Published online Nov 21, 2010. doi: 10.3748/wjg.v16.i43.5462
Revised: July 6, 2010
Accepted: July 13, 2010
Published online: November 21, 2010
AIM: To evaluate if traction-assisted endoscopic mucosal resection (TA-EMR) is feasible and if it enables en bloc resection of colorectal lesions.
METHODS: Seven patients with a total of 12 colorectal adenomas were prospectively enrolled. All lesions were removed by TA-EMR: one hemostatic clip tied to a white silk suture was applied to the base of the lesion to allow traction through the working channel of the colonoscope. A conventional polypectomy snare was mounted over the suture and the lesion was pulled into the snare and resected in one piece.
RESULTS: All 12 lesions (nine sessile) were resected en bloc with free lateral and vertical margins by using this novel technique, including five lesions (5/12, 41.6%) in less-accessible positions, where TA-EMR enabled complete visualization of the base before resection. Mean longest lesion and specimen sizes were 9 mm (range: 6-25 mm) and 11 mm in diameter (range: 7-17 mm), respectively. No serious procedure-related complications were observed.
CONCLUSION: TA-EMR through the endoscope using a hemostatic clip and suture material is technically feasible. Visualization of colorectal lesions in less-accessible locations can be improved.
- Citation: Dauser B, Winkler T, Salehi B, Riss S, Beer F, Herbst F. Traction-assisted endoscopic mucosal resection for polypectomy in the large intestine. World J Gastroenterol 2010; 16(43): 5462-5466
- URL: https://www.wjgnet.com/1007-9327/full/v16/i43/5462.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i43.5462
Endoscopic mucosal resection (EMR) for removal of neoplastic gastrointestinal lesions and early cancers has been established as a minimally invasive technique. Referring to the “adenoma-adenocarcinoma sequence”, benign adenomas are regarded as precursors of cancer and should be completely removed during endoscopic procedures[1].
Besides the conventional inject-lift-cut EMR, i.e. strip biopsy, several variations have been developed such as cap-assisted EMR or EMR using a ligating device to enable safe and complete resection with the polypectomy snare[2-5]. En bloc resection with different EMR techniques is limited to adenomas of 15-20 mm in diameter[6-8]. However, smaller lesions, and particularly those in less-accessible locations, are sometimes hard to be ensnared and are challenging to the endoscopist. To improve visualization and make en bloc resection easier by exerting traction on the lesions while performing EMR or endoscopic submucosal dissection (ESD), several techniques have been described, such as sinker-assisted ESD, percutaneous traction-assisted EMR, magnetic anchor devices, external grasping forceps, thin endoscope-assisted ESD and the S-O-clip device[9-15]. Jeon et al[16] recently have reported peroral traction-assisted ESD for treatment of gastric neoplasms. In this paper, we describe a novel technique for traction-assisted (TA)-EMR through the endoscope, and its early results in treating colorectal lesions.
Between July 2009 and March 2010, seven patients with a total of 12 colorectal lesions were enrolled. There were four female and three male patients, with a mean age of 75 years (range: 61-79 years). All patients had previous endoscopic investigation with histologically confirmed neoplastic adenomas. Each patient had at least one adenoma, which could not be removed by referring gastroenterologists because of its size or location, therefore, patients were sent to our department. We consecutively used the TA-EMR technique for all lesions found during colonoscopy that were > 5 mm in size. Study approval was given by the local ethics committee and written informed consent was obtained from all patients. The study was registered at ClinicalTrials.gov under NCT00966420.
Bowel preparation was done using 3 L polyethylene glycol solution. Colonoscopy was performed with a narrow band imaging video endoscope (CF-H180AI; Olympus, Hamburg Germany). All patients were initially sedated with midazolam (3-5 mg); propofol boluses of 20 mg were given intermittently to maintain sedation.
