Published online Feb 28, 2010. doi: 10.3748/wjg.v16.i8.1008
Revised: December 7, 2009
Accepted: December 14, 2009
Published online: February 28, 2010
AIM: To investigate the preparation, physicochemical characterization and cytotoxicity in vitro of Gemcitabine-loaded poly(ethylene glycol)-block-poly(D,L-lactide) (PEG-PDLLA) nanovesicles.
METHODS: The nanovesicle carriers were prepared from the amphiphilic block copolymer of PEG-PDLLA by a double emulsion technique, and gemcitabine was used as the model drug. The morphology of the nanovesicles was determined by scanning and transmission electron microscopy, and the drug content, drug entrapment and drug-release curve in vitro were detected by UV-Vis-NIR spectrophotometry. Cytotoxicity in the human pancreatic cancer cell line SW1990 was tested by 3-(4,5-dimethyl) ethiazole (MTT) assay.
RESULTS: The gemcitabine-loaded nanovesicles were hollow nanospheres with a mean size of 200.6 nm, drug loading of 4.14% and drug embedding ratio of 20.54%. The nanovesicles showed excellent controlled release that was characterized by a fast initial release during the first 72 h, followed by a slower and continuous release. The MTT assay demonstrated that gemcitabine-loaded nanovesicles exhibited dose-dependent and time-delayed cytotoxicity in the human pancreatic cancer cell line SW1990.
CONCLUSION: Gemcitabine-loaded PEG-PDLLA nanovesicles prepared by a double emulsion technique exhibited good performance for controlled drug release, and had similar cytotoxic activity to free gemcitabine.
-
Citation: Jia L, Zheng JJ, Jiang SM, Huang KH. Preparation, physicochemical characterization and cytotoxicity
in vitro of gemcitabine-loaded PEG-PDLLA nanovesicles. World J Gastroenterol 2010; 16(8): 1008-1013 - URL: https://www.wjgnet.com/1007-9327/full/v16/i8/1008.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i8.1008
Nanovesicles are a new type of drug carrier, which can protect incorporated drugs from in vivo metabolism while enhancing their therapeutic effect and reducing their toxicity. Compared with other drug delivery systems, nanovesicles are more suitable for hydrophilic drugs because of their hollow structure and internal aqueous phase, which can burden high drug loading.
Gemcitabine has been demonstrated to display antitumor activity against a wide variety of cancers, including pancreatic, colon, lung, breast, bladder and ovarian cancer[1-3]. However, gemcitabine is metabolized rapidly in the blood. Thus, a major limitation of this antitumor drug is that gemcitabine has a very short plasma half-life and strong side effects when administered intravenously[4]. As a drug carrier, nanovesicles may promote the efficacy of gemcitabine and reduce its side effects.
Polylactic acid (PLA) is the most widely used synthetic polymer, which is known to be biocompatible and degradable to give the natural product lactic acid[5]. However, nanoparticles based on PLA accumulate blood proteins on their surface as they circulate through the body[6,7]. This nonspecific absorption of proteins attracts attention from immune cells, with the result that nanoparticles are often removed from circulation before reaching their tumor targets. Modification with poly(ethylene glycol) (PEG) chains immobilized on the surface forms a hydrophilic palisade, which creates repulsion between the nanovesicles, and this repulsion can stop the nanovesicles from agglomerating, thus increasing their dispersion stability in aqueous media[8-10]. Furthermore, PEG is able to prevent proteins from adhering to the surface and thus avoids nanovesicles being recognized by macrophages[11,12], which prolongs the circulation time and facilitates nanoparticle uptake by specific cancer cells for cancer therapy[13,14].
However, there have only been a few studies on the incorporation of gemcitabine into PEG-block-poly(D,L-lactide) (PEG-PDLLA) nanovesicles. Therefore, we prepared gemcitabine-loaded nanovesicles, observed their size distribution, morphology and drug-release performance, and carried out a preliminary investigation of their cytotoxicity in vitro.
The amphiphilic block copolymer of PEG-PDLLA was synthesized by Sun Yat-Sen University. Gemcitabine was purchased from Eli Lilly & Co (Indianapolis, IN, United States). RPMI1640 and fetal bovine serum (FBS) were purchased from Gibco (Grand Island, NY, United States).
The human pancreatic cancer cell line SW1990 was obtained from the Second Affiliated Hospital of Sun Yat-Sen University. All cells were cultured as monolayers at 37°C in a 5% CO2/95% humidified atmosphere with RPMI1640 supplemented with 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin and 2 mmol/L L-glutamine.
