Published online Dec 7, 2010. doi: 10.3748/wjg.v16.i45.5732
Revised: June 28, 2010
Accepted: July 5, 2010
Published online: December 7, 2010
AIM: To identify the role of anti-pancreatic antibody (PAB) in the diagnosis of inflammatory bowel diseases (IBD) among Turkish patients, and its frequency in first-degree relatives.
METHODS: PAB and anti-Saccharomyces cerevisiae (ASCA) were examined in serum samples of 214 subjects including patients with Crohn’s disease (CD, n = 64), ulcerative colitis (UC, n = 63), first-degree relatives of patients with CD (n = 25), first-degree relatives of patients with UC (n = 28),and a control group with gastrointestinal symptoms other than (IBD) (n = 34) by indirect immunofluorescence Positivity of PAB and ASCA was compared in terms of Vienna classification, disease activity and medications used.
RESULTS: In terms of PAB positivity, no difference was found between patients with CD (14.1%) and UC (7.9%) however, significant difference was observed between patients with CD and subjects in the control group (P < 0.05). No difference was found between patients with CD and their relatives in terms of ASCA positivity, whereas a significant difference was found between other groups (P < 0.001). Compared to ASCA, the sensitivity of the PAB was 19% (7/37), its specificity was 93% (25/27), positive predictive value was 77% (7/9) and negative predictive value was 45% (25/55). ASCA was found with significantly higher prevalence in patients with CD activity index > 150 (P < 0.05).
CONCLUSION: PAB is valuable in the diagnosis of IBD rather than CD, but cannot be used alone for diagnostic purposes. PAB is not superior to ASCA in CD diagnosis and in detecting CD among relatives of patients with CD.
- Citation: Demirsoy H, Ozdil K, Ersoy O, Kesici B, Karaca C, Alkim C, Akbayir N, Erdem LK, Onuk MD, Beyzadeoglu HT. Anti-pancreatic antibody in Turkish patients with inflammatory bowel disease and first-degree relatives. World J Gastroenterol 2010; 16(45): 5732-5738
- URL: https://www.wjgnet.com/1007-9327/full/v16/i45/5732.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i45.5732
The incidence of Crohn’s disease (CD) and ulcerative colitis (UC) is gradually increasing. Despite clinical, endoscopic, radiological andhistopathological findings, about 10% of patients with CD and UC are misclassified[1]. Moreover, 10% of the cases are not classified and referred to as indeterminate colitis. New treatment options for inflammatory bowel disease (IBD) are available today. Medical treatment and surgical operations to be implemented vary depending on the type of the disease.
Several serological indicators can be used for differential diagnosis[2]. The most commonly used two indicators are anti-Saccharomyces cerevisiae (ASCA) and perinuclear anti-neutrophil cytoplasmic antibodies (pANCA)[3]. Anti-pancreatic antibody (PAB) is another indicator that is currently under investigation in this regard. Serological indicators are not sensitive enough in IBD screening. Therefore, depending on merely serological indicators, diagnosis and treatmentof IBD are not possible. Several studies that areexamining the serological indicators in diagnosis and treatment are underway.
The familial occurrence of IBD is well known. Around 5.5%-22.5% of patients with IBD have another family member also affected with the disease[1-3]. In fact, the most important risk factor for IBD is having a family member with the disease. The relative risk for a sibling of a CD patient to also become affected is 13-36, and for a sibling of a UC patient this risk is 7-17[4], therefore first-degree relatives with no complaints are at risk of developing the disease. Early diagnosis is considered to decrease the disease complications, as well as possible surgical treatment in the long term. Although the search for predictive markers that could identify family members at risk for IBD has been intensive, no such markers have been identified to date. Studies with auto antibodies are being carried out, as these are non-invasive assays. Positive serum antibody findings in first-degree relatives of patients with IBD will, in most cases, indicate potential disease.
To the best of our knowledge, there are no studies that are investigating PAB in CD in Turkey. In our study, PAB was compared with ASCA, which is the most commonly used serological indicator in CD. The five groups included in the study were: patients with CD, patients with UC, first-degree relatives of patients with CD, first-degree relatives of patients with UC, and control subjects. The study aimed to: identify whether PAB plays a role in the differential diagnosis of CD; compare PAB with ASCA; determine the frequency of PAB in first-degree relatives of CD patients who carry potential risk for the disease; and determine whether PAB can contribute to early diagnosis in first-degree relatives.
