Published online Nov 14, 2010. doi: 10.3748/wjg.v16.i42.5272
Revised: June 2, 2010
Accepted: June 9, 2010
Published online: November 14, 2010
The intestinal mucosa is responsible for the absorption of nutrients from the lumen and for the separation of the potentially toxic luminal content (external environment) from the host (internal environment). Disruption of this delicate balance at the mucosal interface is the basis for numerous (intestinal) diseases. Experimental animal studies have shown that gut wall integrity loss is involved in the development of various inflammatory syndromes, including post-operative or post-traumatic systemic inflammatory response syndrome, sepsis, and multiple organ failure. Assessment of gut wall integrity in clinical practice is still a challenge, as it is difficult to evaluate the condition of the gut non-invasively with currently available diagnostic tools. Moreover, non-invasive, rapid diagnostic means to assess intestinal condition are needed to evaluate the effects of treatment of intestinal disorders. This review provides a survey of non-invasive tests and newly identified markers that can be used to assess gut wall integrity.
- Citation: Derikx JP, Luyer MD, Heineman E, Buurman WA. Non-invasive markers of gut wall integrity in health and disease. World J Gastroenterol 2010; 16(42): 5272-5279
- URL: https://www.wjgnet.com/1007-9327/full/v16/i42/5272.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i42.5272
The gut wall forms a physical/anatomical and immunological barrier. The physical/anatomical barrier of the gut is formed by a monolayer of epithelial cells, originating from multipotent stem cells present in the crypt. The epithelial cells, together with the lamina propria, form the mucosa of the intestine[1,2]. The epithelial stem cells differentiate into four major epithelial cells: (1) the absorptive enterocytes, which make up > 80% of all small intestinal epithelial cells; (2) the goblet cells, which produce a variety of mucins and trefoil peptides; (3) the enteroendocrine cells, which export peptide hormones; and (4) the Paneth cells, which secrete a wide variety of antimicrobial peptides.
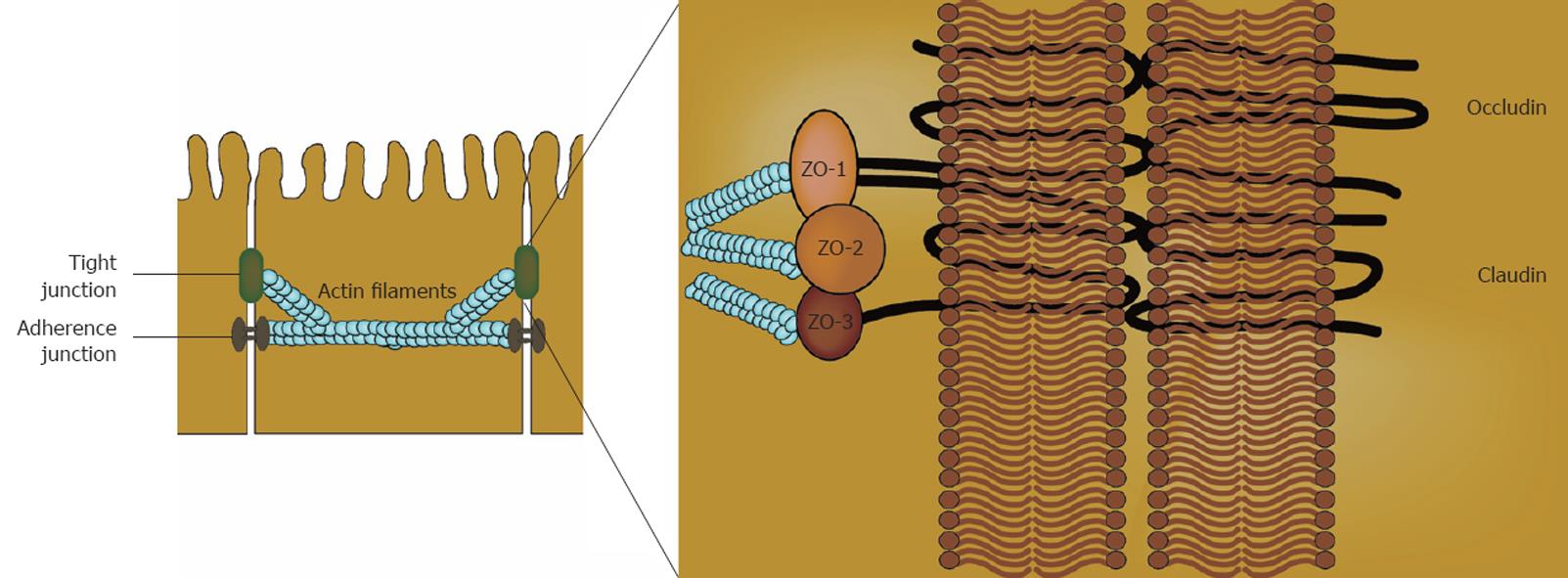
Tight junctions (TJ) are the major complexes responsible for the adherence of intestinal epithelial cells to one another and in this context are an important part of the intestinal barrier[3] (Figure 1). Multiple proteins build up TJ: occludins and members of the claudin family are the major sealing proteins. The sealing proteins interact with cytoplasmic proteins, including zonula-occludin proteins, functioning as adaptors between the TJ proteins and actin and myosin contractile elements within the cells[3]. Breakdown of this barrier potentially leads to translocation of luminal antigens, microbiota, and their toxic products into the circulation. The layers next to the mucosa, the submucosa, muscularis and serosa, are not in direct contact with the lumen. Injury of these layers can result in passage of the luminal content into the abdominal cavity, which is a serious complication.
