Published online Jan 28, 2010. doi: 10.3748/wjg.v16.i4.453
Revised: November 8, 2009
Accepted: November 15, 2009
Published online: January 28, 2010
AIM: To assess the role of IgM and IgG immunohistochemistry (IHC) in the evaluation of autoimmune liver conditions - autoimmune hepatitis (AIH), primary biliary cirrhosis (PBC), and primary sclerosing cholangitis (PSC).
METHODS: Forty one biopsies from untreated patients diagnosed with autoimmune liver disease (AIH, n = 20; PBC, n = 13; PSC, n = 8) and fourteen biopsies of patients with chronic hepatitis C were selected. IgM and IgG-positive plasma cells were counted in each sample.
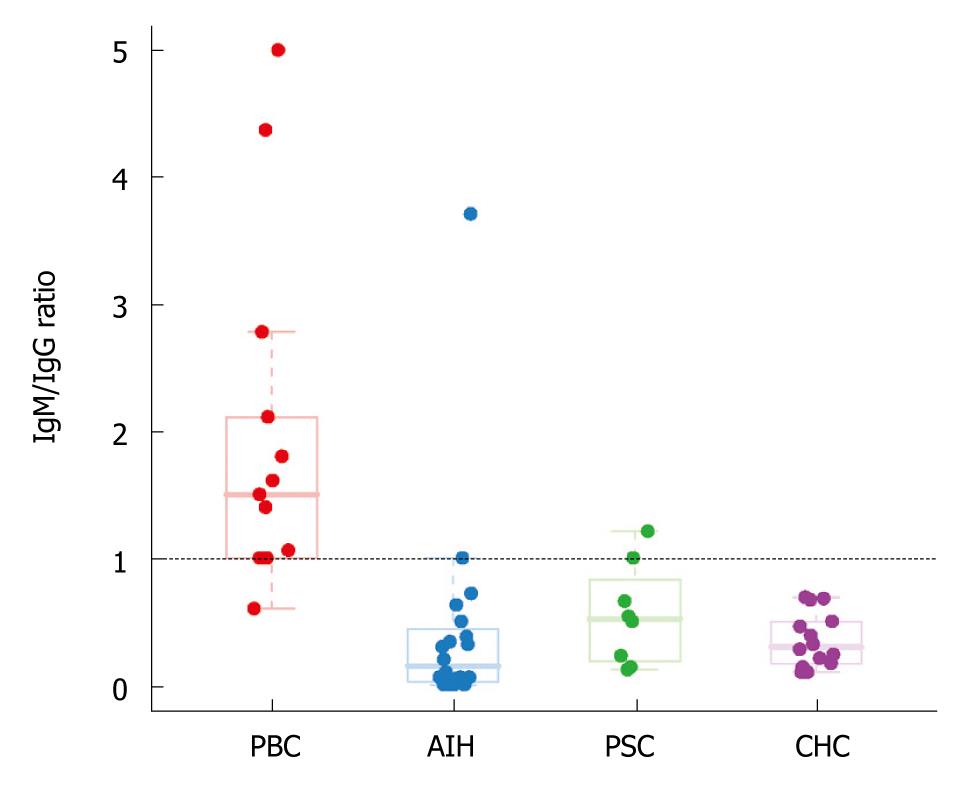
RESULTS: A predominance of IgG-positive plasma cells was seen in AIH (90% of cases), PSC (75% of cases), and chronic hepatitis C (100% of cases), while IgM-positive plasma cells predominated in PBC (92.8% of cases). The IgM /IgG ratio (< 1 or ≥ 1) accurately distinguished PBC from AIH in 90.9% of cases (sensitivity = 92.3%, specificity = 90%), and PBC from either AIH or PSC in 87.8% of cases (sensitivity = 92.3%, specificity = 85.7%).
CONCLUSION: Plasmacytic infiltrates expressing predominantly IgM are characteristic of PBC, while other forms of liver disease analyzed in this study, including AIH, typically show an IgG-predominant plasma cell infiltrate. Our data indicate that IgM and IgG IHC may be a useful tool when PBC is a diagnostic consideration.