Gemcitabine-loaded nanovesicles were prepared by a double emulsion (w/o/w) technique. The preparation was performed as follows: (1) An aqueous solution (2 mg gemcitabine in 0.2 mL pure water) was added dropwise into an organic solution of the polymer (10 mg PEG-PDLLA in 2 mL trichloromethane), and the first emulsion (w/o) was formed by sonication; (2) The emulsion was added dropwise into an organic solution that consisted of 40 mL polyvinyl alcohol (PVA) solution at 0.5% (w/v) and the double emulsion (w/o/w) was obtained by sonication; (3) The residual trichloromethane of the double emulsion was removed completely by vacuum distillation with a rotary evaporator, and free PVA and non-incorporated gemcitabine was removed by dialysis in the pure water overnight; and (4) Nanovesicles obtained as a suspension were purified by filtration through a syringe filter (pore size 0.45 μm), and then subjected to lyophilization to yield the solid nanovesicle samples. We repeatedly prepared three groups of gemcitabine-loaded nanovesicle samples according to the above processes.
Three groups of prepared nanovesicles were redissolved in DMSO. Gemcitabine in the solution was measured by ultraviolet spectroscopy at 275 nm (Perkin-Elmer Lambda 20 UV-Vis spectrophotometer, Beijing, China), and its incorporation efficiency was evaluated by drug loading (DL) and drug embedding ratio (ER). DL (%) = mass of drug in nanovesicles × 100/mass of nanovesicles recovered. ER (%) = mass of drug in nanovesicles × 100/mass of drug used in formulation.
After dilution with purified water, nanovesicle size was determined by photon correlation spectroscopy (PCS) (Malvern Autosizer, United States), at a scattering angle of 90° and a temperature of 25°C. Values are reported as the mean diameter (MD). The morphology of the nanovesicles was observed by transmission electron microscopy (TEM) (JEOL-100CXII, Japan) and scanning electron microscopy (SEM) (XL-30, Holland). A drop of the nanovesicles suspension (10 μL) was placed on copper electron microscopy grids (Formvar coated) and stained with 2% (w/v) phosphotungstic acid solution. After 30 s, the sample was washed with purified water and the excess fluid was removed with a piece of filter paper. The dried sample was then examined.
Phosphate buffer solution (PBS) at pH 7.4 and pH 5 was selected for the release medium. Nanovesicle samples (60 mg each) were resuspended in 5 mL PBS (pH 7.4 or 5) and transferred into a dialysis tubing (MW cut-off: 14 000 Da). The tubing was placed in 45 mL PBS (pH 7.4 or 5). The release study was performed at 37°C in a Shanghai Yiheng Scientific DKZ Incubator Shaker. At selected time intervals, 5 mL buffered solution outside the dialysis tubing was removed for UV-Vis analysis and replaced with 5 mL fresh buffer solution. Gemcitabine concentration was calculated based on the absorbance intensity at 268 nm. The equations were as follows: CpH 7.4 = 34.724A - 0.2971, r2 = 0.9999; CpH 7.4 = 38.317A - 0.3289, r2 = 0.9999 (C: drug concentration; A: absorbance at 268 nm). The cumulative percentage of released gemcitabine (%) could be calculated by the equation: Q (%) = (Vo× Ct + V ×ΣC) × 100%/(W × X) (Q: cumulative percentage of released gemcitabine; Ct: gemcitabine concentration in release medium at each time point; Vo: gross removed volume of release medium; V: volume of sample; W: gross mass of nanovesicles; X: drug content of gemcitabine).
The inhibition of cell growth was evaluated by the 3-(4,5-dimethyl) ethiazole (MTT) method using triplicate assays. Gemcitabine and gemcitabine-loaded nanovesicles were diluted in RPMI1640 at 1.0 × 10-3 mol/L, respectively. Then, 100 μL cell solution with 5 × 104 cells/mL was seeded in 96-well plates (Costar, Cambridge, MA, United States), and allowed to attach overnight. After 24 h incubation, gemcitabine-loaded nanovesicles with concentrations of 10-7, 10-6, 10-5 and 10-4 mol/L were added to the cell cultures. Gemcitabine added at the same concentration acted as a positive control. PBS was added as a negative control. At 24, 48, 72 and 96 h, 20 μL MTT and 200 μL RPMI culture medium were added to each well and incubated at 37°C for 4 h. Then MTT and culture medium were removed and 200 μL DMSO was added. The absorbance of the converted dye, which correlated with the number of viable cells, was determined at 490 nm. Cell viability was determined by the following equation: Cell viability (%) = (Abstest cell/Abscontrol cells) × 100%. Cell inhibitory rate was determined by the following formula: Cell inhibitory rate (%) = (1 - Abstest cell/Abscontrol cells) × 100%.
Statistical analysis was performed with SPSS for Windows version 13.0. Data were expressed as mean ± SD, and were compared using one-way ANOVA. P < 0.05 was considered statistically significant based on a two-tailed test.
