Published online Dec 28, 2010. doi: 10.3748/wjg.v16.i48.6139
Revised: September 13, 2010
Accepted: September 20, 2010
Published online: December 28, 2010
AIM: To investigate the value of duplex Doppler ultrasonography (US) in the assessment of the hemodynamics of the portal and hepatic veins in a cohort of children with chronic liver disease (CLD) and to detect any relationship between the US changes, etiology and severity (or stage) of CLD.
METHODS: We prospectively enrolled 25 children with biopsy-proven CLD. Thirteen had cirrhosis (aged 8.9 ± 2.0 years) and 12 had chronic hepatitis (aged 9.3 ± 2.3 years). Gray scale and color-coded duplex Doppler US were performed for all, as well as 30 healthy age and sex-matched controls. Findings were correlated with clinical, laboratory and histopathological characteristics.
RESULTS: Prominent caudate lobe was detected in 100% of cirrhotics, but none of the chronic hepatitis or controls. Thickened lesser omentum and loss of the triphasic waveform of the hepatic vein were present in 69.2% and 53.8% of cirrhotics vs 33.3% and 8.3% of chronic hepatitis respectively. Portal vein flow velocity was significantly lower (P < 0.0001) and the congestion index was significantly higher (P < 0.005) in both patient groups compared to controls. Child-Pugh’s staging showed a positive correlation with both abnormal hepatic vein waveform and direction of portal blood flow; and a negative correlation with both hepatic and portal vein flow velocities. No correlation with the etiology of CLD could be detected.
CONCLUSION: Duplex Doppler added to grayscale US can detect significant morphologic and portal hemodynamic changes that correlate with the severity (stage) of CLD, but not with etiology.
- Citation: El-Shabrawi MH, El-Raziky M, Sheiba M, El-Karaksy HM, El-Raziky M, Hassanin F, Ramadan A. Value of duplex doppler ultrasonography in non-invasive assessment of children with chronic liver disease. World J Gastroenterol 2010; 16(48): 6139-6144
- URL: https://www.wjgnet.com/1007-9327/full/v16/i48/6139.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i48.6139
The search for a non-invasive biochemical or imaging marker for the severity (stage) and/or etiology of chronic liver disease (CLD) in adults, as well as children, is in extremely active nowadays. Real time ultrasonography (US) has become an integral part of the non-invasive evaluation of the liver in many clinical settings in adults. Color-coded duplex Doppler information regarding the presence or absence of flow and the direction and velocity of that flow can be obtained non-invasively, rapidly and relatively inexpensively[1]. In spite of being evaluated since 1983[2]; the accuracy, sensitivity and specificity of duplex Doppler imaging as a non-invasive diagnostic and prognostic modality for liver cirrhosis, and its correlation to the histopathologic findings as well as the degree of functional impairment of the liver, remains controversial and is still debated by many investigators[3]. Adding duplex Doppler evaluation in numerous studies has clarified the role of this modality in the evaluation of various CLD in adults and children, including liver cirrhosis[4-7]; portal hypertension[8-12]; presence or absence of esophageal varices[13], noninvasive diagnosis of the degree of hepatic fibrosis[14,15], assessment of the portal venous blood flow in cystic fibrosis[16], perioperative monitoring in orthotropic liver transplantation (LTx)[17], prediction of the severity of veno-occlusive disease and assessing its prognosis[18], assessment of the functional hepatic flow and total hepatic flow[19] and investigating the effect of fatty infiltration of the liver on the Doppler waveform pattern in the hepatic veins of obese children[3,20]. Other studies evaluated Doppler US measurement of the blood flow in the hepatic artery, hepatic veins and portal vein as a noninvasive indicator of disease severity in children who had undergone Kasai portoenterostomy for extrahepatic biliary atresia[21] and those who had CLD of unknown etiology[22]. However in the setting of LTx, serial intra- and post-operative Doppler US has largely been accepted as a useful technique for making an early diagnosis of abnormal hemodynamics of the graft circulation. Furthermore, intra-operative Doppler US is used to assess the reconstructed vessels objectively in order to reduce the incidence of vascular complications following LTx[6] and it is gradually replacing the more invasive angiographic techniques[23].
The aim of this study was to investigate the value of abdominal color-coded duplex Doppler US when added to the conventional grayscale scanning in the non-invasive assessment of the splanchnic morphology, as well as hemodynamics of the portal and hepatic veins in a cohort of Egyptian children suffering from CLD. We aimed also to detect any relationship between the US changes, etiology and severity (or stage) of the CLD.