Among outpatients and inpatients who presented to Sisli Etfal Education and Research Hospital Department of Gastroenterology; we enrolled 64 patients with CD, 63 with UC, 25 first-degree relatives of patients with CD, 28 first-degree relatives of patients with UC, and 34 control patients with gastrointestinal symptoms other than IBD. Diagnosis of CD and UC was established by means of clinical, endoscopic and histopathological examinations. Exclusion criteria were; infective enterocolitis (excluded via feces microscopy, culture, serological examination for bacterial and ameba infection, staining of the biopsy with acid-resistant dye and bacterial culture), Behcet’s disease, microscopic colitis and indeterminate colitis. Patients with a diagnosis of IBD for > 6 mo were enrolled. Enrolled patients were categorized according to age, sex, disease type, disease activation, clinical picture and involvement site of the disease.
Patients with UC were classified with proctitis, distal involved colitis, left colon involved colitis, diffuse colitis, and pancolitis. First-degree relatives involved either the siblings or the parents of the patients with IBD. First-degree relatives who had suspected complaints and histories of IBD were excluded. The control group consisted of patients from outpatient clinics who presented with gastrointestinal symptoms other than IBD, and who had no first-degree relative with IBD. Table 1 summarizes the demographical characteristics, disease duration, disease type and sites involvement, and medication being taken for all the enrolled groups.
| CD (n = 64) | CD relatives (n = 25) | UC (n = 63) | UC relatives (n = 28) | Control (n = 34) | |
| Sex | |||||
| Male | 28 (43.8) | 9 (36.0) | 32 (50.8) | 11 (39.3) | 15 (44.1) |
| Female | 36 (56.2) | 16 (64.0) | 31 (49.2) | 17 (60.7) | 19 (55.9) |
| Age (yr, mean ± SD) | 37.93 ± 14.01 | 32.12 ± 14.31 | 38.74 ± 13.13 | 35.35 ± 17.07 | 38.58 ± 15.09 |
| Disease duration (yr, mean ± SD) | 4.37 ± 3.61 | 4.03 ± 3.66 | |||
| Disease location (CD) | |||||
| Colon | 2 (3.1) | ||||
| Ileum | 26 (40.6) | ||||
| Ileocolonic | 36 (56.3) | ||||
| Disease location (UC) | |||||
| Proctitis | 9 (14.3) | ||||
| Distal involvement | 24 (38.1) | ||||
| Left colon invol | 9 (14.3) | ||||
| Diffuse | 2 (3.2) | ||||
| Pancolitis | 19 (30.2) | ||||
| Disease type (CD) | |||||
| Stricturing | 7 (10.9) | ||||
| Penetrating | 6 (9.4) | ||||
| Inflammatory | 51 (79.7) | ||||
| Medications (for CD and UC) | |||||
| No medication | 5 (7.8) | 5 (7.9) | |||
| 5-ASA | 57 (89.06) | 57 (90.4) | |||
| Steroids | 5 (7.8) | 5 (7.9) | |||
| AZA | 13 (20.3) | 8 (12.6) | |||
| Antibiotics | 1 (1.6) | 3 (4.7) |
For the activity of the diseases, CD activity index (CDAI) for CD and Trulove and Witts clinical activity index for UC were used. Medication was classified into seven groups:no medication; 5-aminosalicylic acid (ASA); azathioprine (AZA); 5-ASA + corticosteroid (CS); 5-ASA + AZA; 5-ASA + antibiotics and 5-ASA + CS + AZA.
All tests were conducted by product specialists from Euroimmun AG, Turkey, who were trained at Euroimmun Laboratories in Germany, and were confirmed by a second specialist. Both specialists were blinded to the diagnoses. After being collected from patients, relatives and control group, venous blood samples were centrifuged within 3 h, and serum was separated and were maintained at 80°C until the time of testing. Serum samples to be studied were transported to laboratories in ice boxes.