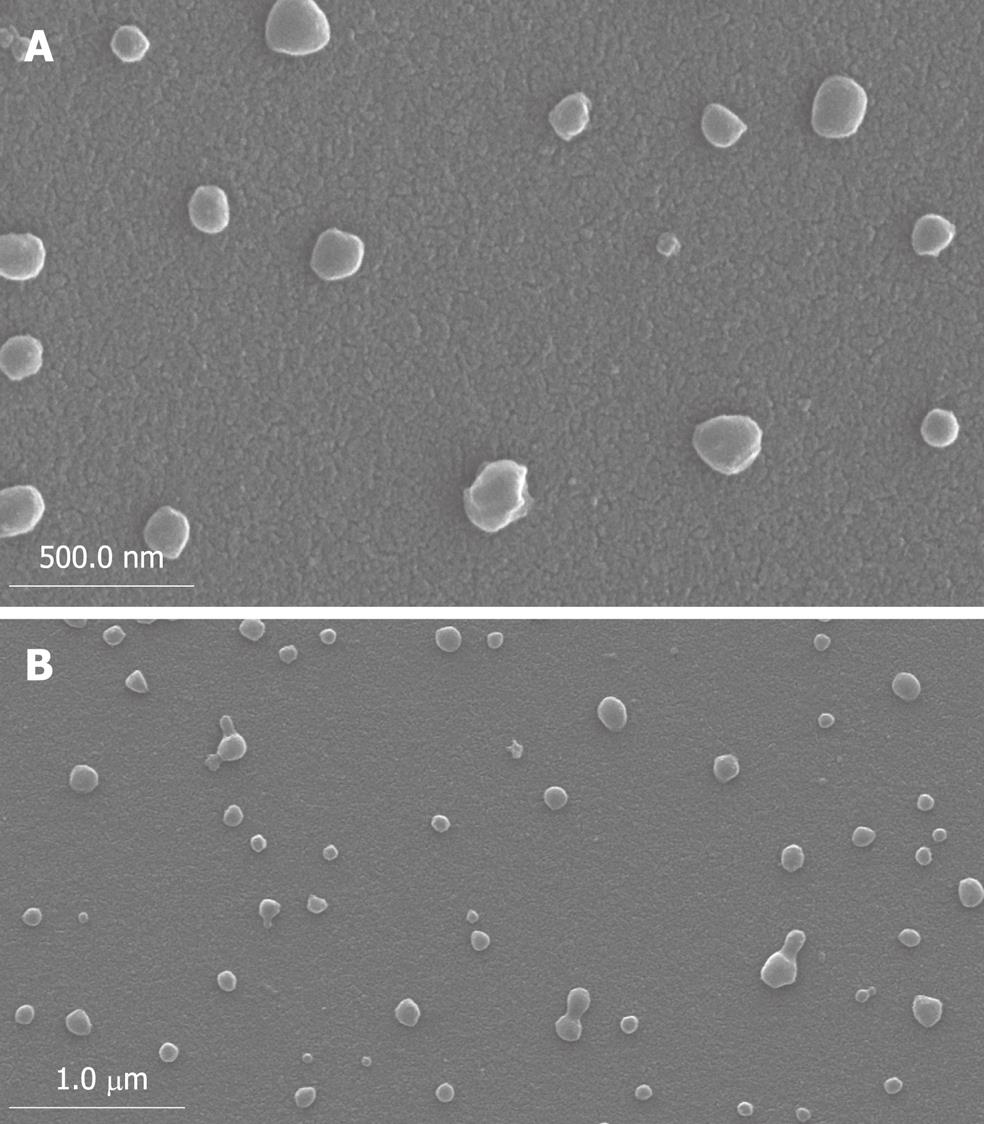
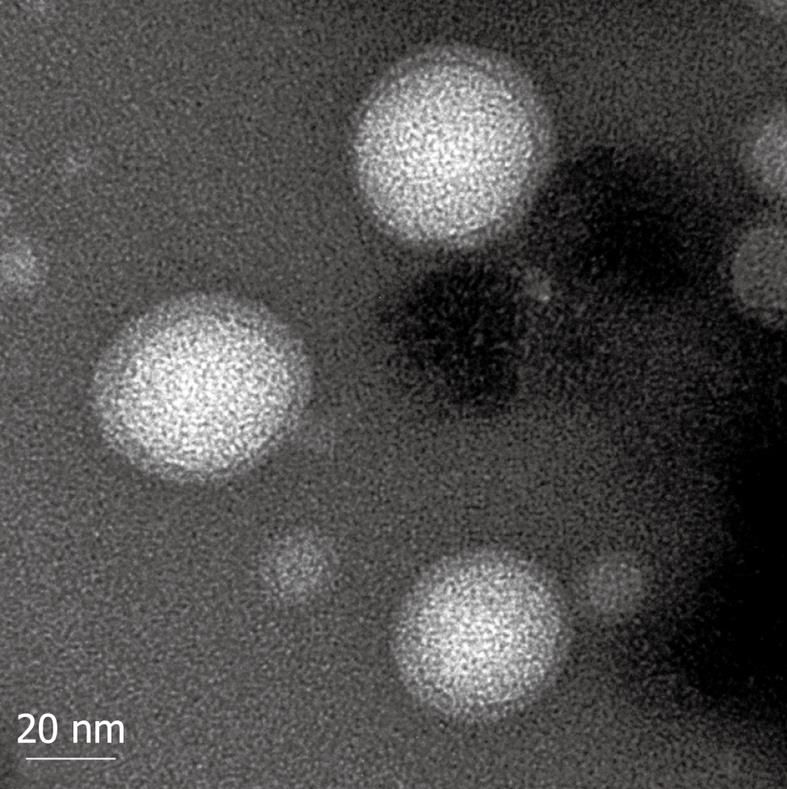
The mean diameter of prepared nanovesicles was 200.6 nm (range: 70-250 nm). As shown by SEM (Figure 1) and TEM (Figure 2), it was clear that the nanovesicles were spherical in shape, and hollow in structure, with a large central cavity in which gemcitabine was loaded.
