercially available software (SPSS version 14.0). Data are presented as the mean ± SE. Means of 2 groups were compared using the Student’s t test (unpaired, two tailed). Means of more than 2 groups were compared using one-way analysis of variance (ANOVA) followed by post-hoc testing using the Least-Significant-Difference test. P < 0.05 was considered statistically significant.
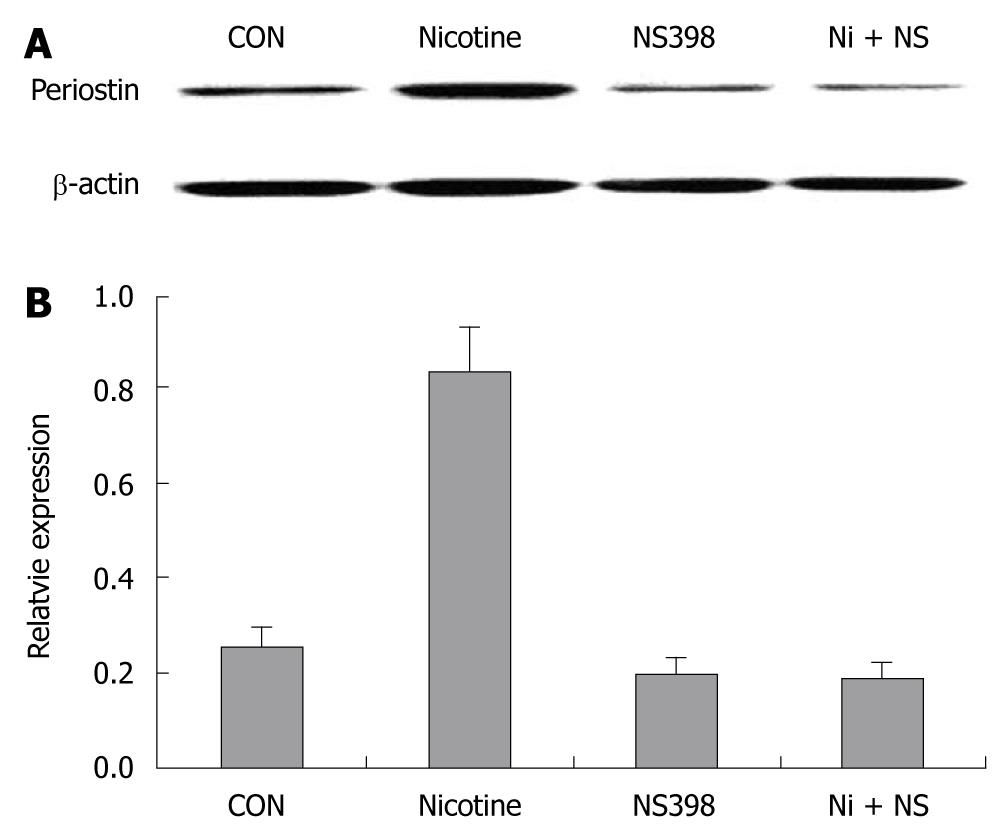
Immunoblotting showed that 200 μg/mL nicotine at 24 h significantly upregulated periostin protein expression in SGC-7901 cells, suggesting that periostin could be a downstream target of nicotine in gastric cancer cells. Pretreatment with the COX-2-specific inhibitor NS 398 almost completely blocked the upregulation of periostin by nicotine in SGC-7901 cells. NS 398 also reduced periostin protein expression in the absence of nicotine in SGC-7901 cells (Figure 1).