We prospectively enrolled 25 children with CLD from the Pediatric Hepatology Unit at Cairo University Children Hospital, Cairo, Egypt. Thirteen patients (group 1) were diagnosed with established cirrhosis (7 girls and 6 boys) with a mean age of 8.9 ± 2.0 years, and 12 (group 2) were diagnosed with chronic hepatitis without cirrhosis (6 girls and 6 boys) with a mean age of 9.3 ± 2.3 years. Thirty healthy child relatives of the patients (13 girls and 17 boys) with a mean age of 8.1 ± 2.2 years were included as a control group. All patients were subjected to: (1) Careful interrogation and thorough physical examination for signs of CLD (jaundice, palmar erythema, bleeding diathesis, hand tremors, hepatomegaly, splenomegaly, ascites and edema); (2) biochemical tests of liver functions; (3) serological markers of viral and autoimmune hepatitis; (4) testing for inborn errors of metabolism when indicated; and (5) percutaneous liver biopsy using the Menghini aspiration technique for histopathological diagnosis, grading and staging of the CLD.
Patients, as well as controls, underwent conventional grayscale US using Toshiba® Sonolayer 2000 apparatus (Toshiba Corporation, Tokyo, Japan) equipped with 3.5 and 5 MHz convex linear transducers. Examination included liver size and echo pattern, portal vein diameter, splenic size and echo pattern, thickness of the lesser omentum in comparison to the aorta as well as detection of ascites. Both patients and controls also underwent color-coded duplex Doppler examination using Toshiba® Sonolayer SSH-60A apparatus (Toshiba Corporation, Tokyo, Japan) with a low frequency (3.5 MHz) transducer, in order to optimize the return of Doppler signals from deeper-lying tissues.
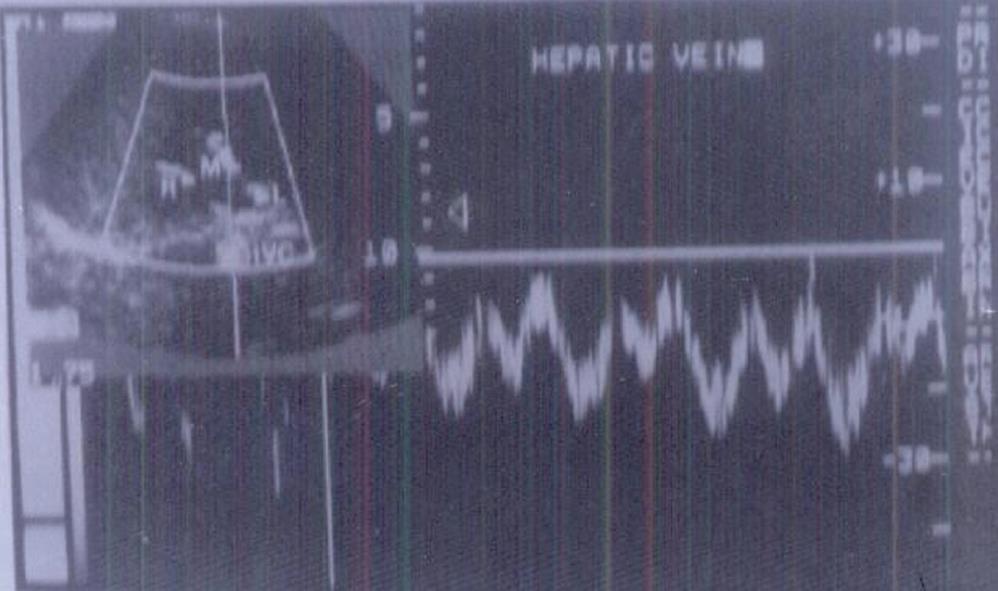
After being fasted overnight for a minimum of 8 h, all children were examined in the supine position. A low pulse repetition frequency was used initially, with manual adjustment when aliasing occurred. Approach for the vein of interest was selected to keep the beam vessel (angle 0) always less than 60o. Doppler recording of the hepatic veins was initially examined using a transverse sub-xiphoid approach, with the probe slightly cephalad. The right intercostal approach was used to obtain a longitudinal view of the middle hepatic vein. Every child was asked to stop breathing for a few seconds, in deep inspiration, during examination to avoid motion artifacts. Measurements were obtained from the hepatic veins at least 2 cm from the confluence with the inferior vena cava, to reduce the possible influence of the changes of flow pattern in the inferior vena cava on hepatic veins hemodynamics. We classified the hepatic vein Doppler waveforms according to Gorka et al[24] into: normal triphasic, abnormal biphasic or monophasic, and those with loss of the reverse-flow. Mean flow velocity of the middle hepatic vein was also measured in cm/s in all patients and controls.
