Published online May 14, 2011. doi: 10.3748/wjg.v17.i18.2338
Revised: November 25, 2010
Accepted: December 2, 2010
Published online: May 14, 2011
AIM: To determine whether absorbable sutures or non-absorbable sutures are better in preventing surgical site infection (SSI), in this paper we discuss the results of a randomized clinical trial which examined the type of sutures used during hepatectomy.
METHODS: All hepatic resections performed from January 2007 to November 2008 at the Department of Surgery at Iizuka Hospital in Japan were included in this study. There were 125 patients randomly assigned to an absorbable sutures (Vicryl) group or non-absorbable sutures (Silk) group.
RESULTS: SSI was observed in 13.6% (17/125) patients participating in this study, 11.3% in the Vicryl group and 15.8% in the Silk group. Incisional SSI including superficial and deep SSI, was observed in 8% of the Vicryl group and 9.5% of the Silk group. Organ/space SSI was observed in 3.2% of the Vicryl group and 6.0% of the Silk group. There were no significant differences, but among the patients with SSI, the period for recovery was significantly shorter for the Vicryl group compared to the Silk group.
CONCLUSION: The incidence of SSI in patients receiving absorbable sutures and silk sutures is not significantly different in this randomized controlled study; however, the period for recovery in patients with SSI was significantly shorter for absorbable sutures.
- Citation: Harimoto N, Shirabe K, Abe T, Yukaya T, Tsujita E, Gion T, Kajiyama K, Nagaie T. Prospective randomized controlled trial investigating the type of sutures used during hepatectomy. World J Gastroenterol 2011; 17(18): 2338-2342
- URL: https://www.wjgnet.com/1007-9327/full/v17/i18/2338.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i18.2338
Despite recent developments in surgery and patient management during the perioperative period, critical complications still develop in a few patients who undergo hepatic resection[1,2]. Surgical site infection (SSI) is one of the most important morbidities of surgery and leads to prolonged hospital stays. Previous studies revealed absorbable suture material in gastrointestinal surgical procedures to reduce the risk of SSI[3,4] although most of those studies have been limited to skin closures. Absorbable sutures are used in most hospitals in the United States and Europe based on the available clinical studies. On the other hand, silk sutures are still used in most hospitals in Japan. A study by Kobayashi et al[5] revealed patients with intraoperative bowel injury, blood loss > 2000 mL, and age > 65 years are at risk of developing SSI after hepatectomy for liver cancers and then all blood vessels and bile ducts were ligated with silk or vessel clips during parenchymal resection. Absorbable sutures are now widely used for abdominal closure in Japan according to the CDC guidelines[6]. During the resection of the liver, silk sutures are generally used for the vessels in Japan, because silk sutures are easier to handle and less expensive than absorbable sutures. However, foreign materials, especially silk, are known to accelerate infection[6,7] and thus lead to a prolonged hospital stay. Togo et al[8] reported the usefulness of absorbable sutures to prevent SSI with a historical control study using an animal model. There has been no known randomized clinical trial for suture material used in the ligation of the cut surface of the liver. In this paper the authors provide the outcome of a randomized clinical trial investigating the type of sutures used during hepatectomy to determine which is better in preventing SSI, absorbable sutures or non-absorbable sutures.