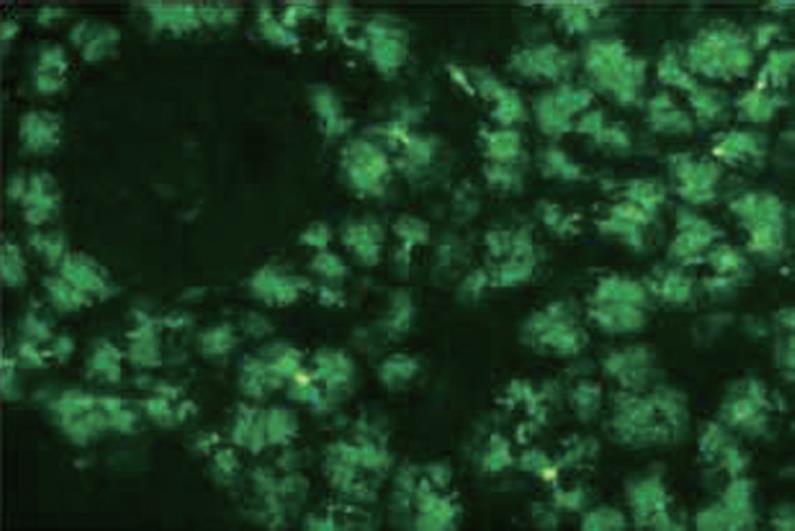
The presence of PAB was determined by indirect immunofluorescence staining in primate pancreas tissue (Figure 1). Kits prepared by Euroimmun AG were used. Substrates that were developed from pancreatic tissue from primates were divided into thin sections (biochips), transferred to slides, and utilized in the kit. The materials in the kits were kept under suitable conditions (4-8°C) until use For dilution and washing steps, we used a solution that was prepared from 10.2 g phosphate buffer, pH 7.2 and 2 mL Tween 20 (organic detergent). Antibodies tend to precipitate after the serum is dissolved, therefore serum samples of each patient were subjected to a string step (vortexing) to ensure homogeneous dispersion in the serum. Dilutions of 1/10 with PBS were prepared for each patient. Prepared dilutions were incubated at room temperature (18-25°C) for 30 min, followed by 5 min washing with PBS. Later, incubation with fluorescence-marked anti-human globulin IgA and IgG conjugates was performed for each patient. After being washed with PBS for 5 min, the slides were prepared for examinations by specialists by adding glycerol, pH 8.4, included in the kit and closing with lamella.
Kits prepared by Euroimmun AG that contained antibodies against S. cerevisiae were used. Storage conditions of this kit were the same as for the PAB kit. The same solutions were used for dilutions and washing steps. However, we used 1/100 dilutions for ASCA rather than 1/10 as in the previous preparation. Dilutions were incubated at room temperature (18-25°C) for 30 min, followed by 5 min washing with PBS. Later, incubation with fluorescence-marked anti-human globulin IgA and IgG conjugates was performed for each patient. After washing with PBS for 5 min, the slides were prepared for examination by specialists by adding glycerol, pH 8.4, included in the kit, and closing with lamella.
Data were evaluated using (SPSS) for Windows, version 9.05 statistical software. Research findings were converted into numeric and percentage distributions and Fischer’s exact χ2 test (used when the number of the object was < 20, or between 20 and 40 but if the least expectant value was < 5) and Pearson’s χ2 test were used to determine the level of significance of correlations between dependent (PAB and ASCA), independent (disease duration, disease onset age, disease type, site of involvement, medication) variables. P < 0.05 was considered statistically significant.
Demographic features of the involved patients are summarized in Table 1.
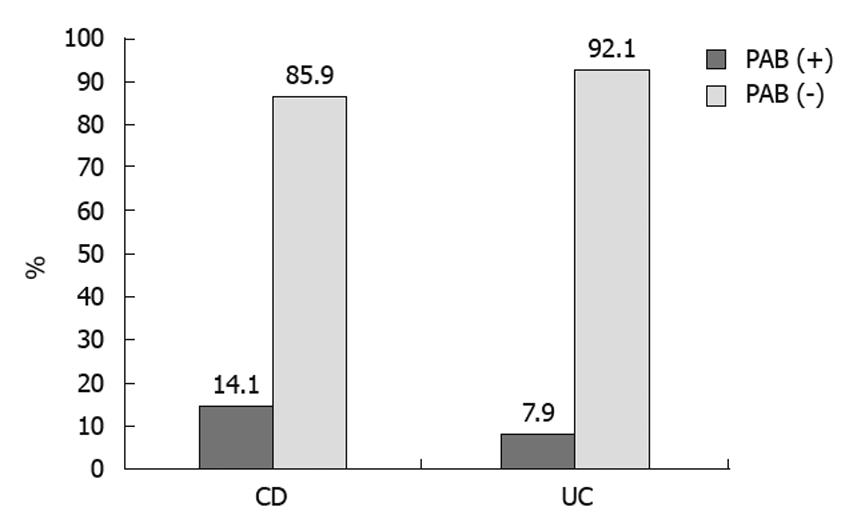
PAB was found to be positive in nine patients with CD (9/64; 14.06%) and five with UC (5/63; 7.93%) (Table 2, Figure 2), but the difference was not statistically significant.
| CD (n = 64) | UC (n = 63) | CD relatives (n = 25) | UC relatives (n = 28) | Control (n = 34) | |
| PAB | 9 (14.1) | 5 (7.9) | 0 (0) | 0 (0) | 0 (0) |
| ASCA | 37 (57.8) | 13 (20.6) | 9 (36) | 5 (17.9) | 6 (17.6) |
ASCA was found to be positive in 57.80% (37/64) of patients with CD, and 20.60% (13/63) of patients with UC, 36% (9/25) of CD relatives, 17.90% (5/28) of UC relatives, and 17.60% (6/34) of the control subjects. A statistically significant difference was found between the patients with CD and UC and between the relatives of patients with UC and the control group (P < 0.001 for both), but there was no significant difference between the patients with CD and their relatives.