The DL and ER in three groups of gemcitabine-loaded nanovesicles are shown in Table 1. The mean value of DL was 4.14% ± 0.13%, and ER was 20.54% ± 0.92%, which indicated good duplication of the nanovesicle preparation.
| Sample | 1 | 2 | 3 | mean ± SD |
| Drug content | 4.23 | 3.99 | 4.19 | 4.14 ± 0.13 |
| Drug encapsulation efficiency | 21.34 | 19.54 | 20.75 | 20.54 ± 0.92 |
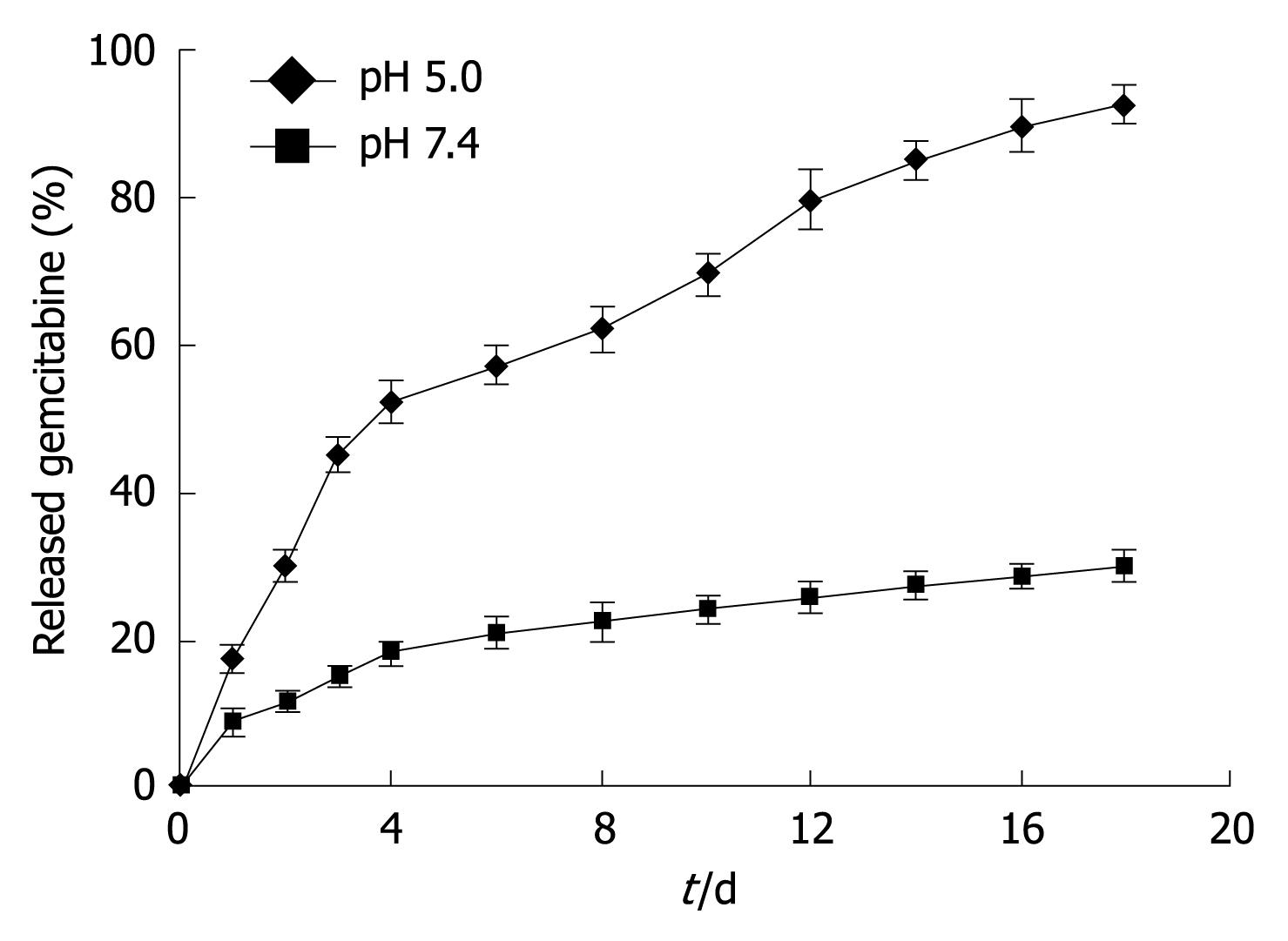
The in vitro release of gemcitabine-loaded nanovesicles in two different buffered solutions (pH 7.4 and 5.0) is shown in Figure 3. A typical two-phase-release was observed in both solutions. A rapid release was observed from gemcitabine-loaded nanovesicles in the first 4 d, and a relatively slower and sustained release was observed in the following days. By comparison with the release at pH 5.0, gemcitabine-release from nanovesicles at pH 7.4 was much slower. In the first 18 d, the cumulative percentage of released gemcitabine at pH 7.4 was < 30%, whereas released gemcitabine at pH 5.0 was 92.70%.