- Citation: Moreira RK, Revetta F, Koehler E, Washington MK. Diagnostic utility of IgG and IgM immunohistochemistry in autoimmune liver disease. World J Gastroenterol 2010; 16(4): 453-457
- URL: https://www.wjgnet.com/1007-9327/full/v16/i4/453.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i4.453
Three major clinicopathologic entities are currently classified as autoimmune liver diseases: autoimmune hepatitis (AIH), primary biliary cirrhosis (PBC), and primary sclerosing cholangitis (PSC). A combination of clinical, laboratory, and pathologic criteria are necessary for the diagnosis of these conditions. Each category of autoimmune liver disease has specific biologic, prognostic, and therapeutic implications; therefore, an accurate diagnosis is essential.
While liver biopsies represent an important part of the diagnostic evaluation of these patients, significant histopathologic overlap exists among the different autoimmune liver diseases. An important histologic finding in liver biopsies, in this setting, is the presence of plasma cells, which are characteristically found in AIH, but are also commonly present in PBC and PSC, as well as in other forms of liver disease[1-6]. Previous studies have shown that assessment of immunoglobulin subclasses in plasma cells by immunohistochemistry (IHC) may be useful in distinguishing PBC from AIH, but the use of such ancillary studies has not yet gained wide acceptance as a diagnostic tool[7-9]. Moreover, limited data are available regarding the immunophenotype of plasma cells in PSC. In this study, we evaluate the predominant plasma cell immunoglobulin subclass present in liver biopsies of patients with well-established AIH, PBC, or PSC and assess the diagnostic utility of IgM and IgG IHC in this setting.
This study was reviewed and approved by our Institutional Review Board. Liver biopsies from untreated patients diagnosed with autoimmune liver disease at our institution from 1993 to 2006 were selected. Inclusion criteria for patients with AIH were: (1) clinical diagnosis of AIH, according to the international autoimmune hepatitis group scoring system[10], including positive serology for AIH-related autoantibodies [antinuclear antibody (ANA), anti-smooth muscle antibody (ASMA), anti-liver-kidney microsomal antibody (anti-LKM-1), or soluble liver antigen antibody (anti-SLA)]; and (2) liver biopsy showing consistent histopathological findings. For PBC, inclusion criteria were: (1) positive anti-mitochondrial antibody (AMA) serology; (2) elevated alkaline phosphatase levels, and (3) consistent histopathological findings. For PSC, inclusion criteria were: (1) elevated alkaline phosphatase levels, and (2) characteristic cholangiographic findings by endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholangiopancreatography (MRCP). Inclusion criteria for all groups also included availability of adequate paraffin-embedded tissue for immunohistochemical analysis. Exclusion criteria for all autoimmune liver disease groups included evidence of viral hepatitis, clinical suspicion for an alternative underlying etiology for the liver disease, and incompatible histopathological findings. For patients with AIH, PBC, and PSC, all biopsies included in this study were taken as part of the initial diagnostic workup. Patients who received any form of immunosuppressive medication (other than inflammatory bowel disease treatment in cases of PSC) or ursodeoxycholic acid previous to the biopsy procedure were excluded. Utilizing the above criteria, 41 patients were included in the study (AIH, n = 20; PBC, n = 13; and PSC, n = 8). For comparison, we also studied 14 patients with chronic hepatitis C with at least focal plasma cells identified on HE sections.
Biopsy samples were considered adequate for the purposes of this study if at least 3 complete portal tracts were present. For each biopsy, the length of each tissue fragment was multiplied by its average width (mm) and the total area of the sample (mm2) was calculated. Subsequently, all cells morphologically consistent with plasma cells showing unequivocal immunohistochemical expression of either IgM or IgG (Cell Marque, 1:20 000) were counted. The absolute number and the concentration of positive cells for each immunoglobulin subtype were assessed. IgM/IgG ratio was analyzed for sensitivity and specificity in the diagnosis of autoimmune liver diseases. Confidence intervals for these values are determined by a score interval[11]. For cases of AIH, PSC, and chronic hepatitis C, the degree of fibrosis was assessed in each case using the Batts-Ludwig staging system. Histological staging of PBC cases was performed using Ludwig’s classification.