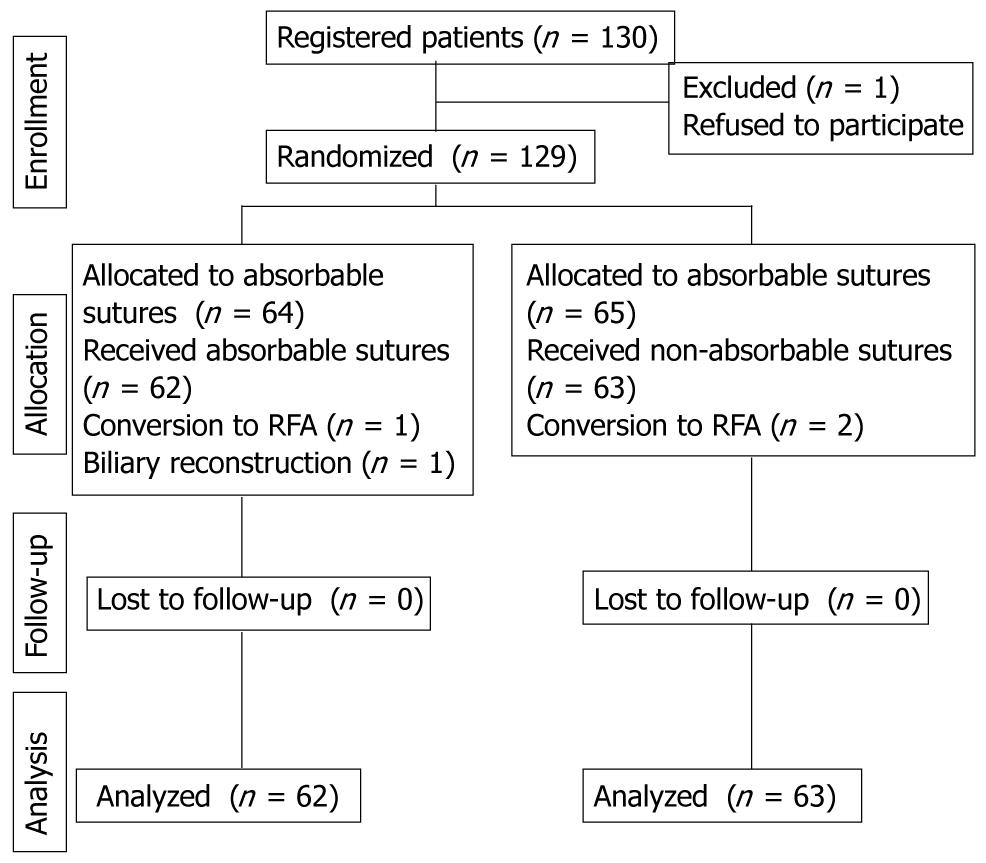
All hepatic resections without biliary reconstruction performed from January 2007 to November 2008 at the Department of Surgery at Iizuka Hospital in Japan were included in this study. Only patients who met the following criteria were enrolled: (1) liver resection without biliary reconstruction; (2) without other malignancy; and (3) until compensated cirrhosis with Child-Pugh class A or B. At the author’s department, the rate of SSI during hepatectomy was 25% according to previous data. The sample size required to detect a difference by chi-square test with 80% power at the 5% significance level was 124 patients. There were 130 patients enrolled in this study. One patient refused to participate in the study. The authors obtained approval from the local ethics committee and obtained informed consent from the other patients. Finally 125 patients were randomly assigned to an absorbable sutures (Vicryl, Johnson & Johnson Corp., Tokyo, Japan) or a non-absorbable sutures (Silk) group. Because the operative procedure used on 4 patients was either conversion to radiofrequency ablation or biliary reconstruction (Figure 1) they were not included in the study. Randomization was achieved by providing sealed envelopes to be opened by the operator before laparotomy. Data analysis was performed for 125 patients.
Liver function was evaluated preoperatively using Child classification and indocyanine green retention test at 15 min (ICGR15) in patients with underlying liver disease. The presence of ascites and ICGR15 test value of more than 40% were considered an absolute contraindication for resection. Hepatitis B surface antigen (HBs Ag) and hepatitis C antibody (HCV Ab) were routinely measured preoperatively.