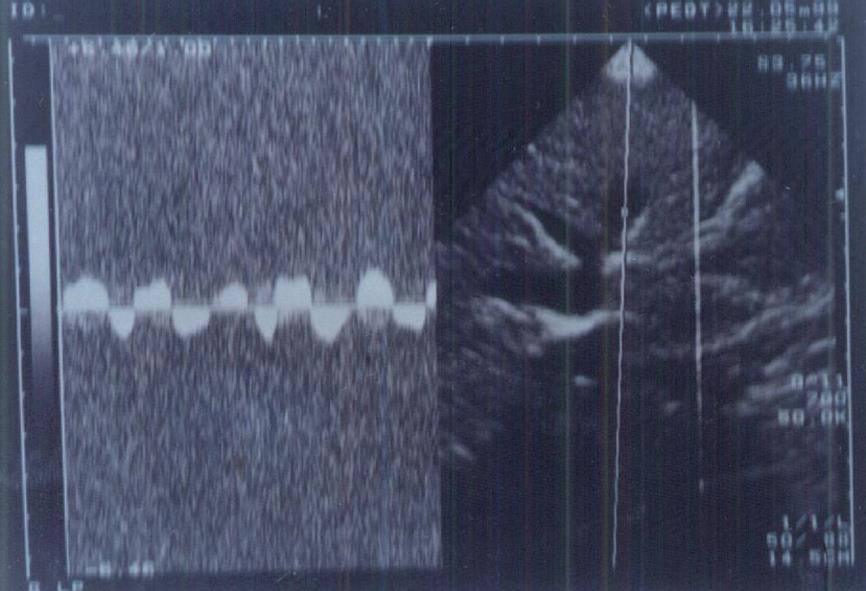
The portal vein was examined from an anterior abdominal subcostal and/or right intercostal approach and scanned longitudinally throughout its entire length. Measurements were obtained in the middle segment between the splenoportal junction and the intrahepatic bifurcation (1-2 cm before the bifurcation). It was examined at a standard point for the diameter, patency, presence or absence of intraluminal echogenic material, direction of flow within the vein and blood flow velocity in cm/s.
The congestion index (CI) is determined by duplex Doppler US. It is the ratio between the cross-sectional area of the portal vein in cm2 and the blood flow velocity in that vein in cm/s, and calculated using the following formula: CI = cross sectional area/blood flow velocity[25,26].
The cross sectional area was calculated using the following formula: Cross sectional area = π× (d2/4); d = diameter of portal vein in cm; and π = 3.14[27].
All data were statistically analyzed using independent samples test, χ2 test, post-Hock test and Armitage[28]. Probability (P value) was considered significant if P < 0.05.
Group 1 included 13 children with established cirrhosis (5 of metabolic etiology and 8 viral hepatitis C or B) and group 2 included 12 with chronic hepatitis and no cirrhosis (5 viral hepatitis C or B and 7 autoimmune). Table 1 shows the demographic and clinical data of the 2 groups. Biochemical tests of liver function showed no significant difference between either group (similar letters meant no significant difference, while dissimilar letters meant a significant difference, the P value is significant).
| Diagnosis | Group 1 (cirrhosis) | Group 2 (chronic hepatitis) |
| Symptoms | ||
| Hematemesis | 2 (15.4) | 3 (25) |
| Jaundice | 4 (38.5) | 3 (25) |
| Dark urine | 5 (38.5) | 4 (33.3) |
| Abdominal distension | 8 (61.7) | 8 (66.7) |
| General physical signs | ||
| Pallor | 5 (38.5) | 3 (25) |
| Jaundice | 5 (38.5) | 4 (33.3) |
| Lower limb edema | 6 (46.2) | 4 (33.3) |
| Abdominal signs | ||
| Hepatomegaly | 7 (53.8) | 9 (75) |
| Splenomegaly | 10 (76.9) | 4 (33.3) |
| Ascites | 5 (38.5) | 3 (25) |
A prominent caudate lobe (as an important US sign) was found in 100% of patients with cirrhosis and none of patients with chronic hepatitis or controls. Thickened lesser omentum (i.e. lesser omentum: aortic diameter ratio > 1:1.7 in children[29]) was present in 69.21% of cirrhotic patients in comparison to 33.3% of patients with chronic hepatitis. Loss of the normal triphasic oscillation of the hepatic vein waveform was detected in 53.8% of group 1 in comparison to 8.3% of group 2 and none of the controls. Abnormal direction of portal blood flow was detected in 46.2% of group 1 and 25% of group 2, and none of the controls (Figures 1 and 2).