Sensitivity and specificity of PAB was 19% (7/37) and 93% (25/27), respectively. Positive predictive value was 77% (7/9) and negative predictive value was 45% (25/55). The likelihood ratio was 2.7 (0.19/10.93) and coherence was 0.50 (7+25/64).
Although frequencies of PAB and ASCA were found to be higher among the patients aged < 0 years than in the older group (Tables 3 and 4), the differences were not statistically significant for PAB or ASCA. The positivity ratios of ASCA and PAB were not significantly different between the patients with UC and CD.
| A1 (n = 43, PAB = 7, 16.27%) | A2 (n = 21, PAB = 2, 9.52%) | |||||||
| B1 (n = 31, PAB = 5, 16.12%) | B2 (n = 6, PAB = 1, 16.66%) | B3 (n = 6, PAB = 1, 16.66%) | B1 (n = 20, PAB = 2, 10%) | B2 (n = 1, PAB = 0, 0%) | B3 (n = 0, PAB = 0, 0%) | |||
| L1 (n = 26, 3.84%) | A1 (n = 18, PAB = 1, 5.55%) | n = 15, PAB = 1, 6.66% | n = 0, PAB = 0, 0% | n = 3, PAB = 0, 0% | A2 (n = 8, PAB = 0, 0%) | n = 7, PAB = 0, 0% | n = 1, PAB = 0, 0% | n = 0, PAB = 0, 0% |
| L2 (n = 2, 0%) | A1 (n = 1, PAB = 0, 0%) | n = 1, PAB = 0, 0% | n = 0, PAB = 0, 0% | n = 0, PAB = 0, 0% | A2 (n = 1, PAB = 0, 0%) | n = 1, PAB = 0, 0% | n = 0, PAB = 0, 0% | n = 0, PAB = 0, 0% |
| L3 (n = 36, 22.22%) | A1 (n = 24, PAB = 6, 25%) | n = 15, PAB = 4, 25% | n = 6, PAB = 1, 16.66% | n = 3, PAB = 1, 33.33% | A2 (n = 12, PAB = 2, 16.66%) | n = 12, PAB = 2, 16.66% | n = 0, PAB = 0, 0% | n = 0, PAB = 0, 0% |
| A1 (n = 43, ASCA = 27, 62.79%) | A2 (n = 21, ASCA = 10, 47.61%) | |||||||
| B1 (n = 31, ASCA = 18, 58.06%) | B2 (n = 6, ASCA = 3, 50%) | B3 (n = 6, ASCA = 6, 100%) | B1 (n = 20, ASCA = 9, 45%) | B2 (n = 1, ASCA = 1, 100%) | B3 (n = 0, ASCA = 0, 0%) | |||
| L1 (18/26, 69.23%) | A1 (n = 18, ASCA = 13, 72.22%) | n = 15, ASCA = 10, 66.66% | n = 0, ASCA = 0, 0% | n = 3, ASCA = 3, 100% | A2 (n = 8, ASCA = 5, 62.5%) | n = 7, ASCA = 4, 57.14% | n = 1, ASCA = 1, 100% | n = 0, ASCA = 0, 0% |
| L2 (0/2, 0%) | A1 (n = 1, ASCA = 0, 0%) | n = 1, ASCA = 0, 0% | n = 0, ASCA = 0, 0% | n = 0, ASCA = 0, 0% | A2 (n = 1, ASCA = 0, 0%) | n = 1, ASCA = 0, 0% | n = 0, ASCA = 0, 0% | n = 0, ASCA = 0, 0% |
| L3 (19/36, 52.77%) | A1 (n = 24, ASCA = 14, 58.33%) | n = 15, ASCA = 8, 53.33% | n = 6, ASCA = 3, 50% | n = 3, ASCA = 3, 100% | A2 (n = 12, ASCA = 5, 41.66%) | n = 12, ASCA = 5, 41.66% | n = 0, ASCA = 0, 0% | n = 0, ASCA = 0, 0% |
When CD activity was classified as mild and severe, no difference was found in PAB positivity (Table 5). The same assessment performed for ASCA yielded no statistically significant difference.
| Disease activity according to CDAI | PAB | ASCA | Total | ||
| Negative | Positive | Negative | Positive | ||
| Mild (< 150 points) | 49 (84.5) | 9 (15.5) | 27 (46.6) | 31 (53.4) | 58 (100) |
| Moderate (150-450 points) | 5 (100) | 0 (0) | 0 (0) | 5 (100) | 5 (100) |
| Severe (> 450 points) | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 1 (100) |
| Total | 55 | 9 | 27 | 37 | 64 |
CD patients were grouped according to medication and classified according to PAB and ASCA positivity. No significant difference was found in patients using corticosteroids or immunosuppressive drugs in terms of PAB and ASCA levels.