Table 2 shows the cell inhibitory rate of gemcitabine-loaded nanovesicles and free gemcitabine at a concentration range of 10-7 to 10-4 mol/L. With increasing drug concentration, pancreatic cancer cells were decreased in the gemcitabine-loaded nanovesicles and free gemcitabine groups. At each drug concentration, the cell inhibitory rate in the gemcitabine-loaded nanovesicles group was lower than that in the free gemcitabine group, however, no statistically significant difference was observed (all P > 0.05).
| Drug concentration (mol/L) | Gemcitabine-loaded nanovesicles | Free gemcitabine | P value |
| Cell inhibitory rate (%) | Cell inhibitory rate (%) | ||
| 10-7 | 18.3 ± 1.6 | 21.7 ± 2.5 | 0.480 |
| 10-6 | 46.2 ± 2.7 | 49.5 ± 4.1 | 0.619 |
| 10-5 | 82.3 ± 5.4 | 78.8 ± 4.2 | 0.592 |
| 10-4 | 93.2 ± 5.3 | 95.2 ± 3.7 | 0.552 |
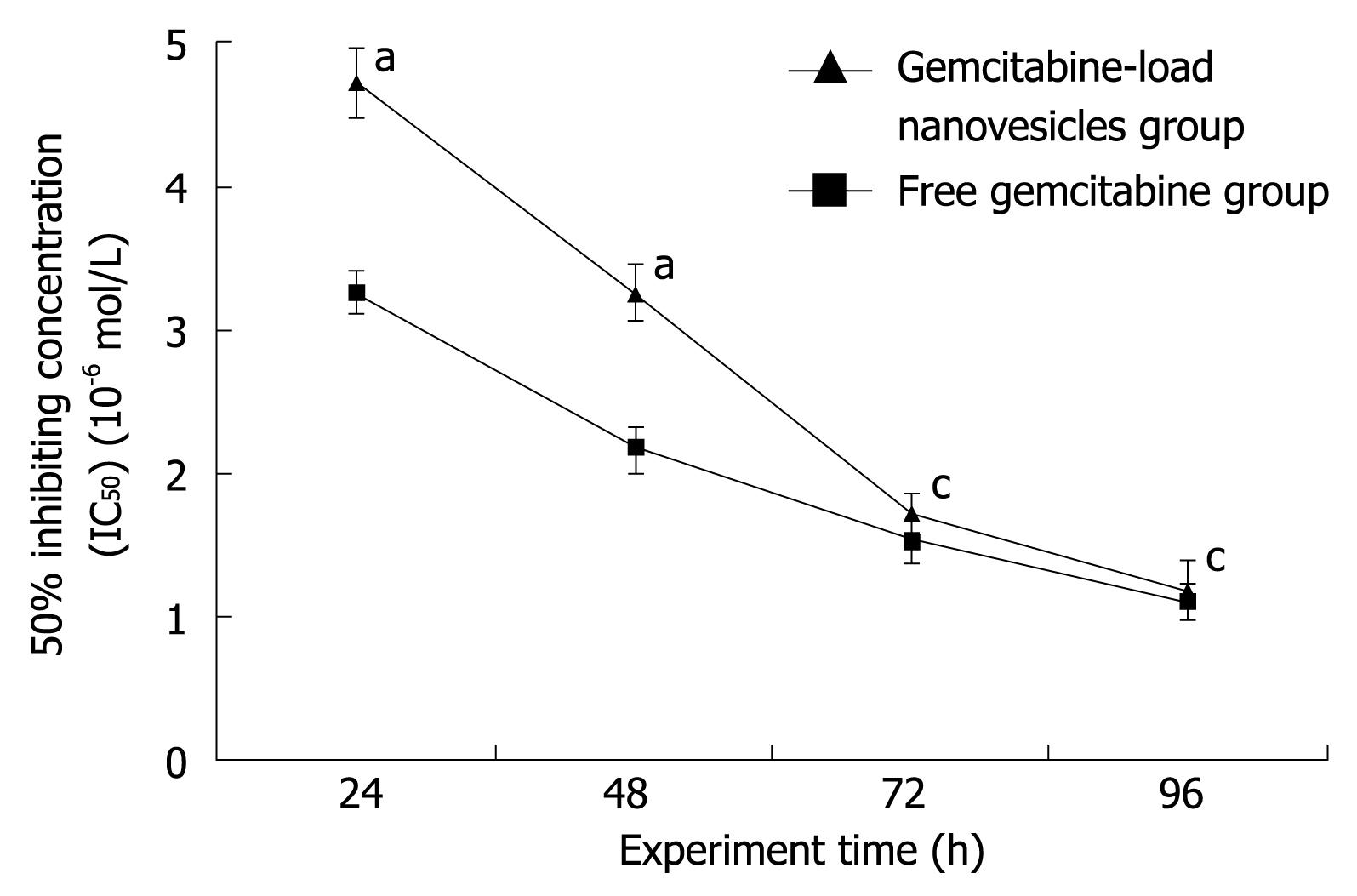
The IC50 (50% inhibiting concentration) of gemcitabine-loaded nanovesicles and free gemcitabine in human SW1990 pancreatic cancer cells at four time points is shown in Figure 4. IC50 gradually decreased with time in both groups. In the first 48 h, IC50 in the gemcitabine-loaded nanovesicles group was significantly higher than that in the free gemcitabine group (P < 0.05). At 72 h, IC50 was also higher in the gemcitabine-loaded nanovesicles group than in the free gemcitabine group, but this difference was not statistically significant (P > 0.05). Up to the end point at 96 h, IC50 was very similar in the two groups.