Surgical technique and intra-operative care were standardized by the same team in this study. These included a J-shaped incision for routine abdominal access, a slow and gentle hepatic dissection using an ultrasonic dissector with coagulator (CUSA Excel, Integra Co., USA), with systematic ligation of all sizable vessels, and close ultrasonographic guidance along the transection line. Cholecystectomy was performed in all patients if the gallbladder was present. Intraoperative bile leakage test was routinely performed to identify bile leakage[9]. With this procedure, we recognized small bile leakage sites on the cut liver surface and could repair these sites by Z-suturing using 6-0PDSII (Johnson & Johnson Corp., Tokyo, Japan). Intraoperative vascular control was achieved using the Pringle maneuver[10]. In order to prevent backflow bleeding central venous pressure was decreased to as low as 5 mm Hg when possible with careful circulating volume and respiratory assistance. When central venous pressure control was insufficient, outflow control was achieved by selective vascular exclusion, or by clamping of the infrahepatic inferior vena cava[11]. One or two closed drains were inserted close to the cut surface of liver parenchyma. Before the subcutaneous tissue was closed, the wound was washed with 1 L saline. Vicryl (Johnson & Johnson Corp., Tokyo, Japan), an absorbable suture material, was used during abdominal wound closure. Drains were removed when no bleeding or bile leakage were observed within 3 d. Bile leakage was defined as the drainage of macroscopic bile from the surgical drains for more than 7 d after surgery[9]. If bile leakage is clinically suspected after the removal of drains, percutaneous drainage is performed. Intravenous ampicillin/sulbactam (ABPC/SBT) 1.5 g was administered 30 min before surgery and additional ABPC/SBT was administered every 3 h. Systemic antibiotics were used for only two days after surgery. During the operation gloves were changed every 3 h.
SSI was defined as a condition in which purulent discharge was observed from any incision or space that was manipulated during an operation with or without microbiological evidence as in the guideline issued by CDC[6] and it was identified prospectively by direct observation of the surgical site. Patients were followed up 30 d after hospital discharge. SSI occurring after hospital discharge was included in this study. Remote infection was defined as a condition in which fever and leukocytosis were present with bacteria in sputum, urine, catheter-tip, blood, or other body fluid/space with or without microbiological evidence.
We analyzed associations between the continuous and categorical clinico-pathologic variables using the Student’s t tests and χ2 tests, respectively. Multivariate analysis was performed with a logistic regression test. A P value less than 0.05 was considered statistically significant.
The clinicopathological factors between the Vicryl and Silk groups were compared (Table 1). No significant differences were recognized during the evaluation of the host factors and the operative factors. SSI was observed in 13.6% (17/125) of patients in this study, 11.3% in the Vicryl group and 15.8% in the Silk group (P = 0.620). Incisional SSI including superficial and deep SSI, were observed in 8% of the Vicryl group and 9.5% of the Silk group (P = 0.999). Organ/space SSI was observed in 3.2% of the Vicryl group and 6.0% of the Silk group (P = 0.680). Organ/space SSI was namely abdominal abscess and percutaneous drainage was needed. Remote infection was observed with an incidence of 3.2% in the Vicryl group and 1.6% in the Silk group (P = 0.546). Pneumonia and urinary tract infection were observed in the Vicryl group and pneumonia in the Silk group. There were no significant differences. There was no hospital mortality in this study.
| Variables | Vicryl group | Silk group | P-value |
| (n = 62) | (n = 63) | ||
| Age | 68 ± 10 | 67 ± 12 | 0.684 |
| Male/female | 37/25 | 41/22 | 0.720 |
| Body mass index | 22.6 ± 3.0 | 23.7 ± 12 | 0.159 |
| Diabetes mellitus (%) | 19.4 | 33.0 | 0.104 |
| HBV (%) | 17.7 | 20.6 | 0.560 |
| HCV (%) | 50.0 | 39.7 | 0.666 |
| Albumin (g/dL) | 3.6 ± 0.5 | 3.6 ± 0.5 | 0.694 |
| Total bilirubin (mg/dL) | 0.7 ± 0.4 | 0.7 ± 0.4 | 0.813 |
| AST (IU/L) | 46 ± 31 | 43 ± 27 | 0.592 |
| Platelet count (x 104/μL) | 21.0 ± 11.0 | 19.7 ± 10.1 | 0.460 |
| ICGR15 (%) | 15.4 ± 9.0 | 16.4 ± 11.9 | 0.589 |
| Child-Pugh A/B | 58/4 | 59/4 | 0.999 |
| HCC/non-HCC | 46/16 | 47/16 | 0.826 |
| Liver cirrhosis (ch/lf/lc) | 15/20/27 | 15/22/26 | 0.948 |
| Anatomical/nonanatomical | 20/42 | 20/43 | 0.618 |
| Operation time (min) | 273 ± 88 | 276 ± 105 | 0.876 |
| Blood loss (g) | 698 ± 894 | 763 ± 1329 | 0.749 |
| Transfusion (%) | 12.9 | 17.4 | 0.620 |
| Bile leakage (%) | 3.2 | 6.3 | 0.680 |
| Total SSI (%) | 11.3 | 15.8 | 0.603 |
| Incisional SSI (%) | 8.0 | 9.5 | 0.999 |
| Organ/space SSI (%) | 3.2 | 6.3 | 0.680 |
| Remote infection (%) | 3.2 | 1.6 | 0.546 |
| Hospital stay (d) | 16 ± 15 | 16 ± 14 | 0.880 |
In the univariate analyses, operation time > 265 min, blood loss > 1000 g, and bile leakage differed significantly as predictive factors of SSI (Table 2). The type of sutures was not significant. Five factors including operation time > 265 min, blood loss > 1000 g, bile leakage and the type of sutures were analyzed with multivariate logistic regression. Results are given in Table 3. Blood loss > 1000 g was the only independent variable among the five factors.