Twenty patients had a diagnosis of AIH (8 males, 12 females, mean age 32, range 4-63), all of whom had positive antinuclear antibody (ANA) serology. Two pediatric patients had positive anti-smooth muscle antibody serology in addition to a positive ANA. Associated autoimmune conditions included thyroid dysfunction (3 patients), rheumatoid arthritis (1 patient), vitiligo (1 patient), and alopecia (1 patient). One patient was diagnosed with drug-induced AIH while receiving minocycline treatment for acne. Fifty percent of AIH patients had early stage fibrosis (stages 0-2) and 50% had advanced fibrosis (stages 3 and 4).
Thirteen patients had a diagnosis of PBC (1 male, 12 females, mean age 52, range 43-71). Associated autoimmune conditions included sicca syndrome (26% of patients), and hypothyroidism (6% of patients). PBC patients were staged as following: stage 1 (3/13, 23%), stage 2 (7/13, 54%), stage 3 (3/13, 23%), stage 4 (1/15, 6.6%). Typical florid duct lesions were seen in 3/13 (23%) of PBC patients (stage 2, two patients; stage 3, one patient).
Eight patients had a diagnosis of PSC (6 males, 2 females, mean age 31, range 7-67), including characteristic findings on endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholangiopancreatography (MRCP). Fifty percent of PSC patients had early stage fibrosis (stages 0-2), and 50% had advanced fibrosis (stages 3 and 4). Typical periductal fibrosis was seen in only 2 cases. Additional histologic findings included mild-moderate chronic lymphoplasmacytic portal inflammation, and bile ductular proliferation (8/10 patients). Associated conditions included ulcerative colitis (4/8, 50%), and Crohn’s disease (2/8, 25%).
Fourteen patients with chronic hepatitis C infection were also included (9 males, 5 females, mean age 50, range 36-66). All patients in this group had documented chronic viral hepatitis C infection by serum polymerase chain reaction (PCR) (genotype 1, n = 11; genotype 2, n = 2; and genotype 3, n = 1). Twelve patients underwent liver biopsy for grading and stating (native liver biopsies). Two patients had recurrent hepatitis C post liver transplantation. Interpretation of liver biopsy in all cases was consistent with hepatitis C (stage 0, n = 1; stage 1, n = 2; stage 2, n = 6; stage 3, n = 3; stage 4, n = 2).
The combined number of IgG and IgM+ plasma cells in AIH (average = 9.75 cells/mm2) was higher than that seen in CHC, PBC or PSC (average = 6.90, 6.19 and 5.16 cells/mm2, respectively, P < 0.05). In all cases, plasma cells were present predominantly within the portal tracts.
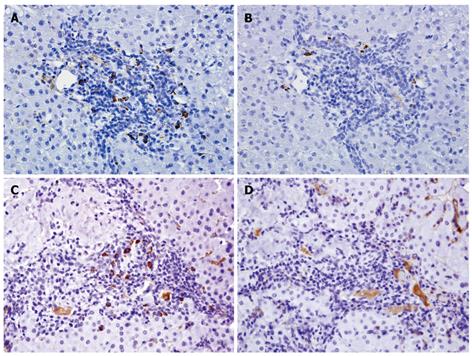
We found a predominance of IgG+ plasma cells in AIH (90% of cases), in PSC (75% of cases), and in chronic hepatitis C (100% of cases), while the majority of plasma cells in PBC were IgM positive (92.3% of cases) (Figures 1 and 2). An IgM/IgG ratio ≥ 1 showed high sensitivity and specificity for the distinction of PBC from other groups included in the study and accurately distinguished PBC from AIH in 90.9% (30/33) of cases, PBC from either AIH or PSC in 87.8% (36/41) of cases, and PBC from all other groups in 90.9% (Table 1). There was no correlation between age or gender and IgM/IgG ratio in any of the study groups.