Tissue damage in IBD is caused by multiple mechanisms that are mediated by the immune system. Although several studies have supported the argument that autoimmune mechanisms are the primary responsible mechanism, the consensus is that autoimmune stimulation does not have direct significance. Therefore, IBD is not actually an autoimmune disease. However, because the pathogenesis is mediated by the immune system, determination of autoantibodies and other immunological indicators is of clinical importance and warrants further research[5].
PAB frequency in patients with CD was found as 14.06% (9/64) and as 7.93% (5/63) for UC patients in the present study. No PAB positivity was noted in the relatives of both CD and UC patients. Although PAB was more positive in CD patients, the difference was not statistically significant. Related studies in the literature have reported PAB frequencies of 15%-40% in CD, 1%-4% in UC, and 1%-4% in control subjects[6-15]. The results of the present study can be considered consistent with those of Greek and Belgian studies that have demonstrated that PAB is present not only in patients with CD, but also in those with UC. Frequency of antibody in patients with UC was reported to be 23.3% in a Belgian study and 24.7% in a Greek study[11-14]. As PAB positivity is also seen in patients with UC, and because the frequency in patients with UC is not significantly different from that in CD patients, PAB seems to be a more suitable indicator for IBD rather than CD. In the light of these findings, PAB cannot be regarded as a screening test for IBD. Nishimori et al [13], Desplat-Jégo et al [15] and Koutroubakis et al [11] have arrived at the same conclusion.
Several serological indicators that are useful in differential diagnosis of UC and CD have been studied recently, and utilization of multiple indicators has been shown to improve differential diagnosis in cases of indeterminate colitis. Some of these indicators are pANCA, ASCA, PAB, OmpC antibody and I-2 and anaerobic coccoid rods antibodies[8-19]. This is why ASCA, together with PAB, was examined in the present study. As shown in Table 2, ASCA positivity was 57.8% (37/64) in CD patients, 20.6% (13/63) in UC patients, 36% (9/25) in the first-degree relatives of patients with CD, 17.9% (5/28) in the first-degree relatives of patients with UC, and 17.6% (6/34) in the control group. These values are much higher than the PAB positivity rates. In terms of these positivity rates, a statistically significant difference was identified between CD and UC, and relatives of UC patients and control subjects (P < 0.001); however, no significant difference was noted between CD patients and their relatives between UC patients and their relatives,or between UC patients and the control group. PAB seems to be a less desirable serological indicator compared to pANCA and ASCA in patients with IBD, as our findings indicate a lower prevalence. However, it can improve diagnosis and the predictive value when used in combination with common indicators.
ASCA, which is found with higher rates of positivity in patients with CD, is reported to be positive also in spondyloarthropathy associated with HLA-B27, which is its disadvantage[8]. In contrast, studies of PAB, have emphasized that it has a high specificity for CD and is positive only in patients with CD[8,17,18].
In a study by Stöcker et al[9], higher rates of PAB positivity were noted in patients with disease duration > 2.5 years compared to those with duration < 2.5 years. Similarly, Klebl et al[16] have also identified a significant relationship between PAB positivity and disease duration (P = 0.04). Koutroubakis et al[11] have compared patients with disease duration less and more than 2 years, and have noted a tendency towards increased PAB positivity in the latter; however, the result was not statistically significant. When PAB frequency was assessed in terms of disease duration in our study, positive findings were identified for 14.3% (5/35) of the patients with disease duration < 3 years compared to 13.8% (4/29) in those with disease duration > 3 years. The results are very close to each other and no difference was detected with the statistical methods used in this study (Fisher’s exact χ2 test).
Another research topic in studies with PAB is the relationship of PAB with disease activity. Most of the earlier studies[7,11,19,20] have failed to identify a correlation between PAB positivity and disease activity, and a parallel was found only by Goischke et al [12]. There were no significant relationships between disease activity and PAB positivity in our study (Fisher’s exact χ2 test).