Next to the physical/anatomical barrier, there is also an immunological barrier. The intestinal epithelium is not merely a static barrier, but participates in immunosurveillance[1,2]. Enterocytes are considered to actively participate as innate immune sensors of microbial pathogens and commensal organisms[4]. Host recognition of microbial components is achieved by so-called pattern recognition receptors (PRRs), such as the NOD-like and Toll-like receptors. Muramyl dipeptide, derived from peptidoglycan, is present in the cell wall of virtually all bacteria and is recognized by NOD2, a PRR expressed in intestinal epithelial cells, including Paneth cells[4]. Crypt Paneth cells secrete defensins, which are antimicrobial peptides, into the villous crypt, maintaining its sterility. Moreover, we recently reported that Paneth cells are equipped with the molecules that recognize and signal endotoxin, one of the most potent immunostimulatory products derived from Gram-negative bacteria[5]. Continuous antimicrobial protection of the crypt is of crucial importance, as the pluripotent stem cells are located there. Damage to stem cells has severe consequences for the maintenance of the homeostasis of normal gut epithelium[6]. Goblet cells secrete mucus, a composition of glycoproteins and water, which provides a filter overlying the intestinal epithelium. Additionally, goblet cells secrete trefoil peptides, small proteins needed for epithelial growth and repair. Furthermore, gut-associated lymphoid tissue is present in the lamina propria and provides immune surveillance. Sampling of luminal antigens occurs by M-cells and dendritic cells, which present antigens to T and B cells, thereby inducing an effector immune response. This response includes secretion of large amounts of IgA by plasma cells. This secretory IgA covers the mucosal surface and has a major role in excluding antigens from passing the epithelium[1,2].
Intestinal permeability is frequently assessed using oral ingestion of small to large-sized probe molecules and measurement of their urinary excretion[7,8]. Large molecules are thought to traverse the epithelium by paracellular pathways via tight junctions between the enterocytes. Permeability of the tight junctions presumably increases in diseased or damaged mucosa, resulting in increased absorption of large molecules. Small molecules are postulated to pass predominantly by transcellular pathways through aqueous pores in the enterocyte membranes that are too small to permit the passage of large molecules. The ratio of urinary excretion of the relatively large molecule is compared with that of the relatively small molecule. When a large and small molecule are combined in the test solution at a fixed concentration ratio, the effects of variables, such as gastric emptying, intestinal transit time, and renal clearance will apply equally to both. Thus, the urinary excretion ratio of these two molecules is expected to be influenced only by the difference in gut permeability for each molecule.
Disaccharides (lactulose) or Poly-ethylene-glycol (PEG)-3350 are frequently used as orally ingested large molecules, while monosaccharides (mannitol, L-rhamnose) or PEG-400 are used as small molecular probes[8]. Subsequently, the renal excretion of the two probes is monitored over a defined interval (mostly 5 h), and permeability is then expressed as the quotient (ratio) of the urinary recovery of the large molecule divided by the small molecule[8]. It is assumed that the probes used are non-fermentable by bacteria in the gastrointestinal lumen and that they are not metabolized in the body. These molecules are also supposed to be excreted in urine in proportion to the amount that has been absorbed through the intestinal mucosa[8]. Thus far, contrasting results have been reported for intestinal permeability tests using dual probe molecules in several studies[7,9]. This is mainly attributed to a number of assumptions that have to be made to interpret the test-results[8,9]. In particular, pathways of intestinal permeation of the different molecules and the mechanisms by which permeability is altered are as yet incompletely understood.
Breakdown of the mucosal barrier potentially leads to translocation of microbiota or their toxic products. Two promising plasma markers, reflecting translocation of bacteria or their products, are D-lactate and endotoxin lipopolysaccharide (LPS), which are metabolic products or components of the commensal bacteria of the gastrointestinal tract. D-lactate is only produced by bacteria as a product of bacterial fermentation[10]. Baseline levels of D-lactate in healthy subjects are very low. Increased levels of D-lactate have been correlated with conditions in which the number of bacteria elevates rapidly, including in patients with bacterial overgrowth due to infection, short bowel syndrome, and mesenteric ischaemia[11]. LPS, the major constituent of the outer membrane of Gram-negative bacteria, is released by Gram-negative bacteria when replicating or dying. Increased circulating LPS levels have been related to an impaired mucosal barrier. The presence of LPS can be measured directly in blood, e.g. by the Limulus Amoebocyte Lysate assay[12]. In addition, anti-LPS antibodies can be measured by endotoxin-core antibody (EndoCAb), an indirect measurement of LPS leakage into the circulation[13]. A drop in levels of circulating anti-LPS antibodies is considered to indicate consumption of antibodies to LPS by exposure to LPS[14].
Any part of the gastrointestinal tract may undergo damage to all layers of the GI wall from a variety of causes, releasing gastric or intestinal contents into the peritoneal cavity, which can cause peritonitis. Symptoms develop suddenly, with severe pain followed shortly by signs of (septic) shock. If a perforation is noted, immediate surgery is necessary, because mortality from peritonitis increases rapidly. The diagnosis of transmural damage (i.e. perforation) of a gastrointestinal organ usually depends on the detection of free intraperitoneal air, which is most often located in the right subphrenic space. Traditionally, a chest X-ray and a plain abdominal X-ray in the upright position, or, more recently, ultrasonography, are the diagnostic tools used to detect free air. However, the sensitivity of these tools is < 80%. Currently, a computed tomography scan is sometimes performed, which can detect free intraperitoneal air as well as small fluid collections and subtle tissue-infiltration at different locations.