Hepatic vein flow velocity showed non-significant negative correlation with liver size (r = -0.125) and weak but significant correlation with both splenic size (r = -0.374) and portal vein diameter (r = -0.304) (Table 2).
| Controls | Group 1 | Group 2 | P value | |
| Liver size in cm | 8.55 ± 0.69 | 10.19 ± 2.33b | 12.2 ± 2.02b,d | < 0.01 |
| Splenic size in cm | 8.43 ± 0.92 | 11.82 ± 1.30b | 10.10 ± 1.50b,d | < 0.01 |
| Portal vein diameter (cm) | 0.61 ± 0.13 | 1.16 ± 0.23b | 0.95 ± 0.22b,d | < 0.01 |
| Hepatic vein flow velocity (cm/s) | 23.1 ± 2.09 | 21.84 ± 1.90 | 22.41 ± 2.02 | > 0.05 |
| Portal vein flow velocity (cm/s) | 31.1 ± 3.92 | 21.23 ± 3.30b | 22.22 ± 1.23b | < 0.01 |
| Congestion index | 4.61 ± 5.75d | 9.50 ± 1.60 | 4.97 ± 1.14d | < 0.01 |
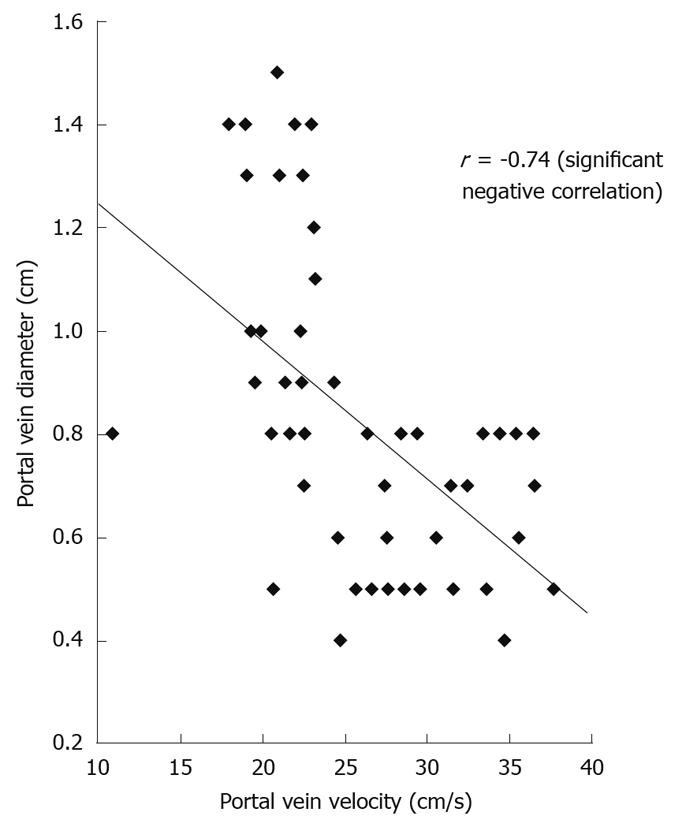
Portal vein flow velocity had weak significant negative correlation with liver size (r = -0.431) and powerful significant negative correlation with both splenic size (r = -0.699) and portal vein diameter (r = -0.743, Figure 3).
CI had a weak significant negative correlation with liver size (r = -0.431) and powerful significant negative correlation with both splenic size (r = -0.699) and portal vein diameter (r = -0.743).