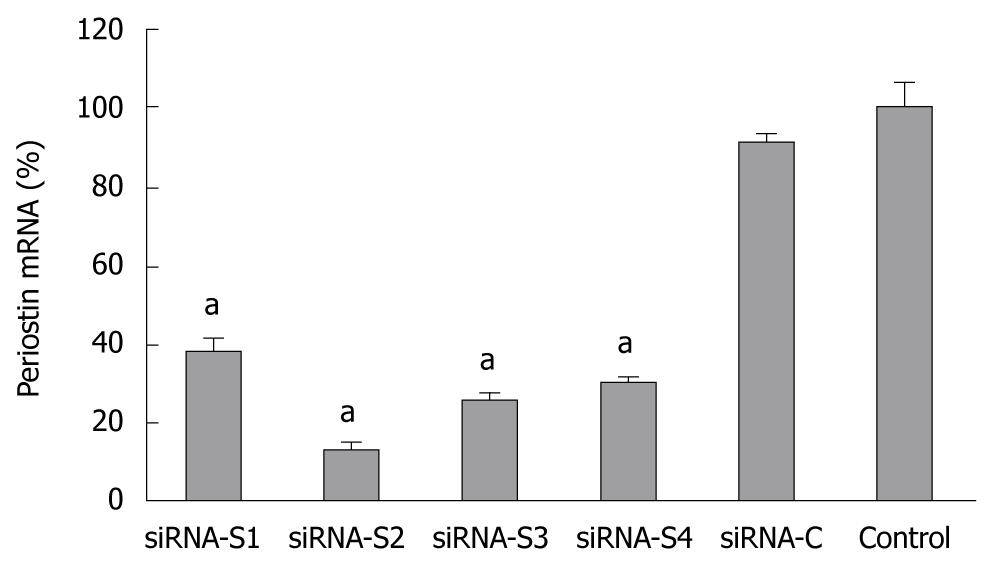
The mRNA level of periostin was significantly reduced in all groups (S1-S4) and was most evident in S2 at 87.2% vs the plasmid control, suggesting that the periostin gene was successfully silenced by siRNA. SGC-7901 cells with successful transfection of the siRNA S2 plasmid were further screened in culture medium supplemented with increasing concentrations of G418 at 400 or 800 μg/mL. The stably silenced cells were finally maintained in culture media with 400 μg/mL G418 (Figure 2).
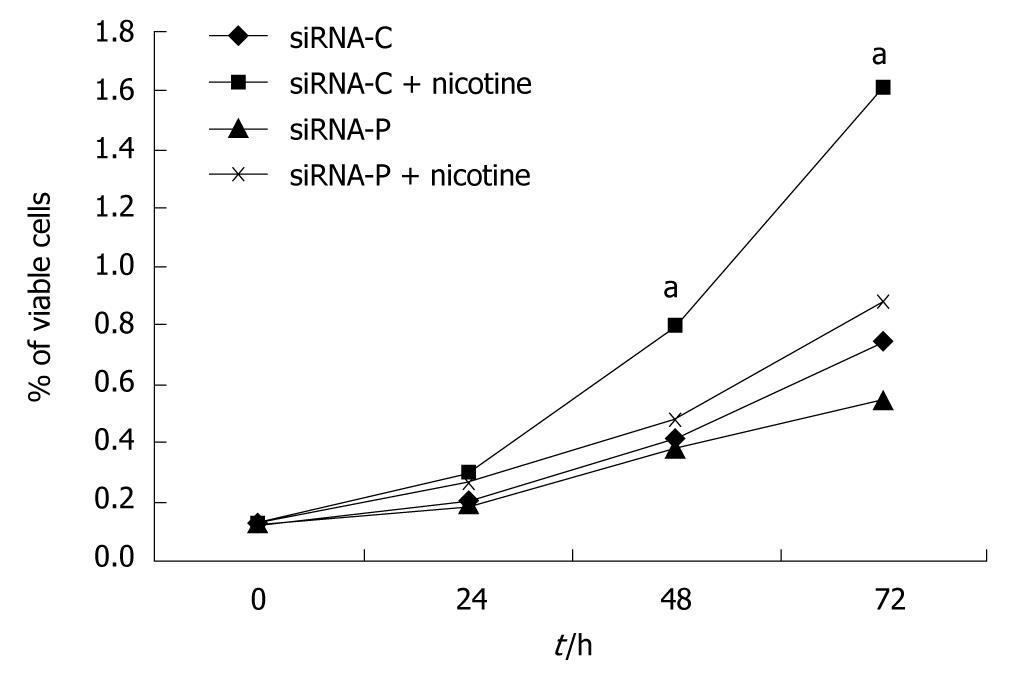
SGC-7901 cells with successful transfection of the control plasmid (siRNA-C) showed slow growth in the MTT assay, but growth was significantly accelerated by nicotine treatment. SGC-7901 cells with successful transfection of the periostin siRNA plasmid (siRNA-P) showed much slower growth vs siRNA-C under both the basal and nicotine-stimulated conditions. Additionally, siRNA-P showed increased proliferation in response to nicotine stimulation, indicating that nicotine-induced cell proliferation could not be fully blocked by silencing periostin in SGC-7901 cells (Figure 3).
