Published online Feb 28, 2010. doi: 10.3748/wjg.v16.i8.1019
Revised: January 4, 2010
Accepted: January 11, 2010
Published online: February 28, 2010
AIM: To compare the influence and clearance effect of enzymatic and non-enzymatic detergents against Escherichia coli (E. coli) biofilm on the inner surface of gastroscopes.
METHODS: Teflon tubes were incubated in a mixture of different detergents and E. coli culture (106 CFU/mL) for 72 h at 15°C, and biofilms on the inner surface of the teflon tubes were analyzed by bacterial count and scanning electron microscopy. To evaluate the clearance effect of detergents, after biofilms were formed on the inner surface of Teflon tubes by 72 h lavage with E. coli culture, tubes were lavaged by enzymatic and non-enzymatic detergents at a speed of 250 mL/min, then biofilms on the inner surface were analyzed by bacterial count and scanning electron microscopy.
RESULTS: Non-enzymatic detergent had a better inhibition function on biofilm formation than enzymatic detergent as it reduced bacterial burden by 2.4 log compared with the control samples (P = 0.00). Inhibition function of enzymatic detergent was not significantly different to that of control samples and reduced bacterial burden by 0.2 log on average (P > 0.05). After lavaging at 250 mL/min for 3 min, no living bacteria were left in the tubes. Scanning electron microscopy observation showed biofilms became very loose by the high shear force effect.
CONCLUSION: Non-enzymatic detergent has a better inhibition effect on biofilm formation at room temperature. High speed pre-lavage and detergents are very important in temporal formed biofilm elimination.
- Citation: Fang Y, Shen Z, Li L, Cao Y, Gu LY, Gu Q, Zhong XQ, Yu CH, Li YM. A study of the efficacy of bacterial biofilm cleanout for gastrointestinal endoscopes. World J Gastroenterol 2010; 16(8): 1019-1024
- URL: https://www.wjgnet.com/1007-9327/full/v16/i8/1019.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i8.1019
The concept of bacterial biofilm was proposed in 1936[1]. Bacteria adhere to wet surfaces easily, then form organized colonies of cells embedded in a self-excreted matrix, which is composed principally of polysaccharide, and the polysaccharide facilitates adhesion to the surface and to each other[1,2]. In clinical medicine, many environments provide optimal conditions for the formation of bacterial biofilm, such as contact lenses, central venous catheters, urinary catheters and so on[3]. Similarly, the presence of biofilm on the surface of gastrointestinal endoscope channels has also been confirmed in recent studies[4-6]. Bacteria residing within biofilms are up to 1000 times more resistant to chemical inactivation than bacteria in suspension[7-9]. Biofilms are not only a reservoir of pathogenic bacteria that can detach, resume their planktonic state, and contaminate the patient, but also a source of endotoxins that may enter the circulation of the patient through ruptured mucosae and cause systemic disorders[10]. It was reported that if the endoscope channels were not cleaned prior to disinfection, the decontamination of endoscopes could become a failure[11,12]. Therefore, it is important to explore a reasonable cleaning agent of gastrointestinal endoscopes to achieve a satisfactory result of anti-biofilm under the present conditions.
In this controlled study, we compared the clearout effect of enzymatic and non-enzymatic detergents against Escherichia coli (E. coli) biofilm on the inner surface of gastroscopes.
A small amount of E. coli ATCC 25922 (Hangzhou Tianhe Biologics Corporation, Zhejiang, China) was taken from slant medium and inoculated into sterile Muller-Hinton (M-H) broth (pH 7.4) to obtain pure bacilli. The poured plate method was used to adjust the concentration of bacteria to 106 CFU/mL.