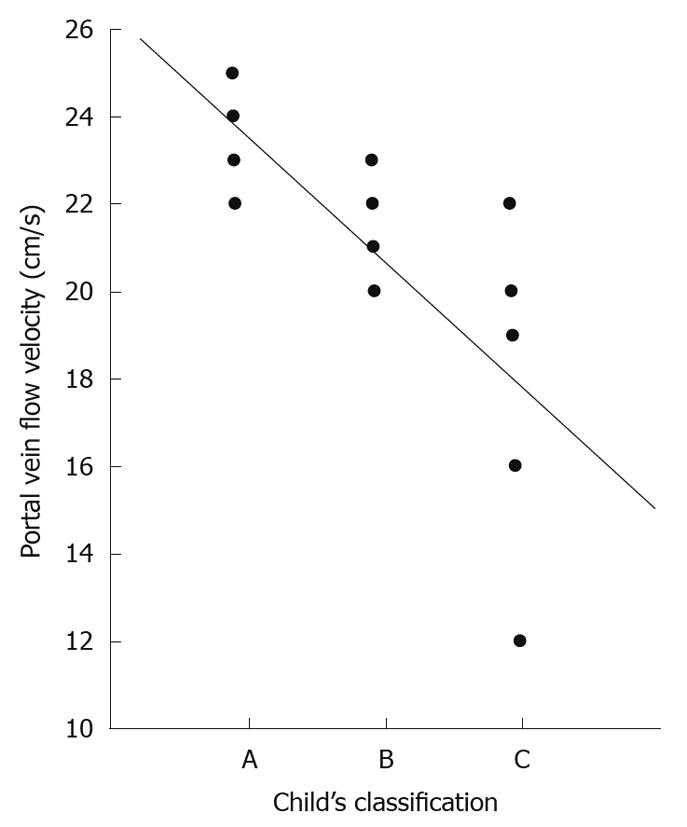
According to Child-Pugh’s[30] classification, 4 (31%) of our cirrhotic patients were Class A; 4 (31%) Class B and 5 (38%) Class C. Those classes showed a powerful significant positive correlation with both abnormal hepatic waveform and abnormal direction of portal blood flow. Also there was a powerful significant negative correlation with both hepatic vein and portal vein flow velocity (r = -0.785 and -0.688, respectively, Figure 4) and weak significant positive correlation with CI (r = -0.595).
Analysis of the US findings according to the etiological categories (metabolic, viral or autoimmune CLD) did not reveal any significant correlations.
In the present study we tried to correlate the splanchnic morphological and hemodynamic parameters of the portal and hepatic veins with the severity of hepatic affection, as evaluated by liver histopathology and Child-Pugh’s classification of cirrhosis[30]. Prominent caudate lobe was a constant finding in all our patients with cirrhosis and not found in chronic hepatitis. The prominence of the caudate lobe in cirrhosis results from marked hyperplasic changes in the regenerative nodules with no cellular or structural atypia. The density of the regenerative hepatocytes becomes much higher, the quantity of bound water larger and free water smaller, increasing the US signals reflected. The reason why only the caudate lobe shows such huge hyperplasia in cirrhosis remains unclear[31].
Thickened lesser omentum (i.e. lesser omentum: aortic diameter more than 1:1.7[29]) is highly-suggestive of portal hypertension and the presence of esophageal varices in children, and allows the detection of portal hypertension earlier than detection of collaterals by Doppler and even earlier than clinical signs[32]. In our study there was a significant increase of lesser omentum thickness in cirrhotic patients than chronic hepatitis.
Koda et al[8], in 1996 described the decrease in portal vein flow velocity with the progress of chronic hepatitis as a sensitive indicator and a useful test with close correlation with the histological degree of liver fibrosis. Schneider et al[33], combined portal vein velocity with hepatic artery pulsatility index as a reliable, non-invasive evaluation of patients with liver cirrhosis. On the other hand Dinç et al[34], reported that portal vein flow velocity and portal vein flow volume alone are not useful parameters for discriminating cirrhotic patients from healthy subjects. In our study we did not measure the hepatic artery pulsatility index; however values of portal vein flow velocity were significantly lower in patients compared to controls and significantly lower in the group with liver cirrhosis than the chronic hepatitis. A significant correlation was also detected between both splenic size and Child-Pugh’s class and the portal flow velocity; the lower the velocity, the larger the splenic size and the worse the Child-Pugh’s class. Therefore in children with cirrhosis, portal flow velocity might be correlated with the severity of portal hypertension and the severity of liver parenchymal dysfunction as worsening Child-Pugh’s class was associated with lower portal vein flow velocity conforming to reports in adults[35].
In the present study CI was significantly higher in the group with liver cirrhosis than both the chronic hepatitis group and the controls. CI showed no significant difference between chronic hepatitis group and controls. CI showed a positive correlation with Child-Pugh’s class of cirrhotics. CI was reported to be a significant parameter in the evaluation of the risk of bleeding varices and prognosis of patients with liver cirrhosis, while in chronic hepatitis patients it was found to be similar to healthy controls and was not related to the grade of hepatic inflammation[36].
Loss of the normal triphasic oscillation of the hepatic vein waveform was detected in 53.8% of our cirrhotics, in comparison to 8.3% of chronic hepatitis and none of the controls. Bolondi et al[37], Ohta et al[38] and Arda et al[39], reported the loss of triphasic oscillation even in early-stage chronic parenchymal liver disease (Child-Pugh’s class A).