Numerous clinical conditions are accompanied by a reduced splanchnic blood flow, including vascular disease, major surgery, and various types of shock. Prolonged hypoperfusion of the splanchnic region will inevitably lead to hypoxic tissue injury. Furthermore, the splanchnic region is an important source and target of inflammatory mediators, which have a major impact on both systemic and regional blood flow and tissue function[15]. Gut mucosal perfusion can be invasively measured by gastric tonometry. Gastric tonometry assesses the pCO2 in the gastric mucosa, taking into account that an increase in tissue CO2 production accompanies anaerobic metabolism, signifying the effectiveness of regional splanchnic perfusion[16].
Functional enterocyte mass is reflected by levels of circulating citrulline, an amino acid not incorporated into proteins[17]. Differentiated small intestinal enterocytes specifically produce citrulline from glutamine, and are responsible for the major part of the total amount of circulating citrulline[18]. Loss of small bowel epithelial cell mass results in declined circulating levels of citrulline, as is shown in haemopoietic stem cell transplant recipients suffering from severe oral and gastrointestinal mucositis following intensive myeloablative therapy[17].
Currently, invasive intestinal biopsies provide the only possibility to detect tight junction breakdown. Endoscopy for biopsy is a time-consuming and invasive procedure.
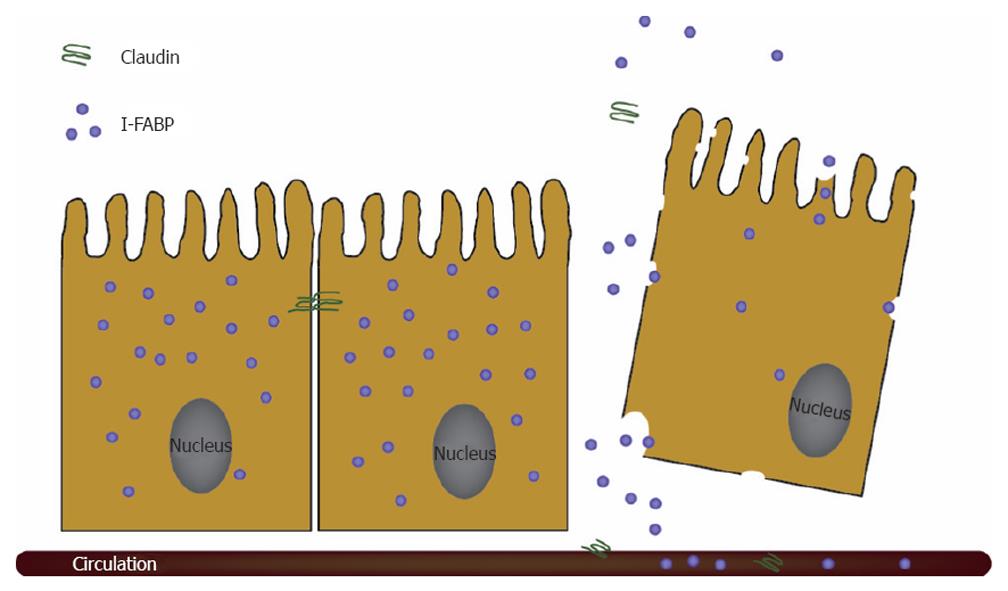
In a recent translational study, using both a rat hemorrhagic shock model and a human setting of patients with active inflammatory bowel disease (IBD), the immunohistochemically visualized loss of claudin-3, the major sealing tight junction protein, from intestinal tissue resulted in the rapid appearance of this protein in the urine[19]. This was the first study to report that measurement of the status of tight junctions can be performed non-invasively (Figure 2).
Measurement of endogenous cytosolic enterocyte proteins in urine or plasma has been shown to be useful to estimate enterocyte damage. Fatty acid binding proteins (FABP) comprise a class of low molecular weight (14-15 kDa) cytosolic proteins found in high concentrations in tissues involved in the uptake and consumption of fatty acids. Three isoforms of FABP are present in the intestine: intestinal (I)-FABP, liver (L)-FABP, and ileal-bile acid binding protein (I-BABP)[20]. I-FABP, L-FABP, and I-BABP are in particular highly expressed in cells present on the tops of the villi. The presence of FABP on the tops of the villi, the initial site of destruction in numerous intestinal diseases, makes circulating FABP potentially useful plasma markers in early stages of intestinal diseases. The kidneys remove approximately 30% of FABP in a single pass, leading to a calculated FABP half-life of 11 min[21]. This emphasizes that FABP is an accurate marker for actual cell damage and that assessment of the urinary concentration is potentially useful in reflecting enterocyte damage, as FABP are rapidly cleared by the kidneys. Especially in neonates and children, this is a great advantage, as blood collection for diagnostic purposes is traumatic for children and a major cause for anaemia in neonates. Intestinal FABP (I-FABP) is primarily limited to mature enterocytes of the small and large intestine[20,22,23]. It circulates in low amounts in the blood stream of healthy individuals. I-FABP is a useful plasma/urinary marker for early enterocyte cell death and levels rise rapidly after episodes of acute intestinal ischaemia and inflammation[20,22,24,25] (Figure 2). The level of circulating I-FABP has been reported to correlate with the histological status of the epithelium after intestinal ischaemia-reperfusion in experimental studies[25,26]. A second gut-specific FABP is Ileal-Bile Acid Binding Protein (I-BABP), which is exclusively present in mature enterocytes of the jejunum and ileum[27]. Enterocytes also contain Liver-FABP (L-FABP), which is localised in the mature enterocytes of the small and large intestine, but is more abundantly present in the liver and to a lesser degree in tubular cells of the kidney[20,23]. Increased circulating and/or urinary L-FABP levels can therefore be derived from other organs than the intestine. Next to FABP, Glutathione S-Transferases (GST) are a family of more or less tissue specific cytosolic enzymes. These proteins are involved in the detoxification of xenobiotic compounds by conjugation to glutathione and grouped into species-dependent families based on their isoelectric point[28]. Alpha and pi GST are found in the small and large intestine[29]. However, these proteins are not organ-specific, as they are also expressed in liver and kidney[30]. Plasma levels of alpha GST are elevated upon ischaemic intestinal damage[31]. In conclusion, I-FABP and I-BABP are the most promising endogenous enterocyte proteins (markers) to assess enterocyte injury, as these proteins are specifically expressed in the gut and released immediately into the circulation upon cell damage.