Gemcitabine is an anticancer nucleoside analogue that is active against various solid tumors. However, with intravenous administration, this drug is inactivated rapidly by enzymatic deamination, and it has a short biological half-life that necessitates the administration of high doses, which leads to unwanted side effects[15-17]. Therefore, as a carrier for gemcitabine delivery, nanovesicles, which are superior to other drug delivery systems for encapsulating hydrophilic drugs and can be subjected to surface modification, are used to overcome the above problems. Many advantages can be found in gemcitabine-loaded nanovesicles, including the possibility of targeting specific sites, controlled drug release, and protection of the encapsulated drug[18-24].
Our prepared nanovesicles were spherical in shape with a hollow structure, and gemcitabine was incorporated in the core of the nanovesicles. Generally, nanovesicles < 500 nm are considered ideal for drug delivery systems, because this size allows more effective systemic circulation than smaller molecules, and they can access places in the human body that larger particles cannot reach. At the same time, nanoparticles of this size can penetrate tumors because of the leaky nature of their microvasculature. This classic effect, which is referred to as the “enhanced permeation and retention effect”, results in prolonged circulation and accumulation of a therapeutic agent within the tumor[25]. Meanwhile, the modification by PEG allows nanoparticles to escape from the vasculature through the leaky endothelial tissue that surrounds the tumor, and therefore accumulate in certain solid tumors[26,27]. As a result, nanoparticles can target the tumor through blood vessels and enhance the anticancer activity of the drug.
As shown in Figure 3, these nanoparticles have a stable release profile. The release of gemcitabine from nanovesicles exhibited a biphasic pattern that was characterized by a fast initial release during the first 72 h, followed by a slower continuous release. The fast release of gemcitabine was probably the result of rapid desorption of gemcitabine from the external layer of the nanovesicles and diffusion of the drug located near the surface, whereas the slower and continuous release may be attributed to slow trans-layer permeation kinetics and diffusion from the interior, together with erosion of the polymer. It has been shown that the aqueous medium slowly penetrates the internal structure of the particles and causes progressive degradation of the polymer chains.
Another phenomenon was that gemcitabine release from nanovesicles at pH 5.0 was much faster than that at pH 7.4, which indicated that faster degradation of nanovesicles appeared at lower pH. This pH-dependent release is of particular interest in achieving tumor-targeted gemcitabine delivery with nanovesicles. It is expected that most of the gemcitabine encapsulated in nanovesicles will remain in the nanovesicle core for a considerable time period, when the injected nanovesicles remain in the plasma at normal physiological conditions (pH 7.4). However, faster release occurs once the nanovesicles reach the solid tumor site, where the pH value is lower than that in normal tissue[28]. In addition, particles are usually internalized inside the cells by endocytosis[22]. Therefore, further accelerated release inside the endosome/lysosome of tumor cells may occur as a result of the decreased pH values.
Moreover, in our study, gemcitabine-loaded nanovesicles were similar to free gemcitabine in terms of their cell inhibitory effect at different drug concentrations. Besides, time-delayed cytotoxicity was exhibited by gemcitabine-loaded nanovesicles in the human pancreatic cancer cell line SW1990. In the first 48 h, IC50 of gemcitabine-loaded nanovesicles was significantly higher than that of free gemcitabine. However, in the following 48 h, IC50 of gemcitabine-loaded nanovesicles gradually approached that of free gemcitabine. Up to the end of our experiment, IC50 was very similar in the two groups. It is estimated that the cell inhibitory effect of gemcitabine-loaded nanovesicles is more obvious during the period of sustained release because of its time-dependent release and delayed nuclear uptake in human SW1990 pancreatic cancer cells, which is consistent with in vitro gemcitabine release studies. Therefore, our future studies will involve in vivo experiments and related research on gemcitabine-loaded nanovesicles.