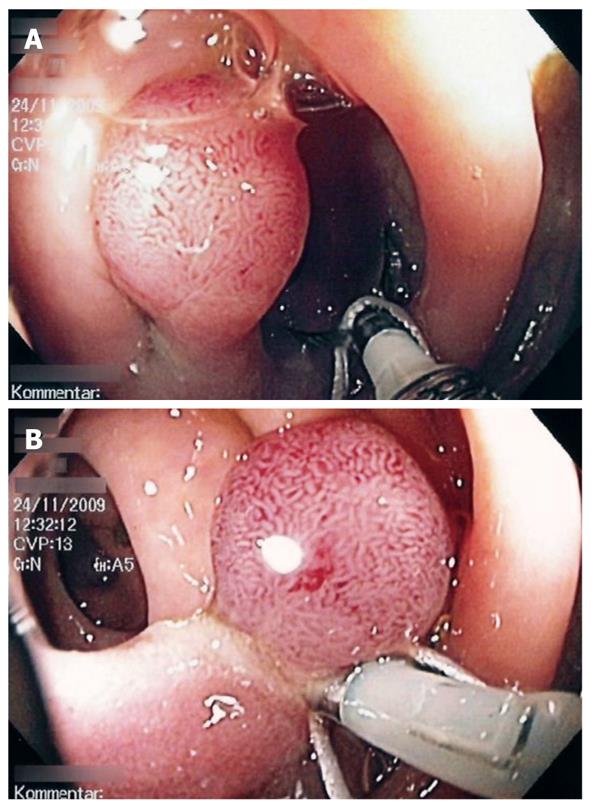
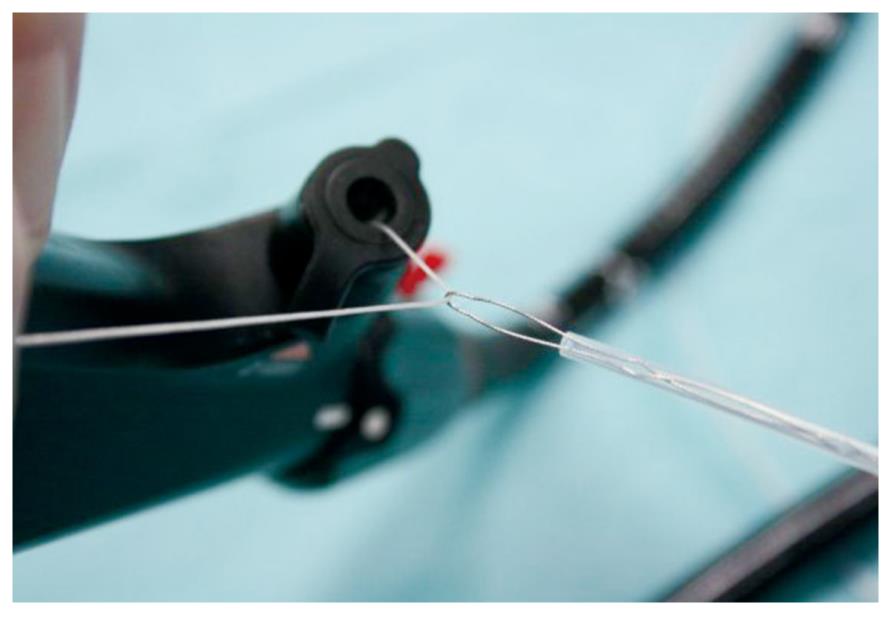
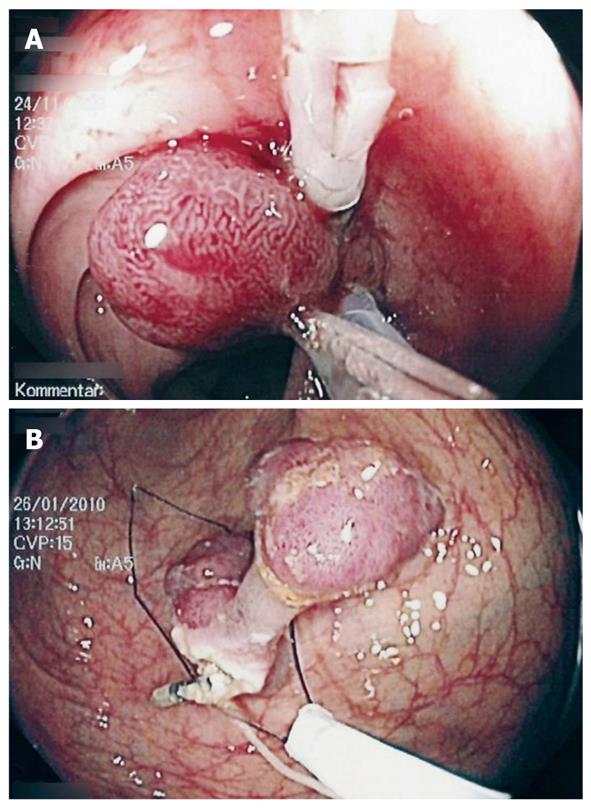
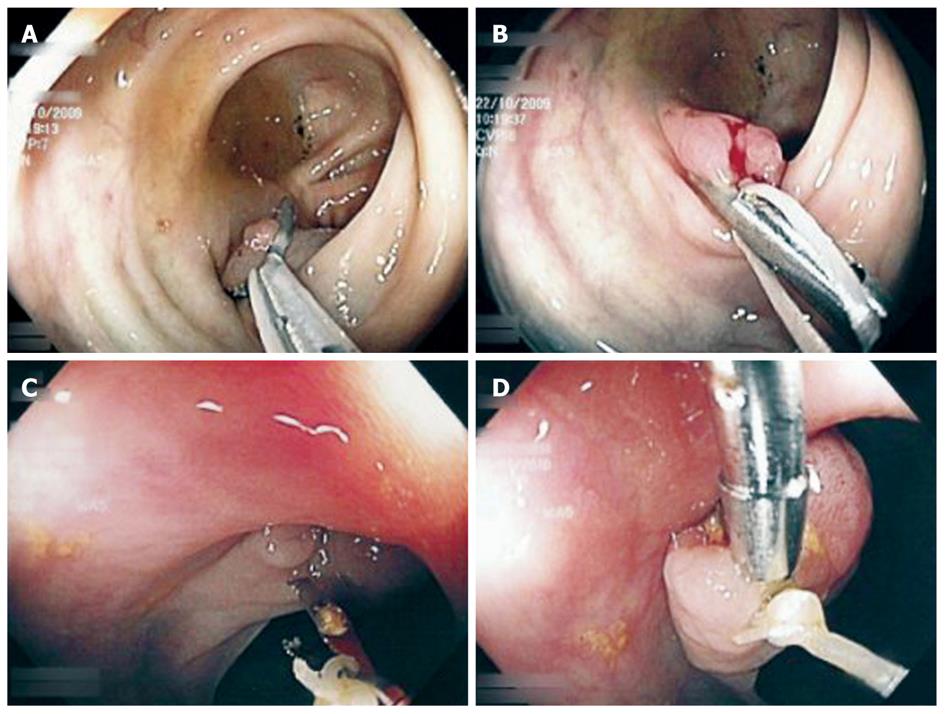
Using a white silk suture (Freka® PEG Gastric Set; Fresenius, Bad Homburg, Germany), a loop was tied and mounted between the branches of the hemoclip (Figure 1). Normal saline solution (2-10 mL, mean: 2 mL) was injected into the submucosal layer to lift the lesion from the muscular layer in the case of sessile adenomas. A reusable hemoclip-applicator device (HX-5LR-1, Olympus) or a Resolution® Clip (Boston Scientific, Ratingen, Germany) was inserted with the tied suture into the working channel, and the clip was anchored within healthy mucosal tissue at the base of the lesion (Figure 2A and B). After removal of the hemoclip-applicator device, an electrosurgical snare (AS-1-S, ASH-1-S; Cook Medical, Moenchengladbach, Germany) was mounted over the suture and brought into the working channel (Figure 3). At the tip of the video endoscope, the snare was delivered and placed over the lesion, which was pulled into the snare towards the endoscope while closing the snare (Figure 4A). Resection was performed using forced coagulating current, output power 60 W (ICC 200; ERBE, Tuebingen, Germany). Resected specimen secured to the suture material was easily removed, together with the endoscope (Figure 4B). The remaining mucosal defect was carefully inspected using narrow band imaging (NBI). Closure of the resection site was not performed routinely.
All specimens were embedded in paraffin wax. Hematoxylin-eosin-stained sections were evaluated by one pathologist (Beer F) who specializes in gastrointestinal pathology.