| Sensitivity (95% CI) | Specificity (95% CI) | Likelihood ratio - positive test (95% CI) | Likelihood ratio - negative test (95% CI) | Accuracy | |
| PBC vs AIH | 92.3% (66.7-98.6) | 90.0% (69.9-97.2) | 9.23 (2.45-34.69) | 0.085 (0.012-0.56) | 90.9% (30/33) |
| PBC vs AIH or PSC | 92.3% (66.7-98.6) | 85.7% (68.5-94.3) | 6.46 (2.57-16.22) | 0.089 (0.013-0.59) | 87.8% (36/41) |
| PBC vs all others | 92.3% (66.7-98.6) | 90.5% (77.9-96.3) | 9.69 (3.76-24.94) | 0.085 (0.012-0.56) | 90.9% (50/55) |
Typical cases of autoimmune liver diseases do not usually represent diagnostic dilemmas from a histopathologic standpoint. However, diagnostic findings are sometimes absent in liver biopsies of autoimmune liver diseases, and consideration of various entities has to be entertained in the differential diagnosis[12-14].
Chronic inflammatory infiltrate, often plasma cell-rich, is a common feature in all autoimmune liver diseases[15]. There is evidence to suggest that the identification of the predominant immunoglobulin (Ig) subclasses within plasma cells in liver biopsies may be useful in the differential diagnosis of autoimmune liver diseases. van Spreeuwel et al[8], in 1984, studied eight patients with PBC and 18 patients with “chronic hepatitis” (which included patients with hepatitis B, as well as patients diagnosed as “chronic persistent hepatitis” and “chronic aggressive hepatitis”). The authors found a significantly higher absolute and relative number of IgM-positive plasma cells, by immunohistochemistry, in patients with PBC compared to patients with chronic hepatitis. The increased number of plasma cells correlated with increased serum levels of IgM in PBC patients.
Milne et al[7], in 1990, studied 14 patients with PBC and 14 patients with “chronic active hepatitis” (which included patients with hepatitis B, ANA-positive hepatitis, and idiopathic chronic hepatitis). Using immunohistochemistry for IgA, IgM, and IgG, the authors found a predominance of IgA and IgG expression in plasma cells in “chronic active hepatitis”, while IgM and IgG represented the main Ig classes seen in cases of PBC. In both studies, all cases of PBC had positive AMA serology, increased alkaline phosphatase levels, and histopathological findings which were consistent with the diagnosis. However, the “chronic hepatitis” group was heterogeneous and included patients with hepatitis B, as well as other forms of chronic hepatitis, then classified as “chronic active hepatitis” and “chronic aggressive hepatitis”, which may have included cases of hepatitis C and autoimmune hepatitis by current diagnostic standards.
Recently, Daniels et al[9] specifically studied the immunophenotype of plasma cells in the differential diagnosis of PBC and AIH. Thirty eight patients with AIH and 18 patients with PBC were included. All cases of AIH showed an IgG-predominant plasma cell infiltrate, while IgM prevailed over IgG in 88% of PBC cases upon qualitative analysis of IHC slides (back-to-back comparison).
In the present study, we included untreated patients meeting strict clinical and pathologic diagnostic criteria for both AIH and PBC. In addition, we also included patients with PSC, which were not included in previous studies, as this condition may also be a diagnostic consideration in this setting. A group of hepatitis C patients was included for comparison.
Our results clearly show that most cases of PBC expressed a characteristic IgM/IgG immunophenotype. There was an obvious predomination of IgM expression in plasma cells of patients with PBC (92.3% of patients), compared to an IgG-predominant expression in the majority of plasma cells in patients with AIH (90% of cases), PSC (75% of cases), and CHC (100% of cases). Among the groups of patients included in this study, the differential diagnosis between AIH and PBC is likely the most challenging from a histopathological perspective. The IgM/IgG ratio (< 1 or ≥ 1) accurately distinguishes PBC from AIH in over 90% of cases (sensitivity = 92.3%, specificity = 90%). A high sensitivity and specificity (92.3% and 90.5%, respectively) was also found for the distinction between PBC and all other groups combined (Table 1).