In summary, using the double emulsion technique, small, spherical and submicron sized (< 210 nm) PEG-PDLLA nanovesicles loaded with gemcitabine were prepared which did not change the drug structure or cytotoxicity. Compared with free gemcitabine, the gemcitabine-loaded nanovesicles elicited a time-dependent improvement in cytotoxic effect that was related to delayed release. This may possibly improve the administration of gemcitabine to pancreatic cancer patients.
Gemcitabine is used widely for the treatment of pancreatic cancer. However, because of its rapid metabolism in the blood, gemcitabine has a very short plasma half-life and strong side effects when administered intravenously. As a drug carrier, nanovesicles may promote the efficacy of gemcitabine and reduce its side effects.
Among the nanoparticle drug delivery systems, nanovesicles are recommended as drug delivery devices for anticancer agents. The double emulsion technique is suitable for the preparation of poly(ethylene glycol)-block-poly(D,L-lactide) (PEG-PDLLA) gemcitabine-loaded nanovesicles.
On the basis of previous data, this is the first study of the preparation, physicochemical characterization and in vitro cytotoxicity of gemcitabine-loaded nanovesicles. The results of the present study showed that the small, spherical and submicron sized gemcitabine-loaded nanovesicles, which were prepared by a double emulsion technique, did not change the drug structure or cytotoxicity. The gemcitabine-loaded nanovesicles elicited a time-dependent improvement in cytotoxic effect that was related to delayed drug release, compared with free gemcitabine.
Gemcitabine-loaded PEG-PDLLA nanovesicles exhibited good controlled drug release, and similar cytotoxic activity to free gemcitabine. This may provide a way of improving the administration of gemcitabine to pancreatic cancer patients.
The double emulsion (w/o/w) technique is a biochemical engineering method. It is performed as follows: an aqueous solution is added to an organic solution of the polymer, and the first emulsion (w/o) is formed by sonication. The emulsion is added to an organic solution, and the double emulsion (w/o/w) is obtained by sonication.
The authors report what is mainly a biochemical engineering study, although the carcinoma cell line utilised is gastrointestinal. The results are fairly limited but interesting, although clinical introduction of this kind of treatment is still many years away.
Peer reviewer: Jamie Murphy, MRCS (Eng), MA, Lecturer in Colorectal Surgery, Centre for Academic Surgery, Royal London Hospital, 3rd Floor Alexandra Wing, London, E1 1BB, United Kingdom
S- Editor Wang JL L- Editor Webster JR E- Editor Lin YP
| 1. | Jia L, Zhang MH, Yuan SZ, Huang WG. Antiangiogenic therapy for human pancreatic carcinoma xenografts in nude mice. World J Gastroenterol. 2005;11:447-450. [Cited in This Article: ] |
| 2. | Kamath A, Yoo D, Stuart OA, Bijelic L, Sugarbaker PH. Rationale for an intraperitoneal gemcitabine chemotherapy treatment for patients with resected pancreatic cancer. Recent Pat Anticancer Drug Discov. 2009;4:174-179. [Cited in This Article: ] |
| 3. | Danesi R, Altavilla G, Giovannetti E, Rosell R. Pharmacogenomics of gemcitabine in non-small-cell lung cancer and other solid tumors. Pharmacogenomics. 2009;10:69-80. [Cited in This Article: ] |
| 4. | Reddy LH, Dubernet C, Mouelhi SL, Marque PE, Desmaele D, Couvreur P. A new nanomedicine of gemcitabine displays enhanced anticancer activity in sensitive and resistant leukemia types. J Control Release. 2007;124:20-27. [Cited in This Article: ] |
| 5. | Chen C, Lv G, Pan C, Song M, Wu C, Guo D, Wang X, Chen B, Gu Z. Poly(lactic acid) (PLA) based nanocomposites--a novel way of drug-releasing. Biomed Mater. 2007;2:L1-L4. [Cited in This Article: ] |
| 6. | Tschon M, Fini M, Giavaresi G, Torricelli P, Rimondini L, Ambrosio L, Giardino R. In vitro and in vivo behaviour of biodegradable and injectable PLA/PGA copolymers related to different matrices. Int J Artif Organs. 2007;30:352-362. [Cited in This Article: ] |
| 7. | Uner M. Preparation, characterization and physico-chemical properties of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): their benefits as colloidal drug carrier systems. Pharmazie. 2006;61:375-386. [Cited in This Article: ] |
| 8. | Porjazoska A, Yilmaz OK, Baysal K, Cvetkovska M, Sirvanci S, Ercan F, Baysal BM. Synthesis and characterization of poly(ethylene glycol)-poly(D,L-lactide-co-glycolide) poly(ethylene glycol) tri-block co-polymers modified with collagen: a model surface suitable for cell interaction. J Biomater Sci Polym Ed. 2006;17:323-340. [Cited in This Article: ] |