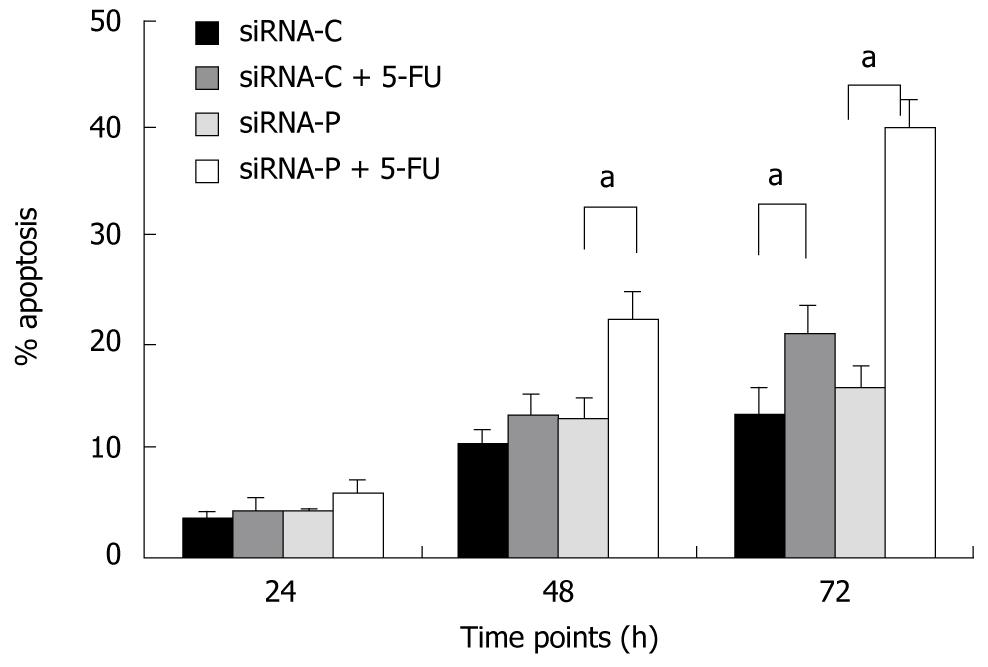
SiRNA-P showed higher sensitivity to 5-fluorouracil (5-FU, 50 mg/L) treatment than did siRNA-C, as revealed by flow cytometry. After 24 h of 5-FU treatment, the apoptotic rate did not differ between the two groups (4.36% vs 6.06%, siRNA-P vs -C). However, after 48 and 72 h, siRNA-P had a significantly higher apoptotic rate than did siRNA-C (13.3% vs 22.1%, 20.5% vs 39.7%) (Figure 4).