Interestingly, in 1 case of autoimmune hepatitis which showed a very high IgM/IgG ratio, similar to that seen in the PBC group, a positive antimitochondrial antibody (titer > 1:160) was detected on clinical follow-up (AMA serology was negative on initial evaluation). A second liver biopsy of this patient showed lymphoplasmacytic portal inflammation and associated prominent ductocentric granulomatous inflammation with significant bile duct destruction (florid duct lesion). A diagnosis of AIH-PBC sequential syndrome was rendered 3 years after the initial biopsy. This patient remained in the AIH group of this study because criteria for AIH were met at the time of initial biopsy. Although only 1 case of overlap syndrome was identified in our database, the fact that plasma cells predominantly expressed IgM in both the initial and the follow-up biopsies is intriguing. This raises the question of whether immunohistochemistry for specific immunoglobulins could provide additional information (i.e. identify a PBC-like pattern) in the setting of suspected overlap syndrome, or even in otherwise typical autoimmune hepatitis.
PSC cases showed a predominance of IgG+ plasma cells in most cases, although a significant number (25%) showed a predominance of IgM+ plasma cells. In most cases of PSC, however, the total number of plasma cells was low, and sampling error may have occurred during the evaluation of individual cases as IgM or IgG-predominant.
Among our chronic hepatitis C samples (15 cases selected from 30 consecutive cases of chronic hepatitis C), a plasmacytic component was present within the lymphocyte-predominant infiltrate in all cases. Because the cases were selected on the basis of presence of plasma cells, an obvious selection bias is present. However, since 15 of 30 consecutive cases were included, it is clear that plasma cells are common in our chronic hepatitis C population. All biopsies from hepatitis C patients showed predominantly IgG-producing plasma cells.
Overall, IgG was the predominant immunoglobulin subclass produced by plasma cells in all groups included in this study, except for the PBC group, in which an IgM-predominant plasma cell population is seen in the majority of cases. From a diagnostic standpoint, we believe that plasma cell-rich infiltrates seen on liver biopsies with IgM/IgG ratio ≥ 1 should alert the pathologist to the possibility of PBC, while an IgM/IgG ratio < 1 renders a diagnosis of PBC significantly less likely. IgG-predominant infiltrates, although characteristically present in AIH, are relatively nonspecific and may be seen in a variety of conditions.
In conclusion, the histological features of the different autoimmune liver diseases may overlap considerably in some cases. While the plasma cell-rich infiltrates seen in several forms of liver disease predominantly express IgG, PBC seems to be an exception, as most plasma cells in these patients express IgM. Our data indicates that a predominantly IgM+ plasma cell infiltrate, although not pathognomonic, should strongly support the diagnosis of PBC. The IgM/IgG ratio is particularly helpful distinguishing PBC from AIH.
Increased numbers of plasma cells may be present on liver biopsies of autoimmune hepatitis, primary biliary cirrhosis, and primary sclerosing cholangitis patients, and the histopathologic distinction of these entities may be difficult in some cases. Identification of characteristic immunohistochemical phenotypes of plasma cells in such cases could be useful in the differential diagnosis between the different entities in this group of diseases.
Previous studies have shown that assessment of immunoglobulin subclasses in plasma cells by immunohistochemistry may be useful in the histopathologic evaluation of autoimmune liver diseases. However, further studies are necessary in order to validate the diagnostic utility of IgM and IgG immunohistochemistry in this specific scenario.
The histopathologic evaluation of autoimmune liver diseases has relied almost exclusively on morphologic features seen on routine stains. In this study, the authors describe the potential application of IgM and IgG immunohistochemistry in the setting of autoimmune liver diseases, especially in the differential diagnosis of PBC from other conditions. The authors have demonstrated that an IgM-predominant plasmacytic infiltrate is typical of PBC but uncommon in other forms of chronic liver disease.