In conclusion, grayscale and color-coded duplex Doppler US are very valuable, non-invasive diagnostic modalities in children with CLD. They could detect splanchnic morphological and portal hemodynamic changes that could be correlated to the degree of liver parenchymal affection but not to the etiology of the CLD. Therefore we recommend their wider application in the assessment of children with CLD.
The search for a non-invasive biochemical or imaging marker for the severity (stage) and/or etiology of chronic liver disease (CLD) in adults as well as children is in extremely active nowadays. Real time ultrasonography (US) has become an integral part of the non-invasive evaluation of the liver in many clinical settings in adults. Color-coded duplex Doppler information regarding the presence or absence of flow and the direction and velocity of that flow can be obtained non-invasively, rapidly and relatively inexpensively.
The aim of the research is to investigate the value of duplex Doppler US in the assessment of the hemodynamics of the portal and hepatic veins in a cohort of children with CLD, and to detect any relationship between the US changes and etiology and severity (or stage) of CLD.
In the present study, the authors tried to correlate the splanchnic morphological and hemodynamic parameters of the portal and hepatic veins with the severity of hepatic affliction. In this study, the authors found that the values of portal vein flow velocity were significantly lower in patients compared to controls and significantly lower in the group with liver cirrhosis than the chronic hepatitis group. A significant correlation was also detected between both splenic size and Child-Pugh’s class and the portal flow velocity; the lower the velocity, the larger the splenic size and the worse the Child-Pugh’s class. Therefore in children with cirrhosis, portal flow velocity might be correlated with the severity of portal hypertension and the severity of liver parenchymal dysfunction.
In conclusion, grayscale and color-coded duplex Doppler US are very valuable, non-invasive diagnostic modalities in children with CLD. They could detect splanchnic morphological and portal hemodynamic changes that could be correlated to the degree of liver parenchymal affection but not to the etiology of the CLD. Therefore the authors recommend their wider application in the assessment of children with CLD.
This article well documented that the grayscale and color-coded duplex Doppler US are very valuable for non-invasive diagnostic modalities in children with CLD, and this will interest the readers.
Peer reviewer: Dr. Herwig R Cerwenka, Professor, Department of Surgery, Medical University of Graz, Auenbruggerplatz 29, A-8036 Graz, Austria
S- Editor Sun H L- Editor Rutherford A E- Editor Zheng XM
| 1. | Schiff E, Sorrell M, Maddrey W. Non invasive imaging. 8th ed. New York: Lippencott-Raven Philadelphia 1999; 276-282. [Cited in This Article: ] |
| 2. | Kawamura S, Miyatake K, Okamoto K, Beppu S, Kinoshita N, Sakakibara H, Nimura Y. Analysis of the portal vein flow with two-dimensional echo-Doppler method. Ultrasound Med Biol. 1983;Suppl 2:511-515. [Cited in This Article: ] |
| 3. | Dietrich CF, Lee JH, Gottschalk R, Herrmann G, Sarrazin C, Caspary WF, Zeuzem S. Hepatic and portal vein flow pattern in correlation with intrahepatic fat deposition and liver histology in patients with chronic hepatitis C. AJR Am J Roentgenol. 1998;171:437-443. [Cited in This Article: ] |
| 4. | Colli A, Cocciolo M, Riva C, Martinez E, Prisco A, Pirola M, Bratina G. Abnormalities of Doppler waveform of the hepatic veins in patients with chronic liver disease: correlation with histologic findings. AJR Am J Roentgenol. 1994;162:833-837. [Cited in This Article: ] |
| 5. | Tarantino L, Giorgio A, de Stefano G, Mariniello N, Perrotta A, Aloisio V V, Forestieri MC, Del Viscovo L, Borsellino G. Reverse flow in intrahepatic portal vessels and liver function impairment in cirrhosis. Eur J Ultrasound. 1997;6:171-177. [Cited in This Article: ] |