| 9. | Yang X, Deng W, Fu L, Blanco E, Gao J, Quan D, Shuai X. Folate-functionalized polymeric micelles for tumor targeted delivery of a potent multidrug-resistance modulator FG020326. J Biomed Mater Res A. 2008;86:48-60. [Cited in This Article: ] |
| 10. | Kommareddy S, Amiji M. Poly(ethylene glycol)-modified thiolated gelatin nanoparticles for glutathione-responsive intracellular DNA delivery. Nanomedicine. 2007;3:32-42. [Cited in This Article: ] |
| 11. | Kim BS, Kim CS, Lee KM. The intracellular uptake ability of chitosan-coated Poly (D,L-lactide-co-glycolide) nanoparticles. Arch Pharm Res. 2008;31:1050-1054. [Cited in This Article: ] |
| 12. | Shuai X, Ai H, Nasongkla N, Kim S, Gao J. Micellar carriers based on block copolymers of poly(epsilon-caprolactone) and poly(ethylene glycol) for doxorubicin delivery. J Control Release. 2004;98:415-426. [Cited in This Article: ] |
| 13. | Craparo EF, Cavallaro G, Bondì ML, Mandracchia D, Giammona G. PEGylated Nanoparticles based on a polyaspartamide. preparation, physico-chemical characterization, and intracellular uptake. Biomacromolecules. 2006;7:3083-3092. [Cited in This Article: ] |
| 14. | Weiss B, Schneider M, Muys L, Taetz S, Neumann D, Schaefer UF, Lehr CM. Coupling of biotin-(poly(ethylene glycol))amine to poly(D,L-lactide-co-glycolide) nanoparticles for versatile surface modification. Bioconjug Chem. 2007;18:1087-1094. [Cited in This Article: ] |
| 15. | Reddy LH, Renoir JM, Marsaud V, Lepetre-Mouelhi S, Desmaële D, Couvreur P. Anticancer efficacy of squalenoyl gemcitabine nanomedicine on 60 human tumor cell panel and on experimental tumor. Mol Pharm. 2009;6:1526-1535. [Cited in This Article: ] |
| 16. | Calvagno MG, Celia C, Paolino D, Cosco D, Iannone M, Castelli F, Doldo P, Frest M. Effects of lipid composition and preparation conditions on physical-chemical properties, technological parameters and in vitro biological activity of gemcitabine-loaded liposomes. Curr Drug Deliv. 2007;4:89-101. [Cited in This Article: ] |
| 17. | Vauthier C, Dubernet C, Chauvierre C, Brigger I, Couvreur P. Drug delivery to resistant tumors: the potential of poly(alkyl cyanoacrylate) nanoparticles. J Control Release. 2003;93:151-160. [Cited in This Article: ] |
| 18. | Swain MD, Octain J, Benson DE. Unimolecular, soluble semiconductor nanoparticle-based biosensors for thrombin using charge/electron transfer. Bioconjug Chem. 2008;19:2520-2526. [Cited in This Article: ] |
| 19. | Katsikogianni G, Avgoustakis K. Poly(lactide-co-glycolide)-methoxy-poly(ethylene glycol) nanoparticles: drug loading and release properties. J Nanosci Nanotechnol. 2006;6:3080-3086. [Cited in This Article: ] |
| 20. | Schaffazick SR, Pohlmann AR, de Cordova CA, Creczynski-Pasa TB, Guterres SS. Protective properties of melatonin-loaded nanoparticles against lipid peroxidation. Int J Pharm. 2005;289:209-213. [Cited in This Article: ] |
| 21. | De Jong WH, Borm PJ. Drug delivery and nanoparticles:applications and hazards. Int J Nanomedicine. 2008;3:133-149. [Cited in This Article: ] |
| 22. | Pinto Reis C, Neufeld RJ, Ribeiro AJ, Veiga F. Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomedicine. 2006;2:8-21. [Cited in This Article: ] |
| 23. | Kunii R, Onishi H, Ueki K, Koyama K, Machida Y. Particle characteristics and biodistribution of camptothecin-loaded PLA/(PEG-PPG-PEG) nanoparticles. Drug Deliv. 2008;15:3-10. [Cited in This Article: ] |
| 24. | Limayem Blouza I, Charcosset C, Sfar S, Fessi H. Preparation and characterization of spironolactone-loaded nanocapsules for paediatric use. Int J Pharm. 2006;325:124-131. [Cited in This Article: ] |
| 25. | Greish K. Enhanced permeability and retention of macromolecular drugs in solid tumors: a royal gate for targeted anticancer nanomedicines. J Drug Target. 2007;15:457-464. [Cited in This Article: ] |
| 26. | van Vlerken LE, Vyas TK, Amiji MM. Poly(ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular delivery. Pharm Res. 2007;24:1405-1414. [Cited in This Article: ] |
| 27. | Juillerat-Jeanneret L. The targeted delivery of cancer drugs across the blood-brain barrier: chemical modifications of drugs or drug-nanoparticles? Drug Discov Today. 2008;13:1099-1106. [Cited in This Article: ] |
| 28. | Gerweck LE, Vijayappa S, Kozin S. Tumor pH controls the in vivo efficacy of weak acid and base chemotherapeutics. Mol Cancer Ther. 2006;5:1275-1279. [Cited in This Article: ] |