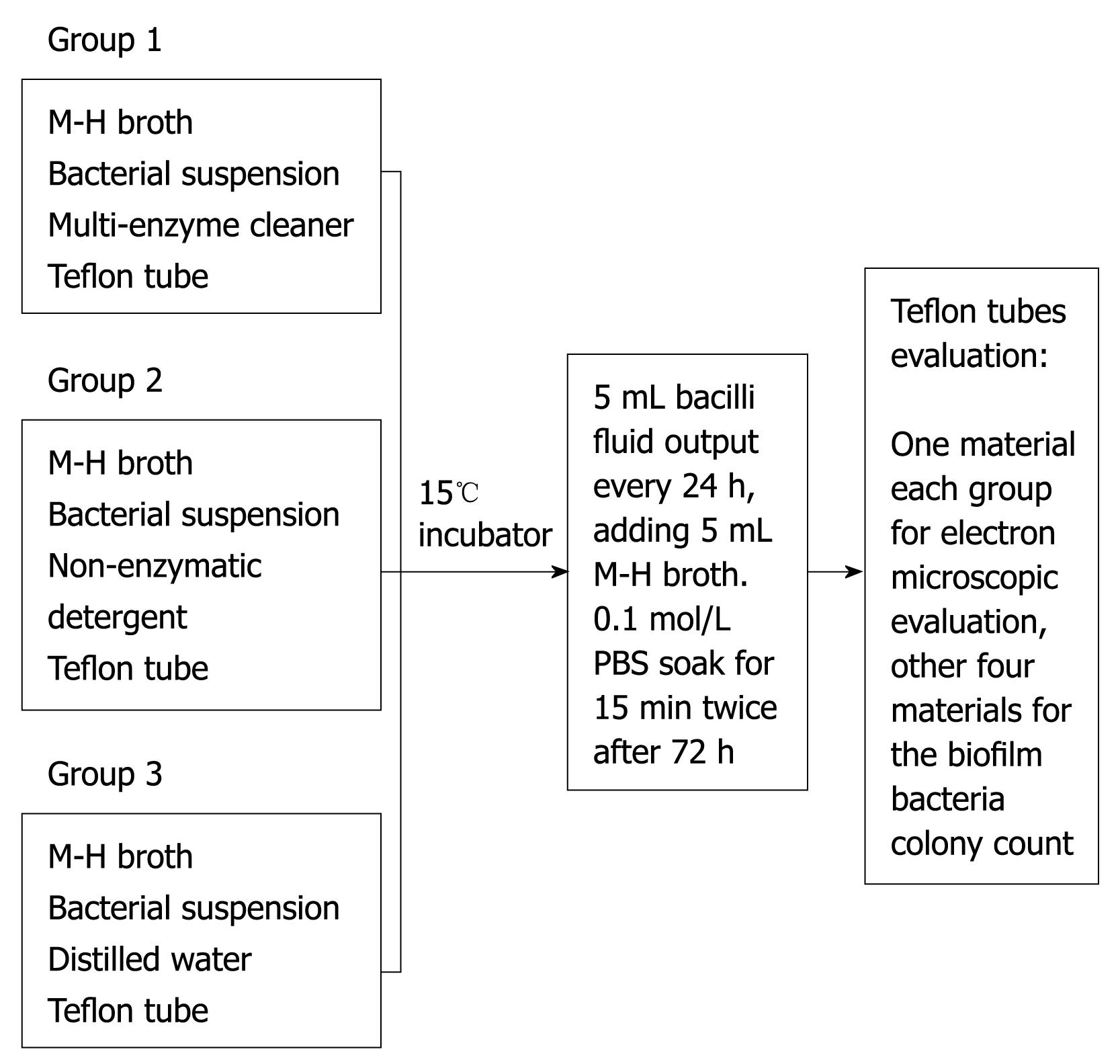
A high temperature sterilized Teflon tube (digestive endoscope lumen material) was cut into 15 pieces with 0.5 cm × 0.5 cm per piece, which were randomly divided into 3 groups: Group 1: enzymatic detergent group; Group 2: non-enzymatic detergent group; Group 3: blank control group. To prepare a sterile biopsy bottle for each group, 5 mL of M-H broth and 100 μL of bacterial suspension with good preparation previously were added into each group. 13 mL of rapid multi-enzyme cleaner (3M Company, Sao Paulo, Minnesota, USA), 25 μL of a non-enzymatic detergent, intercept (Minntech Corporation, Minneapolis, Minnesota, USA) with a 1:200 dilution ratio (recommended maximum dilution ratio of the products) and 20 μL sterile distilled water were added into Group 1, Group 2 and Group 3, respectively, taking care not to overlap the various pieces of material. Then each group was put into a 15°C incubator to incubate. 5 mL bacilli fluid was aspirated by sterile syringe every 24 h, adding 5 mL M-H broth; it was then soaked in 0.1 mol/L phosphate buffered saline (PBS) for 15 min through two stages after 72 h. We discarded the PBS in order to remove bacteria loosely attached to the wall, then Teflon tubes were taken from each bottle with sterile tweezers to prepare for evaluation. One sample from each group was prepared for electron microscopic evaluation, and the other 4 samples were used for the biofilm bacteria colony count (Figure 1).