According to the disease type of CD, PAB was positive in 13.72% of patients with inflammatory type, in 14.28% of patients with stricturing type, and in 16.66% of patients with fistulizing type disease. The differences between the groups mentioned above were not found to be statistically significant, although a higher frequency was noted for the fistulizing type. Antibody frequency has been shown to be higher in fistulizing and stricturing type diseases[11,16]. Klebl et al[16] have found that PAB frequency was 31.5% in stricturing and non-penetrating type, 41.7% in stricturing type, and 31.5% in penetrating type disease. Koutroubakis et al[11], on the other hand, have reported the frequency as 60% in stenotic type, 28.6% in inflammatory type, and 41.2% in fistulizing type disease, with a statistically significant result for stenotic type compared to the other types.
The most problematic cases in differential diagnosis are CD patients with isolated colon involvement, as the sites of involvement are the same as with UC. Sites of involvement in CD patients in this study were ileum for 26, ileocolon for 36 and colon for two patients. There were very few subjects with colon involvement, therefore, it was not possible to perform a comparison with subjects with ileum involvement. PAB positivity of patients with ileocolon involvement was higher than for other groups, but without statistical significance. There were also no significant differences in ASCA results according to site of involvement. These results were in accordance with several other relevant studies. In their studies from 1991 and 1996, Seibold et al did not find a relationship between PAB and site of bowel involvement[7-21]. Klebl et al[16] noted no relationship between PAB positivity and L category by the Vienna classification system. Koutroubakis et al[11] did not detect a significant relationship between disease localization and PAB positivity, but found less frequent PAB positivity in CD with colon involvement as compared to ileum or ileocolon involvement (P = 0.1). Similarly, no relation between PAB and disease localization was identified by Lawrance et al[22].
Another issue investigated in PAB studies is the relationship between PAB and concomitant drugs. Despite the studies by Stöcker et al[9] and Folwaczny et al [10] that have reported a high antibody frequency in patients not using glucocorticosteroids, Seibold et al [7,21], Goischke et al [12] and Klebl et al [16] did not detect any relationship between PAB frequency and drug use, including glucocorticosteroids. In our study, PAB positivity rates showed no difference between CD patients who were and were not taking glucocorticosteroids. Similarly, no statistically significant difference was noted in patients who were and were not using AZA.
Of the patients with IBD in the present study, 5%-10% had a positive familial history. Farmer et al[23] observed that one third of IBD patients had family histories positive for IBD. It is known that the incidence of CD is 14-times higher in complaint-free first-degree relatives of CD patients compared to the general population, and that these individuals are at risk of developing the disease[24] whereas Monsén et al[25] found that the prevalence of CD among first degree relatives 21 times higher than among non-relatives. Some studies have indicated that PAB positivity has a low frequency in the first-degree relatives of patients with CD. Seibold et al [21] have investigated 606 patients and arrived at the conclusion that PAB was a specific indicator for CD, and that PAB was rarely positive in family members of CD patients (2.5%). They concluded that most of the PAB-positive family members were CD patients[5,19]. Folwaczny et al[10] have calculated PAB positivity in first-degree relatives of CD patients as 4%. The same study has reported that there were no significant differences between the incidence of PAB-positivity in the relatives of CD and patients and healthy controls[6]. On the other hand, the findings of Joossens et al [14] are inconsistent with those reported in the above studies; they have found PAB prevalence of 32% in CD, 23.3% in UC and 22.2% in family members of patients with IBD. One of the purposes of the present study was to investigate PAB and ASCA positivity in first-degree relatives of patients with IBD, and to determine whether these indicators would contribute to early detection of the disease in these risk groups. However, PAB was detected in none of the first-degree relatives of IBD patients, nor in any subjects in the control group. ASCA results for the relatives of patients with CD, relatives of UC patients and the control group did not differ significantly, either. Shanahan et al[19] had summarized that autoantibodies were unlikely to have a direct, primary pathogenic role in CD and viewed that autoantibodies as important bridge between clinical and basic science. Müller-Ladner et al[20] had also found a link between pancreatic antibodies and Crohn’s disease. Therefore, we believe it would not be appropriate to use PAB or ASCA alone for determining the potential of CD patients’ relatives to develop CD.
In conclusion, in our study based on the absence of a significant difference between positivity in CD and UC, PAB has a diagnostic value for detecting patients with IBD rather than CD, and it should not be used alone for diagnosis. Also, we can say that investigating PAB and ASCA in first-degree relatives of IBD patients does not offer much benefit in early detection of the disease, and there is no superiority of PAB over ASCA in clinical practice in terms of CD diagnosis and early detection of the disease in patients’relatives. Further studies with novel indicators are still needed in the diagnosis of CD, and early diagnosis in relatives of CD patients.