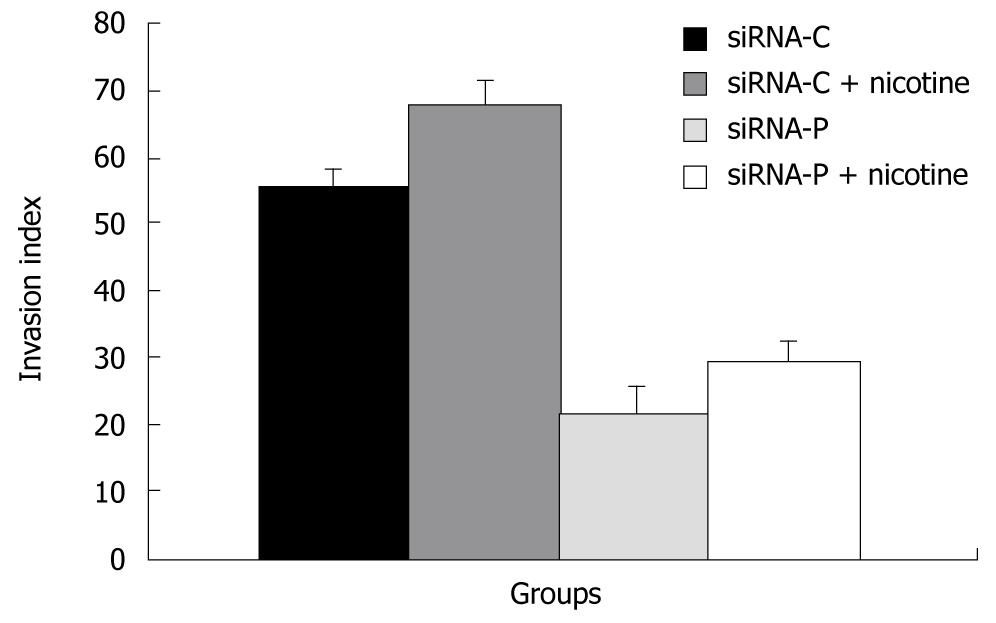
SiRNA-P showed a significantly lower index of invasion (21.8% ± 4.11%) than did siRNA-C (55.4% ± 2.64%), indicating that silencing of periostin can reduce the capability of gastric cancer cells to invade. Similarly, with nicotine treatment, siRNA-P also showed significantly less invasion (29.5% ± 3.11%) than did siRNA-C (29.5% ± 3.11%) (Figure 5).
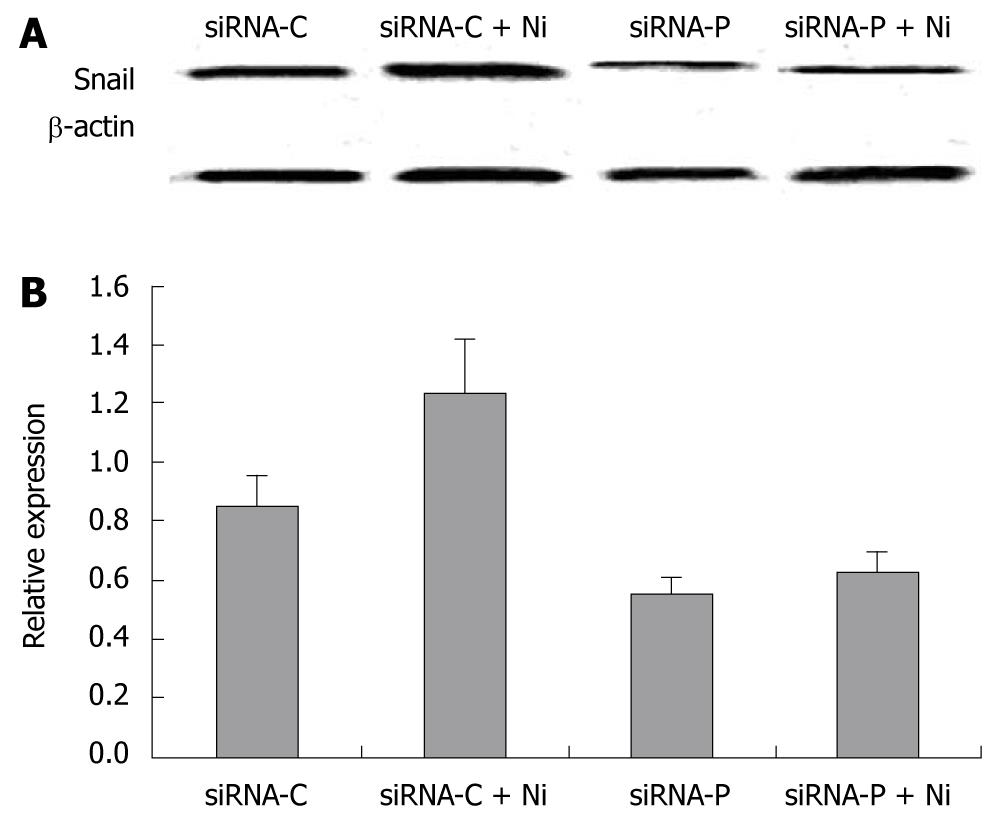
The expression of the EMT marker, Snail, was determined in siRNA-P and siRNA-C by immunoblotting. The Snail protein was significantly decreased in siRNA-P compared to the siRNA-C group, both in nicotine-treated and nicotine–untreated groups. Nicotine treatment significantly increased Snail protein expression in siRNA-C, but not in siRNA-P (Figure 6).