The teflon tubes material was transferred to a sterile Eppendorf (EP) tube, fixed with 1% osmium tetroxide for 1 h, then washed twice (each time for 15 min) in PBS buffer solutions. After washing, they were dehydrated for 15 min using alcohol and 100% acetone, replaced by amyl acetate eater, dried to CO2 critical point, and sprayed gold with an ion sputtering instrument. Then they were scanned by electron microscope photographs (Stereoscan 260, Cambridge, UK). At the same time, another 12 Teflon tubes material in sterile EP tubes containing 1 mL of 0.1 mol/L PBS buffer solution were oscillated ultrasonically (38-47 kHz, 10 min) (Olympus Corporation, Tokyo, Japan), so that biofilm was stripped from the wall. We drew 100 μL PBS buffer solution from the above EP tube, then diluted the sample to 10 000 times with 10 times serial dilution. 0.1 mL diluent was added into a sterile petri dish. M-H agar 20 mL cooled to 50°C was poured into the sterile petri dish, and the samples were mixed and cooled to 37°C. The petri dish was incubated for 24 h, and the growth of colonies and colony counts were examined.
A high-temperature sterilized teflon tube with a length of 120 cm was placed in a sterilized beaker. Media contaminated with E. coli was run through the tube at a flow rate of 250 mL/min to mimic flow conditions (Minntech Co., Minneapolis, Minnesota, USA) during clinical endoscope procedures. The lavage lasted 4.5 h daily for 3 d, then 1/2 bacilli fluid was extracted and poured into the same amount M-H broth every 24 h.
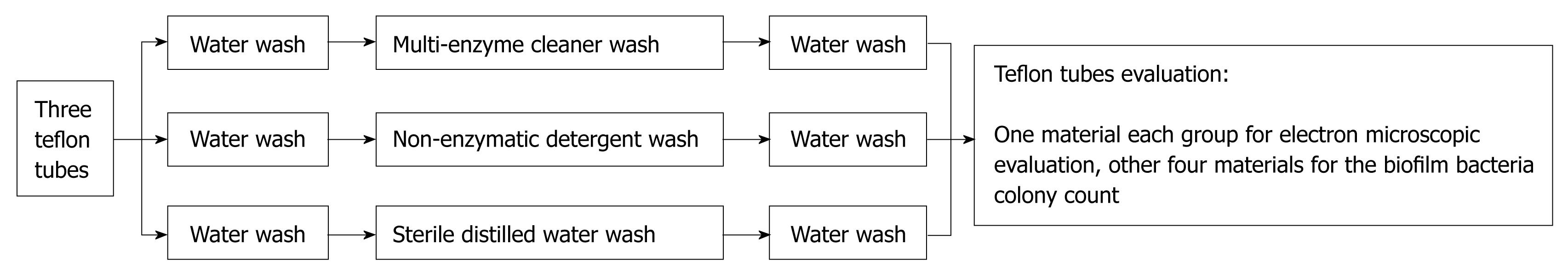
After lavage, the contaminated teflon tube was cut into 3 pieces. Next, the disconnected tubes were randomly divided into an enzyme wash group, a non-enzyme wash group, and a blank control group. According to Endoscope Disinfection Technical Operation Standards of China, after perfusion 3 sections of teflon tube were subjected to water wash (1 min), enzyme wash (3 min), and water wash again (1 min). In Group 1 enzyme wash was with multi-enzyme cleaner, in Group 2 non-enzymatic detergent was used instead of enzyme-containing detergent, and in Group 3 the enzyme wash step was replaced by sterile distilled water. After completing the above steps, tubing was cut with 5 cm spacing interception to produce 5 tubal walls of 0.5 cm × 0.5 cm, including 4 for the biofilm colony count, and 1 for scanning electron microscope observation of the concave wall biofilm (Figure 2).
Statistical analysis was performed with SPSS 13.0 statistical package (SPSS Inc., Chicago, Illinois, USA). The mean value for different groups was compared using Student t-test. P < 0.05 was considered statistically significant.