A broad range of pathologies can lead to intestinal inflammation: neoplasia, IBD, infections, autoimmune diseases (e.g. celiac disease), ischaemia-reperfusion, intestinal hypoperfusion, and e.g. the use of non-steroidal anti-inflammatory drugs. Generally, defects or increased permeability of the mucosal barrier will cause intestinal inflammation in response to the enormous number of bacteria present in the bowel. Recruitment of leukocytes into the intestinal wall is important in the pathogenesis of intestinal inflammation[32]. Activated neutrophils infiltrate the mucosa and their products can be detected in faeces due to release into the intestinal lumen. Obviously, changes in neutrophil release products can also be detected in plasma/serum, but plasma/serum levels are also increased by various conditions other than gut inflammation. Therefore, faecal markers of neutrophils are specific for the detection of inflammatory intestinal diseases. Numerous neutrophil derived proteins present in stool have been studied, including calprotectin, lactoferrin, and elastase[32]. The most promising marker is calprotectin, because of its remarkable resistance to proteolytic degradation and its stability in stool kept at room temperature for at least seven days[33].
Calprotectin, also known as MRP-8/MRP-14 or S100A8/A9 complex, is a 36 kDa calcium and zinc binding heterodimer protein that plays a regulatory role in the inflammatory process. It constitutes about 60% of the soluble proteins in human neutrophilic cytosol and is also found in monocytes, macrophages, and ileal tissue eosinophils. It is released during cell activation or cell death and has antiproliferative, antimicrobial, and immunomodulating functions[32,33].
Faecal calprotectin is nowadays used in clinical practice to evaluate disease activity in the follow-up of patients treated for active IBD[32]. It was found that faecal calprotectin levels correlated well with endoscopic, as well as histological, disease activity of patients with IBD[32]. Moreover, recent studies showed that normalisation of calprotectin levels in patients with established IBD is a strong indicator of mucosal healing. Furthermore, several studies showed that calprotectin was a very sensitive detection marker of inflammation in patients with inflammatory bowel disease (IBD, Crohn’s disease, and ulcerative colitis) compared with healthy controls and patients with irritable bowel syndrome, though not a specific marker, as increased levels were also found in neoplasia, infections, and polyps[32]. Bunn et al[34] confirmed these results on calprotectin levels in children with IBD. Carroll et al[35] stated that faecal calprotectin might be a useful marker of gastrointestinal mucosal inflammation in neonates in a pilot study comparing seven patients with proven necrotising enterocolitis (NEC) with seven healthy peers. This is supported by our recent results[36].
Evaluation of intestinal pathology in patients of all age groups has long been a challenge for clinicians. Numerous patients present with abdominal complaints, which are frequently aspecific and therefore correspond to pathologies of most intra- and even to some extra-abdominal organs. Laboratory tests and imaging techniques are often helpful in revealing disorders of organs, including the liver, pancreas, heart, and kidneys. However, it is still difficult to diagnose intestinal pathology in patients presenting with abdominal complaints[37]. The current standard technique for assessing intestinal status is endoscopy with biopsy. For some diseases this is helpful; however, it is invasive, associated with morbidity, sometimes requires sedation, expensive, and only assesses the function of the biopsied fraction. Moreover, for neutropenic and/or thrombocytopenic patients, the procedure is physically hazardous and often ethically unacceptable. Therefore, a major delay in diagnosis occurs in patients with e.g. NEC, chemotherapy-induced mucositis, acute mesenteric ischaemia, and celiac disease[37,38]. Such a diagnostic delay results in delayed treatment, which is accompanied by higher morbidity and mortality rates. In line with these diagnostic concerns, the follow-up of numerous intestinal diseases is hampered by the absence of rapid, non-invasive diagnostic means to assess intestinal damage for evaluation of the effects of treatment on the recovery of the disorder[37].
Patients undergoing major surgery or sustaining severe trauma are at risk of developing morbidity and mortality from post-operative or post-traumatic systemic inflammatory response syndrome (SIRS), sepsis, and multiple organ failure (MOF). Development of such potentially lethal complications in relatively healthy surgical or trauma patients is poorly understood[7]. Experimental animal studies have generated the hypothesis that the intestines are central in the origin of post-operative and post-traumatic sequelae[39-41]. Human studies have contributed insufficiently to gain insight in the applicability of this hypothesis[7]. Recognition of patients at risk of developing post-operative or post-traumatic SIRS, sepsis, and MOF is important, as patients with these clinical syndromes have the highest noncardiac mortality rate of patients in the intensive care unit (ICU)[39-42].