We observed that nicotine can upregulate periostin protein expression in gastric cancer cells in a COX-2-dependent pathway, which can be blocked by the COX-2-specific inhibitor NS 398. Periostin mRNA expression was significantly decreased by 87.2% in siRNA-P, which was stably silenced with G418 screening. Cell proliferation in siRNA-P was significantly inhibited compared to siRNA-C. Nicotine can promote gastric cancer cell growth, which can be partially blocked by periostin siRNA. Furthermore, siRNA-P had a significantly increased sensitivity to apoptosis induced by the chemotherapy reagent, 5-FU, compared to siRNA-C, especially after 48 and 72 h. Nicotine increased the ability of gastric cancer cells to invade in vitro, and siRNA-P showed decreased invasion in the presence or absence of nicotine compared to the controls. EMT is an important underlying mechanism used by epithelial cancer cells to invade and metastasize. We found that the EMT marker, Snail protein expression, decreased in siRNA-P vs siRNA-C with or without nicotine treatment, indicating that periostin expression could be required for EMT in gastric cancer cells.
Our study revealed that periostin expression was controlled by nicotine. As the most active carcinogen in tobacco, nicotine is well known to promote lung cancer, and its relation to other types of tumors has come under increasing scrutiny. Nicotine has been shown to activate COX-2 in gastric cancer cells, which is related to the growth, survival, invasion, and metastasis of gastric cancer cells[5,7,12]. COX-2 is also an important prognostic factor for gastric cancer. The higher the expression of COX-2 in gastric cancer cells is, the worse the prognosis is[13]. We also found that the regulation of periostin by nicotine was COX-2 dependent. Inhibition of COX-2 almost completely blocked the upregulation of periostin by nicotine, and inhibition of COX-2 itself downregulated periostin expression in gastric cancer cells. These findings indicate that the regulation of periostin by nicotine is completely COX-2 dependent and that periostin expression is partially dependent on COX-2 in gastric cancer cells. Currently, there are only a few reports on the relationship between COX-2 and periostin. It has been shown that activation of the signaling molecule Wnt can concurrently upregulate COX-2 and periostin in mammary epithelial cells[14]. That both COX-2 and periostin are expressed at the same time in gastric cancer cells, along with the relationship of COX-2 to the prognosis of gastric cancer, warrants further investigation to determine whether periostin can serve as a prognostic indicator for gastric cancer.
Our research indicated that periostin gene silencing could significantly increase the sensitivity of gastric cancer cells to 5-FU. A hypoxic environment has been shown to upregulate periostin expression and promote cell survival via activation of the downstream Akt/PKB signaling pathway in non-small cell lung cancer cells[15]. In pancreatic cancer cells, periostin promoted resistance to hypoxia-induced apoptosis through binding to integrin on the cell membrane and activation of the downstream PI3K-AKT signaling pathway, which promotes cell survival[16]. At diagnosis, patients with gastric cancer usually have micro-metastasis of gastric cancer cells into non-gastric areas, such as the liver, abdominal cavity, bone marrow, and peripheral circulation[17-20]. Chemotherapy that does not completely remove these latent cells leads to distant metastasis and the recurrence of tumors. This indicates that tumor cells with metastatic potential may have resistance to apoptosis, and periostin could be an underlying mechanism used to promote invasion, metastasis, and drug resistance in cancer cells via a complex intracellular signaling network.