As shown in Figure 3, the bacterial suspension in the enzymatic detergent group was limpid after culturing for 72 h, related primarily to the multi-enzyme ingredients to digest the organic components in detergent. The bacterial suspension in the non-enzymatic detergent group showed matrix components at the bottom, and the quantity was greater than the control group, which was associated with decomposed bacterial cell components and the organic components of the broth. After counting the number of bacteria in the biofilm, the non-enzymatic detergent had a better inhibition function on biofilm formation and reduced bacterial burden by 2.39 logs compared with water without any detergent (P < 0.05). Enzymatic detergent reduced bacterial burden by 0.23 log compared with water without any detergent, and there was no significant difference between the two groups (P > 0.05) (Table 1). On the surface of teflon tubes from any groups, the remaining biofilm was observed by electron microscopy (Figure 4). It was easier to find biofilm on a rough surface compared with a smooth non-porous surface, which showed that the compatibility of detergent with the inner surface of endoscope channels was important for preventing the build-up of biofilm and removing a mature biofilm. It is generally accepted that smooth non-porous surfaces are the easiest to disinfect.
After lavaging with bacterial suspension and reprocessing with different detergents, no living bacterial matter was left on the surface of teflon tubes. However, at higher magnification, scanning electron microscopy examination showed a few bacterial cells covering the internal surface of teflon tubes (Figure 5). The biofilm cells appeared as small microcolonies in the non-enzyme detergent group and blank group, but as a confluent membrane in the enzyme detergent group. The number of bacteria remaining was smaller in the non-enzyme detergent group than in the blank group.
It has been reported that bacterial biofilm causes about 65% of bacterial infections in the clinic[13]. As research continues, bacterial biofilm is recognized as an ecological community composed of microbes and the irreversibly combined extracellular matrix, not simply a mixture of colonized bacteria and extracellular matrix. Biofilms have different phenotypes according to the difference in microbe growth and genetic transcription[14]. Biofilm induced biological material-related infections occurs widely in clinical departments, for instance, indwelling urinary catheter related infection, indwelling venous catheter related infection, artificial femoral head related infection. Antibiotics do not have a satisfactory effect on the infections due to the resistance of the biofilm[15]. Bacteria within biofilms are up to 1000 times more resistant to antimicrobials than the same bacteria in suspension. Endoscope related infection, as reported in articles[3,16], also occurs in western countries with strict specifications of endoscope reprocessing procedures. Although the poor quality of disinfection of endoscopes is associated with inadequate compliance with reprocessing protocols, another explanation could be more related to the formation of biofilm on endoscope channels. We found that an electron microscope scanning of an obsolete tube confirmed the existance of biofilm on the inner wall in a study.
In this experimental study, we adopted the endoscope lumen material (teflon) as the object of study. In the study on detergent effects on bacterial biofilm formation: biofilm colony count results showed that there was a significant difference in biofilm viable counts between non-enzyme detergent soaking teflon and the blank group (P < 0.05), while there was no significant difference in biofilm viable counts between enzymatic detergent soaking teflon and the control group (P > 0.05). This showed that non-enzyme detergent for biofilm formation had a better inhibitory effect than enzymatic detergent. The electron microscopy results showed that the biofilm was more easily deposited on the surface of non-flat areas, indicating detergent needs a better compatibility to the teflon tube in order to avoid producing a good place to form a biofilm because of the different affinity.
Endoscopes should be washed by water and enzymatic detergents before disinfection, otherwise the disinfectants have an inadequate effect because of the existance of blood, mucus and other organic materials[17]. Some data have revealed that a proper cleaning step could decrease the bacterial cells by 2-5 logs[18]. Our study confirmed the elimination of the number of bacteria within a biofilm by the shearing effect of high speed lavage. In the study for the effects of detergent against bacterial biofilm removal on the tube wall, lavage for 72 h to the teflon tube, then 1 min water wash, 3 min enzyme wash, and 1 min water wash again, produced a biofilm colony count of 0 in all samples of Teflon tube, but there were still differences in scanning electron microscopy; in the nearly 500-fold scan condition, a small amount of E. coli were found, showing small microcolonies for the non-enzyme detergent group and control group, but the number of bacteria remaining was fewer in the non-enzyme detergent group than in blank group. However, there was a confluent membrane of bacterial growth in the enzyme detergent group.