Experimental animal models, resembling the clinical situation of major surgery and trauma, show that haemorrhagic shock leads to disruption of the gut wall integrity, measured by derangement of tight-junctions and elevated circulating levels of FABP, originating from damaged intestinal epithelial cells[43,44]. Moreover, leakage of macromolecules, microbial products, and microbiota from the intestinal lumen into the circulation and mesenteric lymph nodes, spleen, and liver occurs[44]. The inflammatory response to microbial products, such as endotoxins, has been reported to be provoked via various rapidly induced innate immune mechanisms, ranging from Toll Like Receptors to complement activation. Supportive of the importance of the gut in the development of postoperative complications is the observation that protection of the gut wall integrity in hemorrhagically shocked animals by administration of probiotic strains, which inhibit the adhesion of enteric pathogens to intestinal epithelial cells, can result in abrogation of both local and systemic inflammatory responses[45]. However, administration of probiotic strains might also have unwanted side-effects, including disruption of intestinal tight junctions and increase of bacterial translocation[46].
Studies in patients undergoing major gastrointestinal, cardiac, or vascular surgery, investigating the role of the gut in the development of post-operative complications, are largely restricted to data on increased intestinal permeability for sugars, 51Cr-ethylenediaminetetraacetic acid (51Cr-EDTA), and the circulatory levels of endotoxin[14,47-49]. Increased intestinal absorption of sugars or 51Cr-EDTA, indirect measures for gut barrier loss, in patients following major surgery support data obtained from animal studies, indicating that the gut barrier is injured after major surgery[14,48-50]. However, other reports show no changes in intestinal permeability[51]. Moreover, the value of measuring gut integrity with sugar probes is debatable[8,9]. Similar results are reported on the development of endotoxemia. Several authors report on increased circulating levels of endotoxin or reduced values of anti-endotoxin antibody during major surgery, suggesting leakage of gut derived bacterial products from the gut lumen into the circulation due to an impaired epithelial barrier, whereas others show unaltered endotoxin concentrations[49,52]. Data showing better survival in patients with pre-operatively higher anti-endotoxin titres support the theory of intestinal contribution in the development of postoperative complications[53]. In conclusion, the debate regarding involvement of the gut in patients undergoing major surgery is still ongoing.
Three recent studies show the temporary presence of intestinal villous cell damage, measured by increased urinary levels of I-FABP, in patients undergoing cardio-vascular surgery with cardiopulmonary bypass (CPB)[54-56]. Patients with high urinary I-FABP levels developed postoperative gastro-intestinal complications[54]. The use of CPB was shown to be responsible for alterations in blood flow with consequent intestinal mucosal hypoxia and villous tip ischaemia[55,56]. Furthermore, intestinal injury correlated positively with markers of systemic inflammation. We recently reported, in patients undergoing major non-abdominal surgery, a similar influence of reduction in blood pressure on the provocation of intestinal villous cell injury, without the use of extracorporeal circulation[57]. A significant inverse association between mean arterial pressure and succeeding plasma levels of I-FABP and I-BABP was found, which indicates that systemic hypotension is correlated with intestinal mucosal cell injury. Anaesthetics, leading to decreased systemic vascular resistance, mainly cause systemic hypotension. Finally, splanchnic hypoperfusion (measured by gastric mucosal PiCO2 and Pr-aCO2-gap) correlated strongly with intestinal mucosal damage (assessed by plasma I-FABP) at all observed time-points during surgery[57].
In conclusion, the results of these studies show, for the first time, the relation between altered splanchnic perfusion and the development of intestinal mucosal cellular damage in patients undergoing major surgery. Collectively, these findings shed new light on the potential role of intestinal barrier compromise during major surgery, which was deduced from numerous animal studies, but has now been reported, for the first time, in relatively healthy children and adolescents undergoing major (non-abdominal) surgery. Furthermore, these results indicate a need to re-examine currently accepted criteria of haemodynamic parameters, both regarding the use of extra-corporeal circulation and accepted systemic hypotension, in patients undergoing major surgery.
The presence of intestinal damage does not show any cause-and-effect relationship with the development of sepsis or MOF. Moreover, intestinal damage may be part of more generalised tissue damage with epithelial barrier dysfunction in lung, liver, and kidney[7]. These studies are, however, the basis for further research to clarify the onset of intestinal damage. In addition, assessment of intestinal epithelial damage in patients with sepsis or undergoing major surgery might have important clinical implications. Evaluation of intestinal epithelial cell damage as a consequence of splanchnic hypoperfusion, could help to monitor preoperative treatment or therapy in patients with shock directed at restoration of peripheral perfusion and prevention of organ damage.
Relatively new markers for gut wall integrity (including FABP, claudin-3, and calprotectin) might be useful in the early diagnosis of intestinal diseases. The implementation of these markers in daily clinical practice seems only a matter of time. In addition, these markers are used nowadays to unravel the involvement of the gut in patients undergoing major surgery or sustaining major trauma or sepsis. These results led to studies aimed at re-examining accepted criteria of preoperative haemodynamic parameters, both regarding the use of extra-corporeal circulation and accepted systemic hypotension in patients undergoing major surgery[56,57]. We strongly believe that I-FABP can be used as a clinical marker of intestinal hypoperfusion. First of all, there is a strong correlation between gastric mucosal PiCO2 and circulating levels of I-FABP, in patients with postoperative sepsis who were admitted to the ICU[42]. Splanchnic hypoperfusion in the early phase of abdominal sepsis correlated strongly with intestinal mucosal damage. Moreover, elevated plasma I-FABP values on admission to the ICU were associated with a poor outcome in patients with abdominal sepsis. In children with meningococcal sepsis, it was shown that almost half of the patients presented with intestinal epithelial cell damage, shown by increased plasma I-FABP values, at admission to the paediatric ICU. The children who died were characterised by continued presence of gut damage, while in all survivors this injury came to an end within 12 h after starting intensive treatment[58]. A significant proportion (93%) of adult trauma patients rapidly developed intestinal mucosal cell damage, measured by elevation of plasma I-FABP values[59]. The extent of intestinal damage was readily detectable in blood withdrawn on presentation at the emergency department (ER). Interestingly, the highest 10% of I-FABP values at ER belonged to patients with severe abdominal trauma that required acute surgical intervention, such as ruptures of the diaphragm, liver, and spleen. Circulatory concentrations of enterocyte damage marker I-FABP were related to the presence of shock and the extent of general injury, as well as abdominal trauma, indicating that the level of intestinal cell damage was determined by both systemic and local factors. Moreover, early I-FABP values correlated positively with the inflammatory response that developed in the days following trauma[59].