Several antibodies have been associated with inflammatory bowel disease (IBD), with one of the most comprehensively studied being antibodies against anti-Saccharomyces cerevisiae (ASCA). Pancreatic antibodies (PABs) are also newly studied antibodies that are specific for Crohn’s disease (CD) and ulcerative colitis (UC), but their sensitivity alone is low.
PAB in combination with ASCA might increase the sensitivity for detecting CD, especially isolated colonic CD. This study focused on the value of PAB alone or in combination with ASCA for diagnosing IBD, and differentiating CD from UC.
Several serological indicators can be used for differential diagnosis of IBD. PAB is another indicator that is currently under investigation in this regard. Serological indicators are not sensitive enough for IBD screening. Therefore, depending on serological indicators alone, diagnosis and treatment of IBD will not be possible. Several studies that are examining serological indicators for diagnosis and treatment are underway. There are no studies that are investigating PAB in CD in Turkey. The present study aimed to: identify whether PAB plays a role in the diagnosis and differential diagnosis of CD; compare it with ASCA; determine its frequency in first-degree relatives of CD patients who carry a potential risk for the disease; and determine whether it can contribute to early diagnosis.
This study suggest that PAB is a novel indicator that is still needed in the diagnosis of CD, and early diagnosis in relatives of CD patients.
This study aimed to identify the role of PAB in the diagnosis of CD, and determine its frequency in first-degree relatives. The main focus of this work was to measure the frequency of PAB and ASCA in Turkish IBD patients.
Peer reviewers: Dr. Marco Scarpa, PhD, Department of Surgical and Gastroenterological Sciences (Gastroenterology section), University of Padova, Via Giustiniani 2, Padova 35128, Italy; José Manuel Martin-Villa, Professor, PhD, Department of Inmunología, Facultad de Medicina, Universidad Complutense de Madrid, Pabellón V. Planta 4ª, Madrid 28040, Spai
S- Editor Wang JL L- Editor Kerr C E- Editor Lin YP
| 1. | Silverberg MS, Daly MJ, Moskovitz DN, Rioux JD, McLeod RS, Cohen Z, Greenberg GR, Hudson TJ, Siminovitch KA, Steinhart AH. Diagnostic misclassification reduces the ability to detect linkage in inflammatory bowel disease genetic studies. Gut. 2001;49:773-776. [Cited in This Article: ] |
| 2. | Sandborn WJ. Serologic markers in inflammatory bowel disease: state of the art. Rev Gastroenterol Disord. 2004;4:167-174. [Cited in This Article: ] |
| 3. | Lakatos PL, Altorjay I, Szamosi T, Palatka K, Vitalis Z, Tumpek J, Sipka S, Udvardy M, Dinya T, Lakatos L. Pancreatic autoantibodies are associated with reactivity to microbial antibodies, penetrating disease behavior, perianal disease, and extraintestinal manifestations, but not with NOD2/CARD15 or TLR4 genotype in a Hungarian IBD cohort. Inflamm Bowel Dis. 2009;15:365-374. [Cited in This Article: ] |
| 4. | Vermeire S, Van Assche G, Rutgeerts P. Should family members of IBD patients be screened for CARD15/NOD2 mutations? Inflamm Bowel Dis. 2008;14 Suppl 2:S190-S191. [Cited in This Article: ] |
| 5. | Seibold F, Mörk H, Tanza S, Müller A, Holzhüter C, Weber P, Scheurlen M. Pancreatic autoantibodies in Crohn's disease: a family study. Gut. 1997;40:481-484. [Cited in This Article: ] |
| 6. | Targan SR, Landers CJ, Cobb L, MacDermott RP, Vidrich A. Perinuclear anti-neutrophil cytoplasmic antibodies are spontaneously produced by mucosal B cells of ulcerative colitis patients. J Immunol. 1995;155:3262-3267. [Cited in This Article: ] |
| 7. | Seibold F, Weber P, Jenss H, Wiedmann KH. Antibodies to a trypsin sensitive pancreatic antigen in chronic inflammatory bowel disease: specific markers for a subgroup of patients with Crohn's disease. Gut. 1991;32:1192-1197. [Cited in This Article: ] |
| 8. | Shanahan F. Neutrophil autoantibodies in inflammatory bowel disease: are they important? Gastroenterology. 1994;107:586-589. [Cited in This Article: ] |