Details of the polypectomies are shown in Table 1. Altogether, we removed 12 lesions located in the upper rectum (n = 1), at the rectosigmoid junction (n = 1), in the left (n = 8) or right (n = 1) hemicolon, and at the hepatic flexure (n = 1). Seven small adenomas of 6-9 mm diameter (five sessile) and five large adenomas measuring 10-25 mm (four sessile) were included. On five occasions (5/12, 41.6%), the whole extent and the base of the lesion could not be seen due to the adverse position behind a mucosal fold or at an intestinal bend until traction was exerted. All lesions were resected en bloc without any evidence of residual neoplastic tissue using NBI. In addition, histological evaluation confirmed free lateral and circumferential margins (12/12). Advanced pathology, i.e. tubulo-villous adenoma or high-grade dysplasia was found in nine adenomas (9/12, 75%). No neoplastic but hyperplastic tissue was detected once. Mean longest lesion and specimen diameters were 9 mm (range: 6-25 mm) and 11 mm (range: 7-17 mm), respectively. The mean procedure time, starting with injection in the case of sessile adenomas until completion of resection, was 6 min (range: 5-12 min).
| Polyp characteristics | Polyp location | Polyp size | Specimen size (base) | Adverse position | Injection NaCl (mL) | Time (min) | Histology | Grade of dysplasia |
| Protruded | Descending colon | 9 | 11 | No | 0 | 7 | Tubulovillous adenoma, R0 | Intermediate |
| Sessile | Descending colon | 11 | 17 | No | 10 | 6 | Tubular adenoma, R0 | High |
| Sessile | Hepatic flexure | 10 | 15 | Yes | 2 | 6 | Tubulovillous adenoma, R0 | Low |
| Sessile | Sigmoid colon | 12 | 16 | Yes | 5 | 12 | Tubulovillous adenoma, R0 | Intermediate |
| Sessile | Sigmoid colon | 6 | 7 | No | 2 | 10 | Tubulovillous adenoma, R0 | Low |
| Protruded | Sigmoid colon | 8 | 9 | No | 0 | 6 | Tubulovillous adenoma, R0 | Low |
| Sessile | Rectum, upper third | 8 | 10 | No | 2 | 5 | Tubular adenoma, R0 | Low |
| Sessile | Ascending colon | 9 | 12 | No | 4 | 7 | Tubulovillous adenoma, R0 | Low |
| Sessile | Sigmoid colon | 6 | 7 | No | 2 | 6 | Tubular adenoma, R0 | Low |
| Protruded | Sigmoid colon | 25 | 11 | Yes | 0 | 6 | Tubulovillous adenoma, R0 | High |
| Sessile | Sigmoid colon | 10 | 15 | Yes | 2 | 8 | Tubulovillous adenoma, R0 | Low |
| Sessile | Recto-sigmoid junction | 7 | 9 | Yes | 2 | 6 | Hyperplastic adenoma, R0 | / |
No serious procedure-related complications were observed. In one patient (1/12, 8.3%), immediate bleeding after resection had to be controlled using hemostatic clips. All patients were discharged within 1 d after intervention. No delayed complications were observed within 4 wk, when patients were contacted for a final clinical examination.
For all neoplastic colorectal lesions, en bloc removal is desirable as it enables accurate evaluation of completeness of the resected specimen. Moreover, small adenomas might also carry an increased risk of dysplasia, advanced pathology or even carcinoma[17-20].
En bloc or “single piece” snare polypectomy is regarded as the procedure of choice for polyps larger than 5 mm and up to 15-20 mm. For larger lesions, piecemeal resection or ESD has to be carried out. Despite a smaller size, visualization of lesions in less-accessible locations, e.g. behind mucosal folds or at bends in the bowel, may be hampered. Therefore, exact polypectomy snare placement, particularly in sessile lesions, may be aggravated or even impossible. There are only a few studies that have addressed this problem.
Yoshikane et al[21] have described a technique that uses distal attachment to the endoscope to press the semilunar fold at the anal side of the lesion, to bring the base of the polyp within the visual field. Frimberger et al[22] have reported 15 patients treated with polypectomy for problematic polyps, by using a side-viewing duodenoscope as a therapeutic option when polyps were not accessible with the conventional approach. A “suction pseudopolyp technique” for small, flat, non-polypoid lesions of the colon and rectum was published by Pattullo et al[23] 2009. This referred to the fact that ensnaring of such lesions may be technically difficult because of minimal or no protruding tissue.
Achievement of good, direct visualization of the base of the lesion is of utmost importance when performing snare polypectomy, to decrease the risk of incomplete resection and complications, such as perforation. Recently, for ESD, different devices and traction systems have been invented to facilitate the resection procedure as mentioned above[10-15]. These various traction methods have their own unique limitations[16].