Many factors, as recognized and further researched, affect the formation or elimination of biofilms, including the characteristic of the fluid, the species of microbes, surface of the tubes, velocity of flow, temperature and pH value[19]. The conservation of the endoscope should focus on avoiding minor injury to the inner wall of channels, which eases the formation of biofilm. It’s also important to choose a green detergent with more cleansing power, but less corrosion to the tube, not only “with enzyme” or “without enzyme”. According to the manual, the enzymatic detergent has maximum cleansing power at a temperature higher than room temperature. However, we observed the efficiency of detergent at a temperature of 15°C, which is a realistic temperature for use. It was reported that proteolytic enzymes in the detergent increased the risk of occupational asthma, which cannot be improved by avoidance of exposure with enzymes of detergent origin[20].
During our short-term study, the elimination of bacterial biofilm on the inner wall of teflon tubes was studied and residual biofilm was detected by electron microscope scanning. Artificial contamination was performed using the E. coli bacteria only, whereas the actual contamination and the formation of biofilms is produced by miscellaneous bacteria in the clinic. Food residue, mucus, gastric acid and bile existing in the endoscope channels can decrease the shearing of the liquid and, therefore, should be completely removed to prevent the formation of biofilm. In this study, the current cleaning agents could not completely eliminate endoscopic biofilm, which should arouse our attention in the future. Therefore, a thorough cleaning of the endoscope to remove contaminants is one of many important and effective measures to prevent the formation of endoscopic biofilm. At the same time, how to effectively remove biofilm formed on the lumen is a continuing research goal. Further study on the formation and the removal of the biofilm is required and more effective detergents should be explored.
Many clinical environments provide optimal conditions for the formation of bacterial biofilm, including gastrointestinal endoscope channels. Biofilms are not only a reservoir of pathogenic bacteria, but also a source of endotoxins that may enter the circulation of the patient through ruptured mucosae and cause systemic disorders. However, if the endoscope channels are not cleaned prior to disinfection, the decontamination of endoscopes could become a failure because of biofilm formation. Therefore, it is important to explore a reasonable cleaning agent of the gastrointestinal endoscope to achieve a satisfactory result of anti-biofilm under the present conditions.
Endoscope related biofilm infections also occur in western countries with strict specifications of endoscope reprocessing procedures. Therefore, a thorough cleaning of the endoscope to remove contaminants is one of many important and effective measures to prevent the formation of endoscopic biofilm. To understand the effect of current detergents on biofilm, the authors produced a controlled study to compare the clearout effect of enzymatic and non-enzymatic detergents against Escherichia coli biofilm on the inner surface of gastroscopes.
If endoscope channels are not cleaned prior to disinfection, the decontamination of endoscopes could become a failure due to biofilms. Therefore, it is important to explore a reasonable cleaning agent of gastrointestinal endoscopes to achieve a satisfactory result of anti-biofilm under the present conditions. This study aims to draw attention to endoscopic workers to biofilms through the authors’ common efforts to ensure the safety of clinical endoscopic work in the future.
The study results suggest that the current cleaning agents cannot completely eliminate endoscopic biofilm, which should arouse our attention in the future. A thorough cleaning of the endoscope to remove contaminants is one of many important and effective measures to prevent the formation of endoscopic biofilm. How to effectively remove a biofilm that has already formed on the lumen is an ongoing research goal. Further study on the formation and the removal of biofilm is required and more effective detergents should be explored.
Bacterial biofilm: Bacterial biofilm is recognized as an ecological community composed of microbes irreversibly combined with the extracellular matrix, not simply a mixture of colonized bacteria and extracellular matrix.
The study is important as a means of exploring a reasonable cleaning agent of the gastrointestinal endoscope to achieve a satisfactory result of anti-biofilm under the present conditions. It deals with a very important topic on gastrointestinal reprocessing and it can add new information to this topic. It is also interesting and well designed.