| 9. | Stöcker W, Otte M, Ulrich S, Normann D, Stöcker K, Jantschek G. [Autoantibodies against the exocrine pancreas and against intestinal goblet cells in the diagnosis of Crohn's disease and ulcerative colitis]. Dtsch Med Wochenschr. 1984;109:1963-1969. [Cited in This Article: ] |
| 10. | Folwaczny C, Noehl N, Endres SP, Loeschke K, Fricke H. Antineutrophil and pancreatic autoantibodies in first-degree relatives of patients with inflammatory bowel disease. Scand J Gastroenterol. 1998;33:523-528. [Cited in This Article: ] |
| 11. | Koutroubakis IE, Drygiannakis D, Karmiris K, Drygiannakis I, Makreas S, Kouroumalis EA. Pancreatic autoantibodies in Greek patients with inflammatory bowel disease. Dig Dis Sci. 2005;50:2330-2334. [Cited in This Article: ] |
| 12. | Goischke EM, Zilly W. [Clinical importance of organ-specific antibodies in ulcerative colitis and Crohn disease]. Z Gastroenterol. 1992;30:319-324. [Cited in This Article: ] |
| 13. | Nishimori I, Yamamoto Y, Okazaki K, Morita M, Onodera M, Kino J, Tamura S, Yamamoto Y. Identification of autoantibodies to a pancreatic antigen in patients with idiopathic chronic pancreatitis and Sjögren's syndrome. Pancreas. 1994;9:374-381. [Cited in This Article: ] |
| 14. | Joossens S, Vermeire S, Van Steen K, Godefridis G, Claessens G, Pierik M, Vlietinck R, Aerts R, Rutgeerts P, Bossuyt X. Pancreatic autoantibodies in inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:771-777. [Cited in This Article: ] |
| 15. | Desplat-Jégo S, Johanet C, Escande A, Goetz J, Fabien N, Olsson N, Ballot E, Sarles J, Baudon JJ, Grimaud JC. Update on Anti-Saccharomyces cerevisiae antibodies, anti-nuclear associated anti-neutrophil antibodies and antibodies to exocrine pancreas detected by indirect immunofluorescence as biomarkers in chronic inflammatory bowel diseases: results of a multicenter study. World J Gastroenterol. 2007;13:2312-2318. [Cited in This Article: ] |
| 16. | Klebl FH, Bataille F, Huy C, Hofstädter F, Schölmerich J, Rogler G. Association of antibodies to exocrine pancreas with subtypes of Crohn's disease. Eur J Gastroenterol Hepatol. 2005;17:73-77. [Cited in This Article: ] |
| 17. | Sandborn WJ, Loftus EV Jr, Colombel JF, Fleming KA, Seibold F, Homburger HA, Sendid B, Chapman RW, Tremaine WJ, Kaul DK. Evaluation of serologic disease markers in a population-based cohort of patients with ulcerative colitis and Crohn's disease. Inflamm Bowel Dis. 2001;7:192-201. [Cited in This Article: ] |
| 18. | Conrad K, Schmechta H, Klafki A, Lobeck G, Uhlig HH, Gerdi S, Henker J. Serological differentiation of inflammatory bowel diseases. Eur J Gastroenterol Hepatol. 2002;14:129-135. [Cited in This Article: ] |
| 19. | Shanahan F. Antibody 'markers' in Crohn's disease: opportunity or overstatement? Gut. 1997;40:557-558. [Cited in This Article: ] |
| 20. | Müller-Ladner U, Schölmerich J. Pancreatic autoantibodies in Crohn's disease. Eur J Clin Invest. 1999;29:46-47. [Cited in This Article: ] |
| 21. | Seibold F, Scheurlen M, Müller A, Jenss H, Weber P. Impaired pancreatic function in patients with Crohn's disease with and without pancreatic autoantibodies. J Clin Gastroenterol. 1996;22:202-206. [Cited in This Article: ] |
| 22. | Lawrance IC, Hall A, Leong R, Pearce C, Murray K. A comparative study of goblet cell and pancreatic exocine autoantibodies combined with ASCA and pANCA in Chinese and Caucasian patients with IBD. Inflamm Bowel Dis. 2005;11:890-897. [Cited in This Article: ] |
| 23. | Farmer RG, Michener WM, Mortimer EA. Studies of family history among patients with inflammatory bowel disease. Clin Gastroenterol. 1980;9:271-277. [Cited in This Article: ] |
| 24. | Orholm M, Fonager K, Sørensen HT. Risk of ulcerative colitis and Crohn's disease among offspring of patients with chronic inflammatory bowel disease. Am J Gastroenterol. 1999;94:3236-3238. [Cited in This Article: ] |
| 25. | Monsén U, Bernell O, Johansson C, Hellers G. Prevalence of inflammatory bowel disease among relatives of patients with Crohn's disease. Scand J Gastroenterol. 1991;26:302-306. [Cited in This Article: ] |