Published online Sep 21, 2010. doi: 10.3748/wjg.v16.i35.4483
Revised: June 4, 2010
Accepted: June 11, 2010
Published online: September 21, 2010
AIM: To investigate the anti-tumor effects of paeonol in gastric cancer cell proliferation and apoptosis in vitro and in vivo.
METHODS: Murine gastric cancer cell line mouse forestomach carcinoma (MFC) or human gastric cancer cell line SGC-7901 was cultured in the presence or absence of paeonol. Cell proliferation was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, and cell cycle and apoptosis by flow cytometry and TUNEL staining. Tumor growth after subcutaneous implantation of MFC cells in mice was monitored, and the effects of treatment with paeonol were determined.
RESULTS: In vitro, paeonol caused dose-dependent inhibition on cell proliferation and induced apoptosis. Cell cycle analysis revealed a decreased proportion of cells in G0/G1 phase, with arrest at S. Paeonol treatment in gastric cancer cell line MFC and SGC-790 cells significantly reduced the expression of Bcl-2 and increased the expression of Bax in a concentration-related manner. Administration of paeonol to MFC tumor-bearing mice significantly lowered the tumor growth and caused tumor regression.
CONCLUSION: Paeonol has significantly growth-inhibitory and apoptosis-inducing effects in gastric cancer cells both in vitro and in vivo.
-
Citation: Li N, Fan LL, Sun GP, Wan XA, Wang ZG, Wu Q, Wang H. Paeonol inhibits tumor growth in gastric cancer
in vitro andin vivo . World J Gastroenterol 2010; 16(35): 4483-4490 - URL: https://www.wjgnet.com/1007-9327/full/v16/i35/4483.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i35.4483
Gastric cancer is the fourth most common cancer and the second leading cause of cancer-related death in the world. Gastric cancer represents roughly 2% (25 500) of all new cancer cases yearly in the United States[1,2]. However, gastric cancer is much more common in many parts of the world including China. With delayed symptoms, most of gastric cancer patients are often diagnosed at an advanced stage, and the 5-year survival remains less than 20%[1]. Surgical resection remains the only curative treatment. But with high rates of local recurrence and systemic spread (including peritoneal metastases), attempts to improve the outcomes of this disease have incorporated the use of adjuvant chemotherapy. However, the high incidence rate of severe side effects of the drugs used in the chemotherapy limits its therapeutic results. Thus, there has been growing interest in developing more effective chemotherapeutic agents for gastric cancer.
Natural products are potential sources of novel anticancer drugs that may be applicable to cancer chemotherapy. The anti-tumor potential of components from Chinese herbal medicines has been of great interest. With thousands of years of experience, Chinese herbal medicines are considered as a rich source of new therapeutic agents. Paeonol is the main component of a Chinese herbal medicine prepared from the root bark of Paeonia moutan. It has various pharmacological and physiological effects such as sedation, hypnosis, antipyresis, analgesia, anti-oxidation, anti-inflammation, anti-bacteria, immuno-regulation and anti-tumor effects[3-6]. Our previous studies showed that paeonol inhibited the proliferation of different tumor cell lines in vitro and suppressed tumor growth in xenograft tumor models in vivo[7-9].
In this study, we found for the first time that paeonol exerted the impressive biological effects in blocking the growth of gastric cancer cells in vitro, and in an allograft mouse model as well. We also observed that the growth inhibitory effects of paeonol were mediated by induction of apoptotic cell death via increasing the Bax/Bcl-2 ratio. The results of this investigation may provide a scientific explanation for the traditional application of this herbal medicine in gastric cancer therapy.
The compound paeonol with 98% purity was purchased from Tianshi Pharmaceutical Factory of Tongling (Cat. No. 010521) (Tonglin, Anhui, China). For in vivo experiments, paeonol was suspended in 0.5% sodium carboxymethylcellulose (CMC-Na) to the desired concentrations. In vitro, the cells were seeded and treated with the control vehicle (0.1% DMSO), or different concentrations of paeonol for the indicated time. 5-fluorouracil (5-FU) injection was obtained from Shanghai Haipu Pharmaceutical Factory, China (Cat. No. 031109) (Shanghai, China). RPMI-1640 medium was from GIBCO BRL, Life Technologies Inc. (Grand Island, New York, USA), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and acridine orange (AO) were from Sigma Co. (St. Louis, MO, USA). DNA-Prep-Reagents Kit was bought from Beckman Coulter Co. (Miami, FL, USA. Cat. No. 760279K). Rabbit polyclonal antibodies against human Bcl-2 and Bax were all purchased from Lab Vision Corporation (Fremont, California, USA) and streptavidin-biotin-peroxidase (S-P) reagents kit was obtained from Fuzhou Maxim Biotech, Ltd. (Fuzhou, Fujian, China).