Our findings suggest that periostin expression is required for the EMT of gastric cancer cells. Periostin is a mesenchyme-specific gene[21] expressed and secreted by tumor mesenchymal cells[22] and associated with the EMT of tumor cells. In fact, EMT induced by nicotine has been the subject of numerous publications. Nicotine-induced cell invasion and EMT has been shown to relate to the nicotinic acetylcholine receptors on the surface of lung cancer cells[23,24]. Nicotine was recently shown to induce EMT in gastric cancer cell lines through the activation of 5-lipoxygenase[25]. During EMT, epithelial markers decrease, mesenchymal markers increase, and periostin mainly resides in mesenchymal cells[26]. It could be that nicotine-induced periostin upregulation results from EMT, leading to fewer epithelial cells and more mesenchymal cells. However, our results showed that in gastric cancer cells with the periostin gene downregulated by siRNA, there was significant downregulation of the EMT marker gene, Snail, with nicotine treatment, along with the inhibition of cell growth, drug resistance, and tumor invasion. This suggests that periostin changes in gastric cancer cells are not a result of EMT and that, instead, periostin contributes to EMT via a specific pathway.
In this in vitro study, the concentration of nicotine was 200 ng/mL, this was chosen as the most appropriate concentration from our preliminary results. In smokers, venous nicotine levels range from 5 to 15 ng/mL, which is much lower than the level used in our in vitro study[27]. Nicotine, which can be absorbed via respiratory tissues, the skin, and the gastrointestinal tract, takes part in the initiation and promotion of carcinogenesis in the gastrointestinal tract. Because this is a gradual process, a lower in vivo nicotine concentration could enhance the detrimental effects of other carcinogens over a long period of time. In fact, the in vitro effects of nicotine on periostin expression were only observed up to 24 h in this study. Whether this effect is longer lasting will be investigated in subsequent in vivo animal experiments.
In summary, we showed that periostin is a nicotine-regulated gene that contributes to nicotine-induced cell growth, drug resistance, invasion, and EMT in gastric cancer cells. These findings may lead to a new target for the prevention of nicotine-induced gastric cancer.
Gastric cancer has a high occurrence and its relationship with smoking has been a focus of recent research. As the most active carcinogen in cigars, nicotine has been shown to promote gastric cancer cell proliferation and neoangiogenesis in vivo and in vitro. Periostin is a mesenchyme-specific protein that is over-expressed in gastric cancer cells and is related to invasion, distant metastasis and angiogenesis in many tumors.
During the invasion of epithelial-originated malignant cancers, the polarity of epithelial cells disappears along with an increase in migration and mobility, which is known as the epithelial-mesenchymal transition (EMT). Nicotine can enhance invasion and EMT in various tumor cells; however, the relationship between nicotine and EMT-marker periostin has not been unequivocally addressed. In this study, the authors demonstrate that the expression of periostin can be induced by nicotine treatment in gastric cancer cells and that proliferation, drug resistance and invasiveness in gastric cancer cells is induced by nicotine.
Recent reports have highlighted the importance of the EMT and its related molecules, including Snail, in gastrointestinal carcinogenesis and progression. In gastric cancers, EMT is enhanced. This is the first study to report that EMT-related periostin is upregulated by nicotine in gastric cancer cells. Furthermore, The authors’in vitro studies suggest that this protein may be the cause of the proliferation, drug resistance and invasiveness induced by nicotine in this cancer.
By understanding how periostin is induced and by silencing its expression, this study may represent a future strategy for therapeutic intervention in patients with nicotine-associated gastric cancer.
Periostin and Snail are proteins involved in a process called the EMT. EMT occurs when epithelial cells lose polarity and increase migration and mobility. Such a mechanism is crucial in the invasion and metastasis of many epithelial-originated malignant tumors.
The study is entitled “Enhanced proliferation, invasion, and epithelial-mesenchymal transition of nicotine-promoted gastric cancer cells by periostin” presents convincing evidence for the effect of nicotine on the induction of periostin and its effect on EMT of gastric cancerous cell lines.