Our study aimed to evaluate the feasibility of TA-EMR through the endoscope to allow en bloc resection of colorectal lesions. Thereafter, we studied if visualization of colorectal adenomas in less-accessible positions can be improved. The technique described uses commonly available and economical materials and can be performed by a single endoscopist using a flexible endoscope with one working channel. Preparation of the hemoclip by mounting a tied loop of a white silk suture between the branches of the clip is easy. Introduction of the clip device and application of the clip to the base of the lesion is not complicated by the suture material.
By application of traction, the position of the polyps can be improved for ensnaring and especially the whole amount of lesions and the base can be better visualized (Figure 5). Thereafter, the TA-EMR technique can be used to generate a “pseudo-stalk”, and placement of the snare in an adequate distance to the neoplastic tissue is possible.
The materials used are not designed for such an application, therefore, there are some limitations. Assembly of the polypectomy snare over the suture material and bringing it through the working channel can be difficult because the suture has to be gently fixed by the endoscopist at the handle of the endoscope, and the movement of the snare is slightly hindered because of the higher friction due to the suture material in the working channel. Even if it did not happen during this study, there is the possibility that the snare becomes entangled with the clip, or the clip is pulled out of the lesion by excessive traction. We tested clip application ex vivo before starting the study. In the case of clip dislodgment, we suggest removal of the endoscope from the bowel; pulling the (potentially crosswise oriented) clip through the working channel may cause damage to the video endoscope. TA-EMR through the endoscope is technically challenging and positioning the snare over the lesion and the clip sometimes can be awkward. Fixation of the suture material to the end of the clip (instead of between the branches) could facilitate this procedure, but it is presently impossible with the available clip devices. A rigid system instead of the suture material would be helpful because it enables a broader range of movements instead of simple traction.
In conclusion, our preliminary limited experience with TA-EMR through the endoscope is promising. Complete en bloc resection of colorectal lesions is feasible with the TA-EMR technique, and visualization of adenomas in less-accessible positions can be improved.
Endoscopic mucosal resection (EMR) for removal of neoplastic colorectal lesions has become established as minimally invasive therapy. Visualization of lesions in less-accessible locations and complete en bloc resection, especially of flat adenomas, can be challenging or even impossible.
Several elaborated techniques have been described to overcome these technical problems, which necessitates special equipment.
Traction-assisted EMR (TA-EMR) uses commonly available and economic materials. Traction is exerted through the working channel of the endoscope, which protects the bowel against the suture material and allows controlled traction by the endoscopist.
Theoretically, TA-EMR can be performed in every endoscopy unit. Due to the fact that the materials used are not designed for such an application, the technique is challenging. A rigid system instead of the suture material would ease the procedure and allow a broader range of movements.
EMR enables removal of neoplastic lesions in one piece by ensnaring adenomas with a polypectomy snare. Lesions at bends in the bowel or behind mucosal folds potentially cannot be ensnared. To bring adenomas to a better position for EMR, traction can be exerted to allow complete visualization and en bloc resection.
There is an obvious interest in ways to remove difficult polyps, particularly those that lie behind colonic folds. A number of methods have been described to remove these polyps but, thus far, none has been entirely satisfactory. This report describes a relatively simple traction method.
Peer reviewer: Ian C Roberts-Thomson, Professor, Department of Gastroenterology and Hepatology, The Queen Elizabeth Hospital, 28 Woodville Road, Woodville South 5011, Australia
S- Editor Wang YR L- Editor Kerr C E- Editor Ma WH
| 1. | Morson BC. Evolution of cancer of the colon and rectum. Proc Inst Med Chic. 1974;30:145-148. [Cited in This Article: ] |