Murine gastric cancer cell line mouse forestomach carcinoma (MFC) and human gastric cancer cell line SGC-7901 were purchased from Shanghai cell bank, Chinese Academy of Sciences. Six-week-old male healthy Kunming mice were obtained from the Animal Department of Anhui Medical University, and were maintained on a 12 h light/12 h dark cycle from 6:00 AM to 18:00 PM under a regulated environment (20 ± 1°C). Animals were housed in plastic cages with free access to food and water. All procedures followed the guidelines for use of animals in research set by the Association of Laboratory Animal Sciences and the Center for Laboratory Animal Sciences at Anhui Medical University.
Cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and incubated at 37°C in humid atmosphere with 5% CO2.
Cell proliferation assay: Cells were cultured in 96-well plates at a density of 1 × 104 cells with 100 μL medium overnight, and then treated with various concentrations of drugs. After 44 h of drug exposure, MTT solution (5 mg/mL) was added to the plates. The cells were incubated at 37°C for another 4 h. Formazine was solved in 150 μL/well DMSO and absorbance was detected at 490 nm using ELx 800 Strip reader (Bio-Tek, USA). Each drug concentration was tested in five replicates from which the mean, standard deviation and coefficient of variation were calculated. The drug concentration capable of inhibiting cell growth by 50% relative to untreated controls was calculated as follows: Inhibition percentage (%) = (1-A490 nm of experimental well)/A490 nm of control well. The median inhibitory concentration (IC50) (defined as the drug concentration inhibiting cell growth by 50%) was calculated from the dose-response curves.
Cell cycle analysis: Cells were seeded (2 × 105 cells/well) in 6-well plates, treated with or without paeonol for 48 h and then trypsinized, washed in phosphate-buffered saline and fixed in ice-cold 70% ethanol-phosphate-buffered saline. DNA was labeled with propidium iodide. Cells were sorted by flow cytometry, and cell cycle profiles were determined using Mcycle software (Beckman Coulter, Fullerton, CA).
Apoptosis assay: Apoptosis was analyzed by two methods. First, sub-G1 DNA analysis was conducted by flow cytometry. Cells were cultured in 6-well plates. Non-adherent cells were removed by gentle washing, and the medium was removed and replaced with fresh medium containing paeonol at the desired concentrations. After 24 h of drug exposure, cells were collected and centrifuged at 2000 r/min in a 15 mL tube for 5 min, fixed with 70% ethanol for 4 h, washed twice with phosphate buffered saline (PBS), and then 500 μL propidium iodide (PI) staining buffer was added in the dark at room temperature for 30 min according to the procedure of DNA-Prep Coulter reagents kit. A minimum of 1 × 105 cells treated for each group were analyzed using an EPICS XL-MCL Coulter counter. Second, acridine orange fluorescence staining was performed as follows. Cells were seeded overnight in 6-well plates containing cover slips. After incubation with the drug for 48 h, the cover slips were washed twice with PBS, fixed with 95% ethanol for 15 min, acidified with 1% acetic acid for 30 s, dyed with 0.1 mg/mL acridine orange for 10 min, differentiated with 0.1 mol/L CaCl2 for 2 min, and then washed with PBS for 3 times. The cover slips were sealed and observed under fluorescence microscope.
Apoptotic cells in sections of mouse tumor tissues were detected using an in situ apoptosis detection kit (Promega) according to the instructions of the manufacturer. Cells were visualized under light microscope.