In conclusion, evaluation of intestinal tissue damage in the early phase of sepsis is an adequate predictor for survival. Furthermore, the adequacy of treatment of circulatory failure in sepsis is currently monitored using indirect parameters of (peripheral) tissue perfusion and oxygenation, including blood pressure, urinary output, metabolic status (lactate, base deficit) skin temperature, ScvO2, and capillary refill time. However, these parameters do not reflect the actual defects in (peripheral) tissue perfusion and subsequent tissue damage. Assessment of plasma I-FABP levels offers the possibility to monitor the presence of intestinal epithelial cell damage as a consequence of splanchnic hypoperfusion, which could help to monitor treatment directed at restoration of peripheral perfusion and prevention of organ damage. Further studies are needed to clarify the diagnostic potential of assessment of plasma I-FABP in monitoring the treatment of sepsis in the acute phase and during follow-up.
Peer reviewers: Ruben Zamora, PhD, Assistant Professor of Surgery, Department of Surgery, University of Pittsburgh, W1540 Biomedical Science Tower 200 Lothrop St., Pittsburgh, PA 15213, United States; María IT López, Professor, Experimental Biology, University of Jaen, araje de las Lagunillas s/n, Jaén 23071, Spain
S- Editor Wang YR L- Editor Stewart GJ E- Editor Lin YP
| 1. | Nagler-Anderson C. Man the barrier! Strategic defences in the intestinal mucosa. Nat Rev Immunol. 2001;1:59-67. [Cited in This Article: ] |
| 2. | Sansonetti PJ. War and peace at mucosal surfaces. Nat Rev Immunol. 2004;4:953-964. [Cited in This Article: ] |
| 3. | Liu Z, Li N, Neu J. Tight junctions, leaky intestines, and pediatric diseases. Acta Paediatr. 2005;94:386-393. [Cited in This Article: ] |
| 4. | Hisamatsu T, Suzuki M, Reinecker HC, Nadeau WJ, McCormick BA, Podolsky DK. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology. 2003;124:993-1000. [Cited in This Article: ] |
| 5. | Wolfs TG, Derikx JP, Hodin CM, Vanderlocht J, Driessen A, de Bruïne AP, Bevins CL, Lasitschka F, Gassler N, van Gemert WG. Localization of the lipopolysaccharide recognition complex in the human healthy and inflamed premature and adult gut. Inflamm Bowel Dis. 2010;16:68-75. [Cited in This Article: ] |
| 6. | Mukherjee S, Vaishnava S, Hooper LV. Multi-layered regulation of intestinal antimicrobial defense. Cell Mol Life Sci. 2008;65:3019-3027. [Cited in This Article: ] |
| 7. | Soeters PB, Luyer MD, Greve JW, Buurman WA. The significance of bowel permeability. Curr Opin Clin Nutr Metab Care. 2007;10:632-638. [Cited in This Article: ] |
| 8. | Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995;108:1566-1581. [Cited in This Article: ] |
| 9. | Fink MP. Interpreting dual-sugar absorption studies in critically ill patients: what are the implications of apparent increases in intestinal permeability to hydrophilic solutes? Intensive Care Med. 1997;23:489-492. [Cited in This Article: ] |
| 10. | Smith SM, Eng RH, Buccini F. Use of D-lactic acid measurements in the diagnosis of bacterial infections. J Infect Dis. 1986;154:658-664. [Cited in This Article: ] |
| 11. | Poeze M, Froon AH, Greve JW, Ramsay G. D-lactate as an early marker of intestinal ischaemia after ruptured abdominal aortic aneurysm repair. Br J Surg. 1998;85:1221-1224. [Cited in This Article: ] |
| 12. | Caridis DT, Reinhold RB, Woodruff PW, Fine J. Endotoxaemia in man. Lancet. 1972;1:1381-1385. [Cited in This Article: ] |
| 13. | Barclay GR. Endogenous endotoxin-core antibody (EndoCAb) as a marker of endotoxin exposure and a prognostic indicator: a review. Prog Clin Biol Res. 1995;392:263-272. [Cited in This Article: ] |
| 14. | Braun JP, Buhner S, Kastrup M, Dietz E, Langer K, Dohmen PM, Lochs H, Spies C. Barrier function of the gut and multiple organ dysfunction after cardiac surgery. J Int Med Res. 2007;35:72-83. [Cited in This Article: ] |
| 15. | Takala J. Determinants of splanchnic blood flow. Br J Anaesth. 1996;77:50-58. [Cited in This Article: ] |
| 16. | Cerný V, Cvachovec K. Gastric tonometry and intramucosal pH--theoretical principles and clinical application. Physiol Res. 2000;49:289-297. [Cited in This Article: ] |
| 17. | Crenn P, Coudray-Lucas C, Thuillier F, Cynober L, Messing B. Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology. 2000;119:1496-1505. [Cited in This Article: ] |
| 18. | Curis E, Crenn P, Cynober L. Citrulline and the gut. Curr Opin Clin Nutr Metab Care. 2007;10:620-626. [Cited in This Article: ] |