| 2. | Karita M, Tada M, Okita K, Kodama T. Endoscopic therapy for early colon cancer: the strip biopsy resection technique. Gastrointest Endosc. 1991;37:128-132. [Cited in This Article: ] |
| 3. | Kudo S. Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy. 1993;25:455-461. [Cited in This Article: ] |
| 4. | Inoue H, Kawano T, Tani M, Takeshita K, Iwai T. Endoscopic mucosal resection using a cap: techniques for use and preventing perforation. Can J Gastroenterol. 1999;13:477-480. [Cited in This Article: ] |
| 5. | Suzuki H. Endoscopic mucosal resection using ligating device for early gastric cancer. Gastrointest Endosc Clin N Am. 2001;11:511-518. [Cited in This Article: ] |
| 6. | Tanaka S, Haruma K, Oka S, Takahashi R, Kunihiro M, Kitadai Y, Yoshihara M, Shimamoto F, Chayama K. Clinicopathologic features and endoscopic treatment of superficially spreading colorectal neoplasms larger than 20 mm. Gastrointest Endosc. 2001;54:62-66. [Cited in This Article: ] |
| 7. | Saito Y, Fukuzawa M, Matsuda T, Fukunaga S, Sakamoto T, Uraoka T, Nakajima T, Ikehara H, Fu KI, Itoi T. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010;24:343-352. [Cited in This Article: ] |
| 8. | Soetikno R, Kaltenbach T, Yeh R, Gotoda T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol. 2005;23:4490-4498. [Cited in This Article: ] |
| 9. | Kato M. Endoscopic submucosal dissection (ESD) is being accepted as a new procedure of endoscopic treatment of early gastric cancer. Intern Med. 2005;44:85-86. [Cited in This Article: ] |
| 10. | Saito Y, Emura F, Matsuda T, Uraoka T, Nakajima T, Ikematsu H, Gotoda T, Saito D, Fujii T. A new sinker-assisted endoscopic submucosal dissection for colorectal cancer. Gastrointest Endosc. 2005;62:297-301. [Cited in This Article: ] |
| 11. | Kondo H, Gotoda T, Ono H, Oda I, Kozu T, Fujishiro M, Saito D, Yoshida S. Percutaneous traction-assisted EMR by using an insulation-tipped electrosurgical knife for early stage gastric cancer. Gastrointest Endosc. 2004;59:284-288. [Cited in This Article: ] |
| 12. | Gotoda T, Oda I, Tamakawa K, Ueda H, Kobayashi T, Kakizoe T. Prospective clinical trial of magnetic-anchor-guided endoscopic submucosal dissection for large early gastric cancer (with videos). Gastrointest Endosc. 2009;69:10-15. [Cited in This Article: ] |
| 13. | Imaeda H, Iwao Y, Ogata H, Ichikawa H, Mori M, Hosoe N, Masaoka T, Nakashita M, Suzuki H, Inoue N. A new technique for endoscopic submucosal dissection for early gastric cancer using an external grasping forceps. Endoscopy. 2006;38:1007-1010. [Cited in This Article: ] |
| 14. | Uraoka T, Kato J, Ishikawa S, Harada K, Kuriyama M, Takemoto K, Kawahara Y, Saito Y, Okada H. Thin endoscope-assisted endoscopic submucosal dissection for large colorectal tumors (with videos). Gastrointest Endosc. 2007;66:836-839. [Cited in This Article: ] |
| 15. | Sakamoto N, Osada T, Shibuya T, Beppu K, Matsumoto K, Mori H, Kawabe M, Nagahara A, Otaka M, Ogihara T. Endoscopic submucosal dissection of large colorectal tumors by using a novel spring-action S-O clip for traction (with video). Gastrointest Endosc. 2009;69:1370-1374. [Cited in This Article: ] |
| 16. | Jeon WJ, You IY, Chae HB, Park SM, Youn SJ. A new technique for gastric endoscopic submucosal dissection: peroral traction-assisted endoscopic submucosal dissection. Gastrointest Endosc. 2009;69:29-33. [Cited in This Article: ] |
| 17. | Opelka FG, Timmcke AE, Gathright JB Jr, Ray JE, Hicks TC. Diminutive colonic polyps: an indication for colonoscopy. Dis Colon Rectum. 1992;35:178-181. [Cited in This Article: ] |
| 18. | Karita M, Cantero D, Okita K. Endoscopic diagnosis and resection treatment for flat adenoma with severe dysplasia. Am J Gastroenterol. 1993;88:1421-1423. [Cited in This Article: ] |
| 19. | Lieberman D, Moravec M, Holub J, Michaels L, Eisen G. Polyp size and advanced histology in patients undergoing colonoscopy screening: implications for CT colonography. Gastroenterology. 2008;135:1100-1105. [Cited in This Article: ] |
| 20. | Lin WP, Su MY, Ho YP, Hsu CM, Lin CJ, Chiu CT, Chen PC. Treating colorectal polypoid neoplasms during a colonoscopy. Chang Gung Med J. 2005;28:801-807. [Cited in This Article: ] |
| 21. | Yoshikane H, Hidano H, Sakakibara A, Niwa Y, Goto H. Efficacy of a distal attachment in endoscopic resection of colorectal polyps situated behind semilunar folds. Endoscopy. 2001;33:440-442. [Cited in This Article: ] |