Kunming mice were injected with MFC cells (ip), and the second generation of the cells from the mice with ascites was collected under aseptic condition. The collected cells were diluted in normal saline to a concentration of 1 × 1010 cells/L and injected into the right upper flank regions of each mouse. After 7 d, they were weighed and randomized into 5 groups (n = 12-20): A negative control group was given an equal amount of normal saline (intragastric administration), a positive control group was treated with intraperitoneal injection of 5-FU (2.5 mg/kg per day) and three paeonol groups were intragastrically administrated with paeonol (100, 200 and 400 mg/kg per day). Drugs were administrated daily by gavage for 11 d. In addition, we used the mice without tumor injection as normal groups. On day 12, all mice were weighed and killed, and the tumor was removed and weighed.
Cells were cultured in 6-well plates containing cover slips overnight. After exposure to drug for 24 h, the cover slips were washed twice with PBS, fixed with 4% paraformaldehyde solution for 25 min. The standard streptavidinbiotin-peroxidase method was used for immunohistochemical stain. Briefly, endogenous peroxidase activity was blocked by incubation in endogenous peroxidase blocking solution for 10 min followed by incubation with normal non-immune serum for 10 min at room temperature. Samples were incubated overnight at 4°C with primary antibodies against Bcl-2 and Bax, washed with PBS and incubated with biotinylated secondary anti-rabbit antibody for 30 min at 37°C. After washing, the samples were incubated with streptavidin-peroxidase at 37°C for 20 min. Diaminobenzidine/hydrogen peroxidase was used as a chromogen and samples were counterstained with hematoxylin. As negative control, PBS was used instead of primary antibody and other steps were followed in the same way.
The immunohistochemical results were quantitatively analyzed by a biological image analysis system which consists of Nikon ECLIPSE 80i biology microscope, Nikon Digital Camera DXM 1200F, ACT-1 version 2.63 software (Japan), and JEOA 801D morphologic biological image analysis software version 6.0 (Jie Da Technologies, Inc, China). Samples were observed in three randomly selected optical fields under microscopy (× 400) and the average optical density was measured.
Data were expressed as mean ± SD. Analysis of variance test was used for determining differences between groups, and P < 0.05 was regarded as statistically significant.
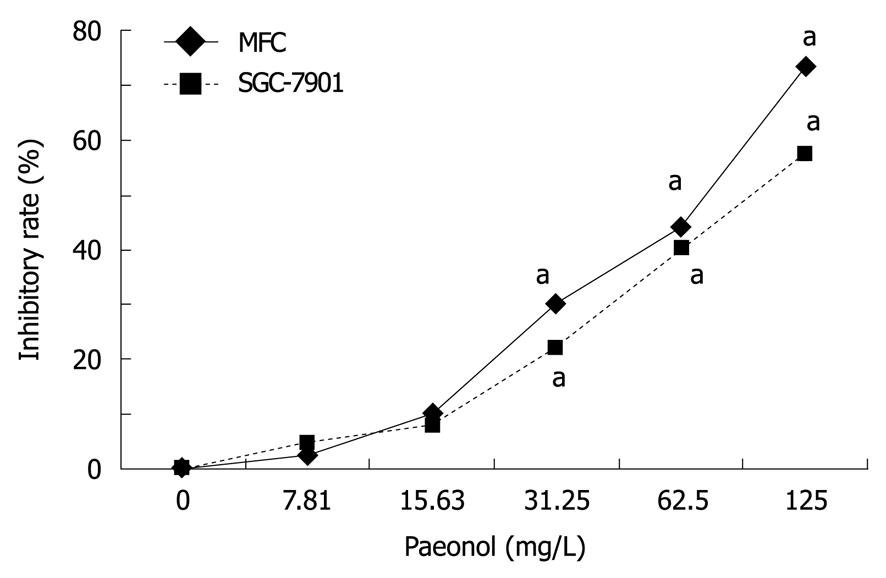
To evaluate the effects of paeonol on cell viability, gastric cancer cell line MFC or SGC-7901 cells were cultured with varying concentrations of paeonol. Exposure to paeonol for 48 h produced a dose-dependent suppression of cell proliferation in both MFC and SGC-790 cells (Figure 1). The IC50 of paeonol on MFC and SGC-790 cells were 60.10 and 82.60 mg/L, respectively.