| Variables | SSI(+) | SSI(-) | P-value |
| (n = 17) | (n = 108) | ||
| Age | 68 ± 10 | 67 ± 11 | 0.667 |
| Male/female | 13 /4 | 65/43 | 0.287 |
| Body mass index | 24.3 ± 5.2 | 23.0 ± 3.9 | 0.208 |
| Diabetes mellitus (%) | 35.3 | 25.0 | 0.384 |
| HBV (%) | 23.5 | 18.5 | 0.703 |
| HCV (%) | 64.7 | 41.7 | 0.560 |
| Albumin (g/dL) | 3.6 ± 0.4 | 3.6 ± 0.5 | 0.977 |
| Total bilirubin (mg/dL) | 0.7 ± 0.3 | 0.7 ± 0.4 | 0.938 |
| AST (IU/L) | 56 ± 22 | 43 ± 29 | 0.070 |
| Platelet count (x 104/μL) | 20.7 ± 7.3 | 20.4 ± 11.0 | 0.904 |
| ICGR15 (%) | 19.9 ± 8.3 | 15.3 ± 10.7 | 0.101 |
| Child-Pugh A/B | 17/0 | 100/8 | 0.597 |
| HCC/non-HCC | 15/2 | 78/30 | 0.638 |
| Liver cirrhosis (ch/lf/lc) | 2/7/8 | 28/35/45 | 0.435 |
| Anatomical/nonanatomical | 8/9 | 32/76 | 0.228 |
| Operation time | |||
| > 265 min (%) | 76.5 | 44.4 | < 0.050 |
| Blood loss | |||
| > 1000 g (%) | 41.2 | 18.5 | < 0.050 |
| Transfusion (%) | 11.8 | 15.7 | 0.999 |
| Bile leakage (%) | 35.3 | 0 | < 0.010 |
| Vicryl/silk | 7/10 | 55/53 | 0.603 |
| Hospital stay (d) | 29 ± 23 | 15 ± 12 | < 0.010 |
| Adjusted odds ratios (95% CI) | P value | |
| Blood loss | ||
| > 1000 g | 7.598 (1.396-41.364) | < 0.010 |
Among the patients with SSI, the period for recovery in the Vicryl group was significantly shorter compared to the Silk group (Table 4).
| Vicryl | 28 ± 11 d |
| Silk | 54 ± 30 d |
Togo et al[8] reported the usefulness of absorbable sutures to prevent SSI with a historical control study using an animal model. This is the only report that the use of absorbable sutures contributed significantly to the prevention of development of SSI. In our prospective study, the incidence of SSI in the Vicryl group was lower compared to the Silk group for both incisional SSI and organ/space SSI, however there were no other significant differences. Watanabe et al[12] reported the use of absorbable sutures in seromuscular suturing or intra-abdominal ligation was associated with a significantly lower incidence of SSI than non-absorbable sutures in the lower alimentary tract procedure, but not in the upper. The incidence of SSI is low so any significant difference is difficult to observe. The duration for recovery from SSI was significantly shorter in the Vicryl group than in the Silk group. Since SSI increases treatment costs and diminishes patient satisfaction markedly, the use of an absorbable suture is recommended in view of the medical economy and patient’s quality of life.