| 6. | Someda H, Moriyasu F, Fujimoto M, Hamato N, Nabeshima M, Nishikawa K, Okuma M, Tanaka K, Ozawa K. Vascular complications in living related liver transplantation detected with intraoperative and postoperative Doppler US. J Hepatol. 1995;22:623-632. [Cited in This Article: ] |
| 7. | Kasztelan-Szczerbińska B, Jargiełło T, Słomka M, Szczerbo-Trojanowska M, Celiński K, Szczerbiński M. Diagnostic value of portal blood velocity measurements in the assessment of the severity of liver cirrhosis. Ann Univ Mariae Curie Sklodowska Med. 2003;58:286-290. [Cited in This Article: ] |
| 8. | Koda M, Murawaki Y, Kawasaki H, Ikawa S. Portal blood velocity and portal blood flow in patients with chronic viral hepatitis: relation to histological liver fibrosis. Hepatogastroenterology. 1996;43:199-202. [Cited in This Article: ] |
| 9. | Uno A, Ishida H, Konno K, Ohnami Y, Naganuma H, Niizawa M, Hamashima Y, Masamune O. Portal hypertension in children and young adults: sonographic and color Doppler findings. Abdom Imaging. 1997;22:72-78. [Cited in This Article: ] |
| 10. | Westra SJ, Zaninovic AC, Vargas J, Hall TR, Boechat MI, Busuttil RW. The value of portal vein pulsatility on duplex sonograms as a sign of portal hypertension in children with liver disease. AJR Am J Roentgenol. 1995;165:167-172. [Cited in This Article: ] |
| 11. | Kozaiwa K, Tajiri H, Yoshimura N, Ozaki Y, Miki K, Shimizu K, Harada T, Okada S. Utility of duplex Doppler ultrasound in evaluating portal hypertension in children. J Pediatr Gastroenterol Nutr. 1995;21:215-219. [Cited in This Article: ] |
| 12. | Gorka W, Kagalwalla A, McParland BJ, Kagalwalla Y, al Zaben A. Diagnostic value of Doppler ultrasound in the assessment of liver cirrhosis in children: histopathological correlation. J Clin Ultrasound. 1996;24:287-295. [Cited in This Article: ] |
| 13. | Liu CH, Hsu SJ, Liang CC, Tsai FC, Lin JW, Liu CJ, Yang PM, Lai MY, Chen PJ, Chen JH. Esophageal varices: noninvasive diagnosis with duplex Doppler US in patients with compensated cirrhosis. Radiology. 2008;248:132-139. [Cited in This Article: ] |
| 14. | Hirata M, Akbar SM, Horiike N, Onji M. Noninvasive diagnosis of the degree of hepatic fibrosis using ultrasonography in patients with chronic liver disease due to hepatitis C virus. Eur J Clin Invest. 2001;31:528-535. [Cited in This Article: ] |
| 15. | Bernatik T, Strobel D, Hahn EG, Becker D. Doppler measurements: a surrogate marker of liver fibrosis? Eur J Gastroenterol Hepatol. 2002;14:383-387. [Cited in This Article: ] |
| 16. | Vergesslich KA, Götz M, Mostbeck G, Sommer G, Ponhold W. Portal venous blood flow in cystic fibrosis: assessment by Duplex Doppler sonography. Pediatr Radiol. 1989;19:371-374. [Cited in This Article: ] |
| 17. | Gatecel C, Dupuy P, Fratacci MD, Ozier Y, Houssin D, Chapuis Y, Payen D. [Peroperative monitoring of hepatic arterial (DSAH) and portal venous (DSVP) output in orthotropic liver transplantation in man]. Ann Fr Anesth Reanim. 1989;8 Suppl:R53. [Cited in This Article: ] |
| 18. | Lassau N, Auperin A, Leclere J, Bennaceur A, Valteau-Couanet D, Hartmann O. Prognostic value of doppler-ultrasonography in hepatic veno-occlusive disease. Transplantation. 2002;74:60-66. [Cited in This Article: ] |
| 19. | Xingjiang W, Weiwei D, Jianmin C, Jianming H, Jieshou L. Functional hepatic flow can predict the hepatic reserve function in surgical cirrhotic patients. J Invest Surg. 2009;22:178-182. [Cited in This Article: ] |
| 20. | Uzun H, Yazici B, Erdogmus B, Kocabay K, Buyukkaya R, Buyukkaya A, Yazgan O. Doppler waveforms of the hepatic veins in children with diffuse fatty infiltration of the liver. Eur J Radiol. 2009;71:552-556. [Cited in This Article: ] |
| 21. | Kardorff R, Klotz M, Melter M, Rodeck B, Hoyer PF. Prediction of survival in extrahepatic biliary atresia by hepatic duplex sonography. J Pediatr Gastroenterol Nutr. 1999;28:411-417. [Cited in This Article: ] |