Peer reviewer: Ourania M Andrisani, PhD, Professor, B038 Hansen Bldg, Center for Cancer Research, Purdue University, West Lafayette, IN 47907, United States
S- Editor Tian L L- Editor Webster JR E- Editor Ma WH
| 1. | Hatakeyama M. Helicobacter pylori and gastric carcinogenesis. J Gastroenterol. 2009;44:239-248. [Cited in This Article: ] |
| 2. | La Torre G, Chiaradia G, Gianfagna F, De Lauretis A, Boccia S, Mannocci A, Ricciardi W. Smoking status and gastric cancer risk: an updated meta-analysis of case-control studies published in the past ten years. Tumori. 2009;95:13-22. [Cited in This Article: ] |
| 3. | Ladeiras-Lopes R, Pereira AK, Nogueira A, Pinheiro-Torres T, Pinto I, Santos-Pereira R, Lunet N. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control. 2008;19:689-701. [Cited in This Article: ] |
| 4. | Shin VY, Cho CH. Nicotine and gastric cancer. Alcohol. 2005;35:259-264. [Cited in This Article: ] |
| 5. | Shin VY, Wu WK, Ye YN, So WH, Koo MW, Liu ES, Luo JC, Cho CH. Nicotine promotes gastric tumor growth and neovascularization by activating extracellular signal-regulated kinase and cyclooxygenase-2. Carcinogenesis. 2004;25:2487-2495. [Cited in This Article: ] |
| 6. | Shin VY, Wu WK, Chu KM, Koo MW, Wong HP, Lam EK, Tai EK, Cho CH. Functional role of beta-adrenergic receptors in the mitogenic action of nicotine on gastric cancer cells. Toxicol Sci. 2007;96:21-29. [Cited in This Article: ] |
| 7. | Shin VY, Wu WK, Chu KM, Wong HP, Lam EK, Tai EK, Koo MW, Cho CH. Nicotine induces cyclooxygenase-2 and vascular endothelial growth factor receptor-2 in association with tumor-associated invasion and angiogenesis in gastric cancer. Mol Cancer Res. 2005;3:607-615. [Cited in This Article: ] |
| 8. | Siriwardena BS, Kudo Y, Ogawa I, Kitagawa M, Kitajima S, Hatano H, Tilakaratne WM, Miyauchi M, Takata T. Periostin is frequently overexpressed and enhances invasion and angiogenesis in oral cancer. Br J Cancer. 2006;95:1396-1403. [Cited in This Article: ] |
| 9. | Puppin C, Fabbro D, Dima M, Di Loreto C, Puxeddu E, Filetti S, Russo D, Damante G. High periostin expression correlates with aggressiveness in papillary thyroid carcinomas. J Endocrinol. 2008;197:401-408. [Cited in This Article: ] |
| 10. | Zhang Y, Zhang G, Li J, Tao Q, Tang W. The expression analysis of periostin in human breast cancer. J Surg Res. 2010;160:102-106. [Cited in This Article: ] |
| 11. | Li JS, Sun GW, Wei XY, Tang WH. Expression of periostin and its clinicopathological relevance in gastric cancer. World J Gastroenterol. 2007;13:5261-5266. [Cited in This Article: ] |
| 12. | Yan W, Shao R. Transduction of a mesenchyme-specific gene periostin into 293T cells induces cell invasive activity through epithelial-mesenchymal transformation. J Biol Chem. 2006;281:19700-19708. [Cited in This Article: ] |
| 13. | Shin VY, Jin HC, Ng EK, Yu J, Leung WK, Cho CH, Sung JJ. Nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induce cyclooxygenase-2 activity in human gastric cancer cells: Involvement of nicotinic acetylcholine receptor (nAChR) and beta-adrenergic receptor signaling pathways. Toxicol Appl Pharmacol. 2008;233:254-261. [Cited in This Article: ] |
| 14. | Haertel-Wiesmann M, Liang Y, Fantl WJ, Williams LT. Regulation of cyclooxygenase-2 and periostin by Wnt-3 in mouse mammary epithelial cells. J Biol Chem. 2000;275:32046-32051. [Cited in This Article: ] |