The characterization of plasma cells in liver biopsies of autoimmune liver disease patients by IgM and IgG immunohistochemistry may be very useful in selected cases and may serve as an adjunct method to conventional histopathologic evaluation.
Autoimmune liver disease refers to a group of chronic liver conditions that includes autoimmune hepatitis, primary biliary cirrhosis, and primary sclerosing cholangitis.
The authors have drawn attention to the preponderance of IgM-positive plasma cells in liver biopsies of patients with PBC, thereby providing a tool for differentiating PBC from a series of other liver conditions.
Peer reviewer: Shmuel Odes, MD, Professor, Head of the Inflammatory Bowel Diseases Unit, Department of Gastroenterology and Hepatology, Soroka University Hospital, Faculty of Health Sciences, Ben Gurion University of the Negev, PO Box 151, Beer Sheva 84101, Israel
S- Editor Wang YR L- Editor O’Neill M E- Editor Ma WH
| 1. | Washington MK. Autoimmune liver disease: overlap and outliers. Mod Pathol. 2007;20 Suppl 1:S15-S30. [Cited in This Article: ] |
| 2. | Ludwig J. The pathology of primary biliary cirrhosis and autoimmune cholangitis. Baillieres Best Pract Res Clin Gastroenterol. 2000;14:601-613. [Cited in This Article: ] |
| 3. | Talwalkar JA, Lindor KD. Primary biliary cirrhosis. Lancet. 2003;362:53-61. [Cited in This Article: ] |
| 4. | Czaja AJ. Autoimmune hepatitis. Clin Liver Dis. 2002;6:xi-xii. [Cited in This Article: ] |
| 5. | Krawitt EL. Autoimmune hepatitis. N Engl J Med. 2006;354:54-66. [Cited in This Article: ] |
| 6. | Wiesner RH, LaRusso NF. Clinicopathologic features of the syndrome of primary sclerosing cholangitis. Gastroenterology. 1980;79:200-206. [Cited in This Article: ] |
| 7. | Milne DS, Horne CH. Immunoglobulin classes in biopsies of autoimmune liver disease. Histopathology. 1990;16:283-286. [Cited in This Article: ] |
| 8. | Van Spreeuwel JP, Van Gorp LH, Nadorp JH, Janssens AR, Lindeman J, Meijer CJ. Immunoglobulin-containing cells in liver biopsies of patients with chronic hepatitis and primary biliary cirrhosis. Histopathology. 1984;8:559-566. [Cited in This Article: ] |
| 9. | Daniels JA, Torbenson M, Anders RA, Boitnott JK. Immunostaining of plasma cells in primary biliary cirrhosis. Am J Clin Pathol. 2009;131:243-249. [Cited in This Article: ] |
| 10. | Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet VJ. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929-38. [Cited in This Article: ] |
| 11. | Agresti A, Coull B. Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat. 1998;52:119-126. [Cited in This Article: ] |
| 12. | Czaja AJ, Carpenter HA. Autoimmune hepatitis with incidental histologic features of bile duct injury. Hepatology. 2001;34:659-665. [Cited in This Article: ] |
| 13. | Bach N, Thung SN, Schaffner F. The histological features of chronic hepatitis C and autoimmune chronic hepatitis: a comparative analysis. Hepatology. 1992;15:572-577. [Cited in This Article: ] |
| 14. | Zen Y, Harada K, Sasaki M, Tsuneyama K, Matsui K, Haratake J, Sakisaka S, Maeyama S, Yamamoto K, Nakano M. Are bile duct lesions of primary biliary cirrhosis distinguishable from those of autoimmune hepatitis and chronic viral hepatitis? Interobserver histological agreement on trimmed bile ducts. J Gastroenterol. 2005;40:164-170. [Cited in This Article: ] |
| 15. | Krawitt EL. Autoimmune hepatitis. N Engl J Med. 2006;354:54-66. [Cited in This Article: ] |