The incidence of SSI as a form of postoperative infection was previously reported to be 20%-25%[8,13,14]. In our clinical experience, the incidence of SSI was observed to be 13.1% in this study and the rate of occurrence is decreasing every year because of SSI surveillance and development of surgical techniques such as closed suction drain and the prevention of bile leakage. Togo et al[8] reported the incidence of postoperative infection gradually decreased significantly with additional countermeasures. A larger sample size as well as a multi-institutional study is required for a randomized controlled study.
Intraoperative blood loss, long operation time and bile leakage were associated with SSI in our study. Both transfusion and operation time are reported to be risk factors of SSI in hepatectomy or other surgery[5,15]. Transfusion could induce immunosuppression in postoperative patients by reduction of the natural killer cell and cytotoxic T-cell populations[16,17]. Operation time could influence the concentration of systemic antibiotics, leading to the incidence of SSI. Bile leakage was observed at 4.8% (6/125) in this study. Generally the incidence of bile leakage was 4.0%-7.2% in recently reported large series[9,18-20]. The presence of bile, blood, and devitalized tissues in the dead space after hepatectomy may provide the ideal environment for bacterial growth and development of organ/space SSI, which frequently results in liver failure. To reduce organ /space SSI, it is most important to prevent bile leakage.
In conclusion, there were no significant differences between absorbable sutures and silk sutures on the incidence of SSI during the resection of the liver in this randomized control study; however, the period for recovery in patients with SSI was significantly shorter for absorbable sutures. Further study is needed to provide evidence on the use of absorbable or non-absorbable suture materials in a multi-institutional randomized clinical trial.
Surgical site infection (SSI) is one of the most important morbidities of surgery and leads to prolonged hospital stays. In this paper the authors provide the outcome of a randomized clinical trial investigating the type of sutures used during hepatectomy to determine which is better in preventing SSI, absorbable sutures or non-absorbable sutures.
During the resection of the liver, silk sutures are generally used for the vessels in Japan, because silk sutures are easier to handle and less expensive than absorbable sutures.
This is a randomized clinical trial investigating the type of sutures used during hepatectomy.
The incidence of SSI was not significantly different between absorbable sutures and silk sutures in this randomized controlled study; however, the period for recovery in patients with SSI was significantly shorter for absorbable sutures.
Vicryl is an absorbable suture material and silk is a non-absorbable suture material, which is widely used in Japan.
Authors report prospective randomized study of surgical site infection comparing silk to vicryl ties in liver resection.
Peer reviewer: Eddie K Abdalla, MD, Professor, Department of Surgical Oncology, PO Box 301402, Houston, TX 77230-1402, United States
S- Editor Tian L L- Editor O’Neill M E- Editor Ma WH
| 1. | Taketomi A, Kitagawa D, Itoh S, Harimoto N, Yamashita Y, Gion T, Shirabe K, Shimada M, Maehara Y. Trends in morbidity and mortality after hepatic resection for hepatocellular carcinoma: an institute's experience with 625 patients. J Am Coll Surg. 2007;204:580-587. [Cited in This Article: ] |
| 2. | Shirabe K, Kajiyama K, Harimoto N, Gion T, Tsujita E, Abe T, Wakiyama S, Nagaie T, Maehara Y. Early outcome following hepatic resection in patients older than 80 years of age. World J Surg. 2009;33:1927-1932. [Cited in This Article: ] |