| 19. | Thuijls G, Derikx JP, de Haan JJ, Grootjans J, de Bruïne A, Masclee AA, Heineman E, Buurman WA. Urine-based detection of intestinal tight junction loss. J Clin Gastroenterol. 2010;44:e14-e19. [Cited in This Article: ] |
| 20. | Pelsers MM, Hermens WT, Glatz JF. Fatty acid-binding proteins as plasma markers of tissue injury. Clin Chim Acta. 2005;352:15-35. [Cited in This Article: ] |
| 21. | van de Poll MC, Derikx JP, Buurman WA, Peters WH, Roelofs HM, Wigmore SJ, Dejong CH. Liver manipulation causes hepatocyte injury and precedes systemic inflammation in patients undergoing liver resection. World J Surg. 2007;31:2033-2038. [Cited in This Article: ] |
| 22. | Lieberman JM, Sacchettini J, Marks C, Marks WH. Human intestinal fatty acid binding protein: report of an assay with studies in normal volunteers and intestinal ischemia. Surgery. 1997;121:335-342. [Cited in This Article: ] |
| 23. | Derikx JP, Vreugdenhil AC, Van den Neucker AM, Grootjans J, van Bijnen AA, Damoiseaux JG, van Heurn LW, Heineman E, Buurman WA. A pilot study on the noninvasive evaluation of intestinal damage in celiac disease using I-FABP and L-FABP. J Clin Gastroenterol. 2009;43:727-733. [Cited in This Article: ] |
| 24. | Kanda T, Fujii H, Tani T, Murakami H, Suda T, Sakai Y, Ono T, Hatakeyama K. Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology. 1996;110:339-343. [Cited in This Article: ] |
| 25. | Derikx JP, Matthijsen RA, de Bruïne AP, van Bijnen AA, Heineman E, van Dam RM, Dejong CH, Buurman WA. Rapid reversal of human intestinal ischemia-reperfusion induced damage by shedding of injured enterocytes and reepithelialisation. PLoS One. 2008;3:e3428. [Cited in This Article: ] |
| 26. | Gollin G, Marks C, Marks WH. Intestinal fatty acid binding protein in serum and urine reflects early ischemic injury to the small bowel. Surgery. 1993;113:545-551. [Cited in This Article: ] |
| 27. | Watanabe K, Hoshi N, Tsuura Y, Kanda T, Fujita M, Fujii H, Ono T, Suzuki T. Immunohistochemical distribution of intestinal 15 kDa protein in human tissues. Arch Histol Cytol. 1995;58:303-306. [Cited in This Article: ] |
| 28. | Beckett GJ, Hayes JD. Glutathione S-transferases: biomedical applications. Adv Clin Chem. 1993;30:281-380. [Cited in This Article: ] |
| 29. | Coles BF, Chen G, Kadlubar FF, Radominska-Pandya A. Interindividual variation and organ-specific patterns of glutathione S-transferase alpha, mu, and pi expression in gastrointestinal tract mucosa of normal individuals. Arch Biochem Biophys. 2002;403:270-276. [Cited in This Article: ] |
| 30. | Campbell JA, Corrigall AV, Guy A, Kirsch RE. Immunohistologic localization of alpha, mu, and pi class glutathione S-transferases in human tissues. Cancer. 1991;67:1608-1613. [Cited in This Article: ] |
| 31. | Khurana S, Corbally MT, Manning F, Armenise T, Kierce B, Kilty C. Glutathione S-transferase: a potential new marker of intestinal ischemia. J Pediatr Surg. 2002;37:1543-1548. [Cited in This Article: ] |
| 32. | Lundberg JO, Hellström PM, Fagerhol MK, Weitzberg E, Roseth AG. Technology insight: calprotectin, lactoferrin and nitric oxide as novel markers of inflammatory bowel disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:96-102. [Cited in This Article: ] |
| 33. | Fagerhol MK. Calprotectin, a faecal marker of organic gastrointestinal abnormality. Lancet. 2000;356:1783-1784. [Cited in This Article: ] |
| 34. | Bunn SK, Bisset WM, Main MJ, Golden BE. Fecal calprotectin as a measure of disease activity in childhood inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2001;32:171-177. [Cited in This Article: ] |
| 35. | Carroll D, Corfield A, Spicer R, Cairns P. Faecal calprotectin concentrations and diagnosis of necrotising enterocolitis. Lancet. 2003;361:310-311. [Cited in This Article: ] |
| 36. | Thuijls G, Derikx JP, van Wijck K, Zimmermann LJ, Degraeuwe PL, Mulder TL, Van der Zee DC, Brouwers HA, Verhoeven BH, van Heurn LW. Non-invasive markers for early diagnosis and determination of the severity of necrotizing enterocolitis. Ann Surg. 2010;251:1174-1180. [Cited in This Article: ] |
| 37. | Dewar DH, Ciclitira PJ. Clinical features and diagnosis of celiac disease. Gastroenterology. 2005;128:S19-S24. [Cited in This Article: ] |
| 38. | Oldenburg WA, Lau LL, Rodenberg TJ, Edmonds HJ, Burger CD. Acute mesenteric ischemia: a clinical review. Arch Intern Med. 2004;164:1054-1062. [Cited in This Article: ] |
| 39. | Moore FA. The role of the gastrointestinal tract in postinjury multiple organ failure. Am J Surg. 1999;178:449-453. [Cited in This Article: ] |
| 40. | Fink MP, Delude RL. Epithelial barrier dysfunction: a unifying theme to explain the pathogenesis of multiple organ dysfunction at the cellular level. Crit Care Clin. 2005;21:177-196. [Cited in This Article: ] |