| 22. | Tüney D, Aribal ME, Ertem D, Kotiloğlu E, Pehlivanoğlu E. Diagnosis of liver cirrhosis in children based on colour Doppler ultrasonography with histopathological correlation. Pediatr Radiol. 1998;28:859-864. [Cited in This Article: ] |
| 23. | Kok T, Peeters PM, Hew JM, Martijn A, Koetse HA, Bijleveld CM, Slooff MJ. Doppler ultrasound and angiography of the vasculature of the liver in children after orthotopic liver transplantation: a prospective study. Pediatr Radiol. 1995;25:517-524. [Cited in This Article: ] |
| 24. | Gorka W, al Mulla A, al Sebayel M, Altraif I, Gorka TS. Qualitative hepatic venous Doppler sonography versus portal flowmetry in predicting the severity of esophageal varices in hepatitis C cirrhosis. AJR Am J Roentgenol. 1997;169:511-515. [Cited in This Article: ] |
| 25. | Moriyasu F, Nishida O, Ban N, Nakamura T, Sakai M, Miyake T, Uchino H. "Congestion index" of the portal vein. AJR Am J Roentgenol. 1986;146:735-739. [Cited in This Article: ] |
| 26. | Horn JR, Zierler B, Bauer LA, Reiss W, Strandness DE Jr. Estimation of hepatic blood flow in branches of hepatic vessels utilizing a noninvasive, duplex Doppler method. J Clin Pharmacol. 1990;30:922-929. [Cited in This Article: ] |
| 27. | Chawla Y, Santa N, Dhiman RK, Dilawari JB. Portal hemodynamics by duplex Doppler sonography in different grades of cirrhosis. Dig Dis Sci. 1998;43:354-357. [Cited in This Article: ] |
| 28. | Armitage P, Berry G. Statistical methods in medical research. 3rd ed. London: Blackwell scientific publication 1994; 234-239. [Cited in This Article: ] |
| 29. | Wagai T, Park SE. Screening of upper abdominal organs by digital linear array real time scanner. Ultrasound and cancer. Princeton: Excerpta Medica 1982; 587-591. [Cited in This Article: ] |
| 30. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [Cited in This Article: ] |
| 31. | Gabata T, Matsui O, Kadoya M, Yoshikawa J, Mitchell DG, Ueda K, Kawamori Y, Takashima T. Giant hyperplasia of the caudate lobe of the cirrhotic liver: correlation with an anomaly of the caudate portal branch. Abdom Imaging. 1999;24:153-156. [Cited in This Article: ] |
| 32. | Shneider BL, Groszmann RJ. Portal hypertension. Liver disease in children. St. Louis: Mosby-Yearbook 1994; 249-253. [Cited in This Article: ] |
| 33. | Schneider AW, Kalk JF, Klein CP. Hepatic arterial pulsatility index in cirrhosis: correlation with portal pressure. J Hepatol. 1999;30:876-881. [Cited in This Article: ] |
| 34. | Dinç H, Sari A, Resit Gümele H, Cihanyurdu N, Baki A. Portal and splanchnic haemodynamics in patients with advanced post-hepatitic cirrhosis and in healthy adults. Assessment with duplex Doppler ultrasound. Acta Radiol. 1998;39:152-156. [Cited in This Article: ] |
| 35. | Taourel P, Blanc P, Dauzat M, Chabre M, Pradel J, Gallix B, Larrey D, Bruel JM. Doppler study of mesenteric, hepatic, and portal circulation in alcoholic cirrhosis: relationship between quantitative Doppler measurements and the severity of portal hypertension and hepatic failure. Hepatology. 1998;28:932-936. [Cited in This Article: ] |
| 36. | Walsh KM, Leen E, MacSween RN, Morris AJ. Hepatic blood flow changes in chronic hepatitis C measured by duplex Doppler color sonography: relationship to histological features. Dig Dis Sci. 1998;43:2584-2590. [Cited in This Article: ] |
| 37. | Bolondi L, Li Bassi S, Gaiani S, Zironi G, Benzi G, Santi V, Barbara L. Liver cirrhosis: changes of Doppler waveform of hepatic veins. Radiology. 1991;178:513-516. [Cited in This Article: ] |
| 38. | Ohta M, Hashizume M, Tomikawa M, Ueno K, Tanoue K, Sugimachi K. Analysis of hepatic vein waveform by Doppler ultrasonography in 100 patients with portal hypertension. Am J Gastroenterol. 1994;89:170-175. [Cited in This Article: ] |
| 39. | Arda K, Ofelli M, Calikoglu U, Olçer T, Cumhur T. Hepatic vein Doppler waveform changes in early stage (Child-Pugh A) chronic parenchymal liver disease. J Clin Ultrasound. 1997;25:15-19. [Cited in This Article: ] |