Peer reviewer: Antonello Trecca, MD, Digestive Endoscopy and Gastroenterology, Usi Group, Via Machiavelli, 22, 00185 Rome, Italy
S- Editor Wang JL L- Editor O’Neill M E- Editor Ma WH
| 1. | Zobell CE, Anderson DQ. Observations on the multiplication of bacteria in different volumes of stored seawater and the influence of oxygen tension and solid surfaces. Biol Bull Woods Hole. 1936;71:324-342. [Cited in This Article: ] |
| 2. | Vickery K, Pajkos A, Cossart Y. Removal of biofilm from endoscopes: evaluation of detergent efficiency. Am J Infect Control. 2004;32:170-176. [Cited in This Article: ] |
| 3. | Donlan RM. Biofilms and device-associated infections. Emerg Infect Dis. 2001;7:277-281. [Cited in This Article: ] |
| 4. | Alvarado CJ, Reichelderfer M. APIC guideline for infection prevention and control in flexible endoscopy. Association for Professionals in Infection Control. Am J Infect Control. 2000;28:138-155. [Cited in This Article: ] |
| 5. | Nelson DB. Recent advances in epidemiology and prevention of gastrointestinal endoscopy related infections. Curr Opin Infect Dis. 2005;18:326-330. [Cited in This Article: ] |
| 6. | MacKay WG, Leanord AT, Williams CL. Water, water everywhere nor any a sterile drop to rinse your endoscope. J Hosp Infect. 2002;51:256-261. [Cited in This Article: ] |
| 7. | Ntsama-Essomba C, Bouttier S, Ramaldes M, Dubois-Brissonnet F, Fourniat J. Resistance of Escherichia coli growing as biofilms to disinfectants. Vet Res. 1997;28:353-363. [Cited in This Article: ] |
| 8. | Cochran WL, McFeters GA, Stewart PS. Reduced susceptibility of thin Pseudomonas aeruginosa biofilms to hydrogen peroxide and monochloramine. J Appl Microbiol. 2000;88:22-30. [Cited in This Article: ] |
| 9. | Gilbert P, McBain AJ. Biofilms: their impact on health and their recalcitrance toward biocides. Am J Infect Control. 2001;29:252-255. [Cited in This Article: ] |
| 10. | Bloss R, Kampf G. Test models to determine cleaning efficacy with different types of bioburden and its clinical correlation. J Hosp Infect. 2004;56 Suppl 2:S44-S48. [Cited in This Article: ] |
| 11. | Marion K, Pasmore M, Freney J, Delawari E, Renaud F, Costerton JW, Traeger J. A new procedure allowing the complete removal and prevention of hemodialysis biofilms. Blood Purif. 2005;23:339-348. [Cited in This Article: ] |
| 12. | Pajkos A, Vickery K, Cossart Y. Is biofilm accumulation on endoscope tubing a contributor to the failure of cleaning and decontamination? J Hosp Infect. 2004;58:224-229. [Cited in This Article: ] |
| 13. | Chicurel M. Bacterial biofilms and infections. Slimebusters. Nature. 2000;408:284-286. [Cited in This Article: ] |
| 14. | Habash M, Reid G. Microbial biofilms: their development and significance for medical device-related infections. J Clin Pharmacol. 1999;39:887-898. [Cited in This Article: ] |
| 15. | Trautner BW, Darouiche RO. Catheter-associated infections: pathogenesis affects prevention. Arch Intern Med. 2004;164:842-850. [Cited in This Article: ] |
| 16. | Pajkos A, Deva AK, Vickery K, Cope C, Chang L, Cossart YE. Detection of subclinical infection in significant breast implant capsules. Plast Reconstr Surg. 2003;111:1605-1611. [Cited in This Article: ] |
| 17. | Zühlsdorf B, Emmrich M, Floss H, Martiny H. Cleaning efficacy of nine different cleaners in a washer-disinfector designed for flexible endoscopes. J Hosp Infect. 2002;52:206-211. [Cited in This Article: ] |
| 18. | Chu NS, Favero M. The microbial flora of the gastrointestinal tract and the cleaning of flexible endoscopes. Gastrointest Endosc Clin N Am. 2000;10:233-244. [Cited in This Article: ] |
| 19. | Marion K, Freney J, James G, Bergeron E, Renaud FN, Costerton JW. Using an efficient biofilm detaching agent: an essential step for the improvement of endoscope reprocessing protocols. J Hosp Infect. 2006;64:136-142. [Cited in This Article: ] |
| 20. | Tripathi A, Grammer LC. Extrinsic allergic alveolitis from a proteolytic enzyme. Ann Allergy Asthma Immunol. 2001;86:425-427. [Cited in This Article: ] |