| 15. | Ouyang G, Liu M, Ruan K, Song G, Mao Y, Bao S. Upregulated expression of periostin by hypoxia in non-small-cell lung cancer cells promotes cell survival via the Akt/PKB pathway. Cancer Lett. 2009;281:213-219. [Cited in This Article: ] |
| 16. | Baril P, Gangeswaran R, Mahon PC, Caulee K, Kocher HM, Harada T, Zhu M, Kalthoff H, Crnogorac-Jurcevic T, Lemoine NR. Periostin promotes invasiveness and resistance of pancreatic cancer cells to hypoxia-induced cell death: role of the beta4 integrin and the PI3k pathway. Oncogene. 2007;26:2082-2094. [Cited in This Article: ] |
| 17. | Nomura T, Kamio Y, Takasu N, Moriya T, Takeshita A, Mizutani M, Hachiya O, Hirai I, Kimura W. Intrahepatic micrometastases around liver metastases from gastric cancer. J Hepatobiliary Pancreat Surg. 2009;16:493-501. [Cited in This Article: ] |
| 18. | Dalal KM, Woo Y, Kelly K, Galanis C, Gonen M, Fong Y, Coit DG. Detection of micrometastases in peritoneal washings of gastric cancer patients by the reverse transcriptase polymerase chain reaction. Gastric Cancer. 2008;11:206-213. [Cited in This Article: ] |
| 19. | Fujita Y, Terashima M, Hoshino Y, Ohtani S, Kashimura S, Kanzaki N, Osuka F, Kogure M, Gotoh M. Detection of cancer cells disseminated in bone marrow using real-time quantitative RT-PCR of CEA, CK19, and CK20 mRNA in patients with gastric cancer. Gastric Cancer. 2006;9:308-314. [Cited in This Article: ] |
| 20. | Koga T, Tokunaga E, Sumiyoshi Y, Oki E, Oda S, Takahashi I, Kakeji Y, Baba H, Maehara Y. Detection of circulating gastric cancer cells in peripheral blood using real time quantitative RT-PCR. Hepatogastroenterology. 2008;55:1131-1135. [Cited in This Article: ] |
| 21. | Coutu DL, Wu JH, Monette A, Rivard GE, Blostein MD, Galipeau J. Periostin, a member of a novel family of vitamin K-dependent proteins, is expressed by mesenchymal stromal cells. J Biol Chem. 2008;283:17991-18001. [Cited in This Article: ] |
| 22. | Kanno A, Satoh K, Masamune A, Hirota M, Kimura K, Umino J, Hamada S, Satoh A, Egawa S, Motoi F. Periostin, secreted from stromal cells, has biphasic effect on cell migration and correlates with the epithelial to mesenchymal transition of human pancreatic cancer cells. Int J Cancer. 2008;122:2707-2718. [Cited in This Article: ] |
| 23. | Dasgupta P, Rizwani W, Pillai S, Kinkade R, Kovacs M, Rastogi S, Banerjee S, Carless M, Kim E, Coppola D. Nicotine induces cell proliferation, invasion and epithelial-mesenchymal transition in a variety of human cancer cell lines. Int J Cancer. 2009;124:36-45. [Cited in This Article: ] |
| 24. | Davis R, Rizwani W, Banerjee S, Kovacs M, Haura E, Coppola D, Chellappan S. Nicotine promotes tumor growth and metastasis in mouse models of lung cancer. PLoS One. 2009;4:e7524. [Cited in This Article: ] |
| 25. | Shin VY, Jin HC, Ng EK, Sung JJ, Chu KM, Cho CH. Activation of 5-lipoxygenase is required for nicotine mediated epithelial-mesenchymal transition and tumor cell growth. Cancer Lett. 2010;292:237-245. [Cited in This Article: ] |
| 26. | Fukushima N, Kikuchi Y, Nishiyama T, Kudo A, Fukayama M. Periostin deposition in the stroma of invasive and intraductal neoplasms of the pancreas. Mod Pathol. 2008;21:1044-1053. [Cited in This Article: ] |
| 27. | Wu WK, Cho CH. The pharmacological actions of nicotine on the gastrointestinal tract. J Pharmacol Sci. 2004;94:348-358. [Cited in This Article: ] |