| 41. | Clark JA, Coopersmith CM. Intestinal crosstalk: a new paradigm for understanding the gut as the "motor" of critical illness. Shock. 2007;28:384-393. [Cited in This Article: ] |
| 42. | Derikx JP, Poeze M, van Bijnen AA, Buurman WA, Heineman E. Evidence for intestinal and liver epithelial cell injury in the early phase of sepsis. Shock. 2007;28:544-548. [Cited in This Article: ] |
| 43. | de Haan JJ, Lubbers T, Hadfoune M, Luyer MD, Dejong CH, Buurman WA, Greve JW. Postshock intervention with high-lipid enteral nutrition reduces inflammation and tissue damage. Ann Surg. 2008;248:842-848. [Cited in This Article: ] |
| 44. | Yang R, Han X, Uchiyama T, Watkins SK, Yaguchi A, Delude RL, Fink MP. IL-6 is essential for development of gut barrier dysfunction after hemorrhagic shock and resuscitation in mice. Am J Physiol Gastrointest Liver Physiol. 2003;285:G621-G629. [Cited in This Article: ] |
| 45. | Ukena SN, Singh A, Dringenberg U, Engelhardt R, Seidler U, Hansen W, Bleich A, Bruder D, Franzke A, Rogler G. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One. 2007;2:e1308. [Cited in This Article: ] |
| 46. | Luyer MD, Buurman WA, Hadfoune M, Speelmans G, Knol J, Jacobs JA, Dejong CH, Vriesema AJ, Greve JW. Strain-specific effects of probiotics on gut barrier integrity following hemorrhagic shock. Infect Immun. 2005;73:3686-3692. [Cited in This Article: ] |
| 47. | Kanwar S, Windsor AC, Welsh F, Barclay GR, Guillou PJ, Reynolds JV. Lack of correlation between failure of gut barrier function and septic complications after major upper gastrointestinal surgery. Ann Surg. 2000;231:88-95. [Cited in This Article: ] |
| 48. | Ohri SK, Somasundaram S, Koak Y, Macpherson A, Keogh BE, Taylor KM, Menzies IS, Bjarnason I. The effect of intestinal hypoperfusion on intestinal absorption and permeability during cardiopulmonary bypass. Gastroenterology. 1994;106:318-323. [Cited in This Article: ] |
| 49. | Riddington DW, Venkatesh B, Boivin CM, Bonser RS, Elliott TS, Marshall T, Mountford PJ, Bion JF. Intestinal permeability, gastric intramucosal pH, and systemic endotoxemia in patients undergoing cardiopulmonary bypass. JAMA. 1996;275:1007-1012. [Cited in This Article: ] |
| 50. | Doig CJ, Sutherland LR, Sandham JD, Fick GH, Verhoef M, Meddings JB. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am J Respir Crit Care Med. 1998;158:444-451. [Cited in This Article: ] |
| 51. | Malagon I, Onkenhout W, Klok G, van der Poel PF, Bovill JG, Hazekamp MG. Gut permeability in paediatric cardiac surgery. Br J Anaesth. 2005;94:181-185. [Cited in This Article: ] |
| 52. | Soong CV, Halliday MI, Barclay GR, Hood JM, Rowlands BJ, Barros D'Sa AA. Intramucosal acidosis and systemic host responses in abdominal aortic aneurysm surgery. Crit Care Med. 1997;25:1472-1479. [Cited in This Article: ] |
| 53. | Bennett-Guerrero E, Ayuso L, Hamilton-Davies C, White WD, Barclay GR, Smith PK, King SA, Muhlbaier LH, Newman MF, Mythen MG. Relationship of preoperative antiendotoxin core antibodies and adverse outcomes following cardiac surgery. JAMA. 1997;277:646-650. [Cited in This Article: ] |
| 54. | Holmes JH 4th, Lieberman JM, Probert CB, Marks WH, Hill ME, Paull DL, Guyton SW, Sacchettini J, Hall RA. Elevated intestinal fatty acid binding protein and gastrointestinal complications following cardiopulmonary bypass: a preliminary analysis. J Surg Res. 2001;100:192-196. [Cited in This Article: ] |
| 55. | Morariu AM, Loef BG, Aarts LP, Rietman GW, Rakhorst G, van Oeveren W, Epema AH. Dexamethasone: benefit and prejudice for patients undergoing on-pump coronary artery bypass grafting: a study on myocardial, pulmonary, renal, intestinal, and hepatic injury. Chest. 2005;128:2677-2687. [Cited in This Article: ] |
| 56. | Hanssen SJ, Derikx JP, Vermeulen Windsant IC, Heijmans JH, Koeppel TA, Schurink GW, Buurman WA, Jacobs MJ. Visceral injury and systemic inflammation in patients undergoing extracorporeal circulation during aortic surgery. Ann Surg. 2008;248:117-125. [Cited in This Article: ] |
| 57. | Derikx JP, van Waardenburg DA, Thuijls G, Willigers HM, Koenraads M, van Bijnen AA, Heineman E, Poeze M, Ambergen T, van Ooij A. New Insight in Loss of Gut Barrier during Major Non-Abdominal Surgery. PLoS One. 2008;3:e3954. [Cited in This Article: ] |
| 58. | Derikx JP, Bijker EM, Vos GD, van Bijnen AA, Heineman E, Buurman WA, van Waardenburg DA. Gut mucosal cell damage in meningococcal sepsis in children: relation with clinical outcome. Crit Care Med. 2010;38:133-137. [Cited in This Article: ] |