| 3. | Kronborg O. Polyglycolic acid (Dexon) versus silk for fascial closure of abdominal incisions. Acta Chir Scand. 1976;142:9-12. [Cited in This Article: ] |
| 4. | Adams IW, Bell MS, Driver RM, Fry WG. A comparative trial of polyglycolic acid and silk as suture materials for accidental wounds. Lancet. 1977;2:1216-1217. [Cited in This Article: ] |
| 5. | Kobayashi S, Gotohda N, Nakagohri T, Takahashi S, Konishi M, Kinoshita T. Risk factors of surgical site infection after hepatectomy for liver cancers. World J Surg. 2009;33:312-317. [Cited in This Article: ] |
| 6. | Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20:250-278; quiz 279-280. [Cited in This Article: ] |
| 7. | Elek SD, Conen PE. The virulence of Staphylococcus pyogenes for man; a study of the problems of wound infection. Br J Exp Pathol. 1957;38:573-586. [Cited in This Article: ] |
| 8. | Togo S, Kubota T, Takahashi T, Yoshida K, Matsuo K, Morioka D, Tanaka K, Shimada H. Usefulness of absorbable sutures in preventing surgical site infection in hepatectomy. J Gastrointest Surg. 2008;12:1041-1046. [Cited in This Article: ] |
| 9. | Yamashita Y, Hamatsu T, Rikimaru T, Tanaka S, Shirabe K, Shimada M, Sugimachi K. Bile leakage after hepatic resection. Ann Surg. 2001;233:45-50. [Cited in This Article: ] |
| 10. | Rahbari NN, Koch M, Mehrabi A, Weidmann K, Motschall E, Kahlert C, Büchler MW, Weitz J. Portal triad clamping versus vascular exclusion for vascular control during hepatic resection: a systematic review and meta-analysis. J Gastrointest Surg. 2009;13:558-568. [Cited in This Article: ] |
| 11. | Otsubo T, Takasaki K, Yamamoto M, Katsuragawa H, Katagiri S, Yoshitoshi K, Hamano M, Ariizumi S, Kotera Y. Bleeding during hepatectomy can be reduced by clamping the inferior vena cava below the liver. Surgery. 2004;135:67-73. [Cited in This Article: ] |
| 12. | Watanabe A, Kohnoe S, Shimabukuro R, Yamanaka T, Iso Y, Baba H, Higashi H, Orita H, Emi Y, Takahashi I. Risk factors associated with surgical site infection in upper and lower gastrointestinal surgery. Surg Today. 2008;38:404-412. [Cited in This Article: ] |
| 13. | Togo S, Matsuo K, Tanaka K, Matsumoto C, Shimizu T, Ueda M, Morioka D, Nagano Y, Endo I, Shimada H. Perioperative infection control and its effectiveness in hepatectomy patients. J Gastroenterol Hepatol. 2007;22:1942-1948. [Cited in This Article: ] |
| 14. | Wu CC, Yeh DC, Lin MC, Liu TJ, P'eng FK. Prospective randomized trial of systemic antibiotics in patients undergoing liver resection. Br J Surg. 1998;85:489-493. [Cited in This Article: ] |
| 15. | Mynster T, Christensen IJ, Moesgaard F, Nielsen HJ. Effects of the combination of blood transfusion and postoperative infectious complications on prognosis after surgery for colorectal cancer. Danish RANX05 Colorectal Cancer Study Group. Br J Surg. 2000;87:1553-1562. [Cited in This Article: ] |
| 16. | Gascón P, Zoumbos NC, Young NS. Immunologic abnormalities in patients receiving multiple blood transfusions. Ann Intern Med. 1984;100:173-177. [Cited in This Article: ] |
| 17. | Kaplan J, Sarnaik S, Gitlin J, Lusher J. Diminished helper/suppressor lymphocyte ratios and natural killer activity in recipients of repeated blood transfusions. Blood. 1984;64:308-310. [Cited in This Article: ] |
| 18. | Viganò L, Ferrero A, Sgotto E, Tesoriere RL, Calgaro M, Capussotti L. Bile leak after hepatectomy: predictive factors of spontaneous healing. Am J Surg. 2008;196:195-200. [Cited in This Article: ] |
| 19. | Tanaka S, Hirohashi K, Tanaka H, Shuto T, Lee SH, Kubo S, Takemura S, Yamamoto T, Uenishi T, Kinoshita H. Incidence and management of bile leakage after hepatic resection for malignant hepatic tumors. J Am Coll Surg. 2002;195:484-489. [Cited in This Article: ] |
| 20. | Nagano Y, Togo S, Tanaka K, Masui H, Endo I, Sekido H, Nagahori K, Shimada H. Risk factors and management of bile leakage after hepatic resection. World J Surg. 2003;27:695-698. [Cited in This Article: ] |