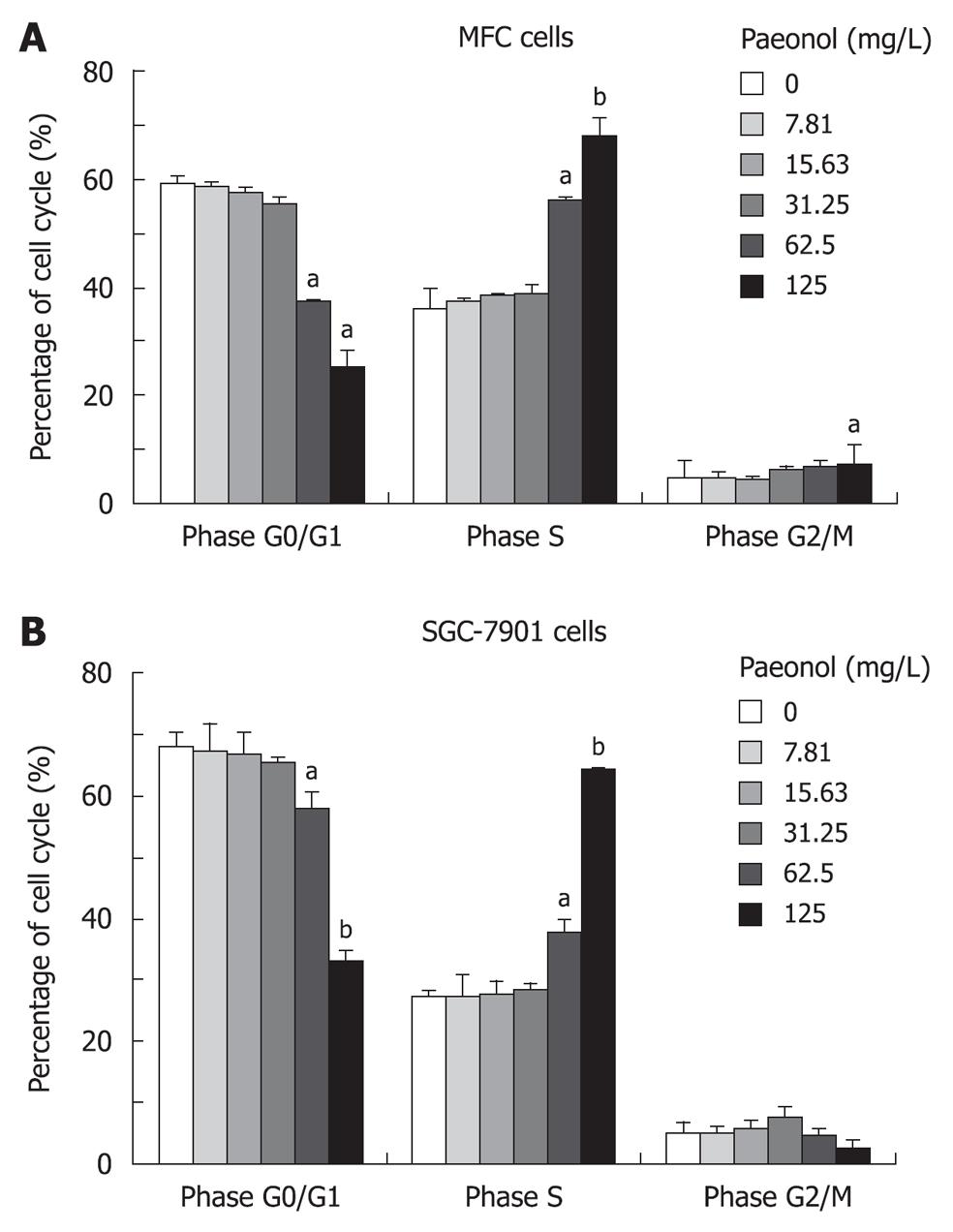
To determine whether paeonol decreased viability of cultured gastric cancer cells by inhibiting cell growth, we investigated the effect of paeonol on cell cycle distribution. After propidium iodide staining and flow cytometric analysis, paeonol treated gastric cancer cells revealed a dose-dependent increase accumulation in the number of cells in S phase at 24 h. Concomitant with this blocking, there was a dose-dependent decrease in the number of cells accumulating in the G0/G1 phase, and also a decrease in G2/M phase but only in MFC cell line, which is consistent with the finding that paeonol blocks the cell cycle at the S phase (Figure 2).
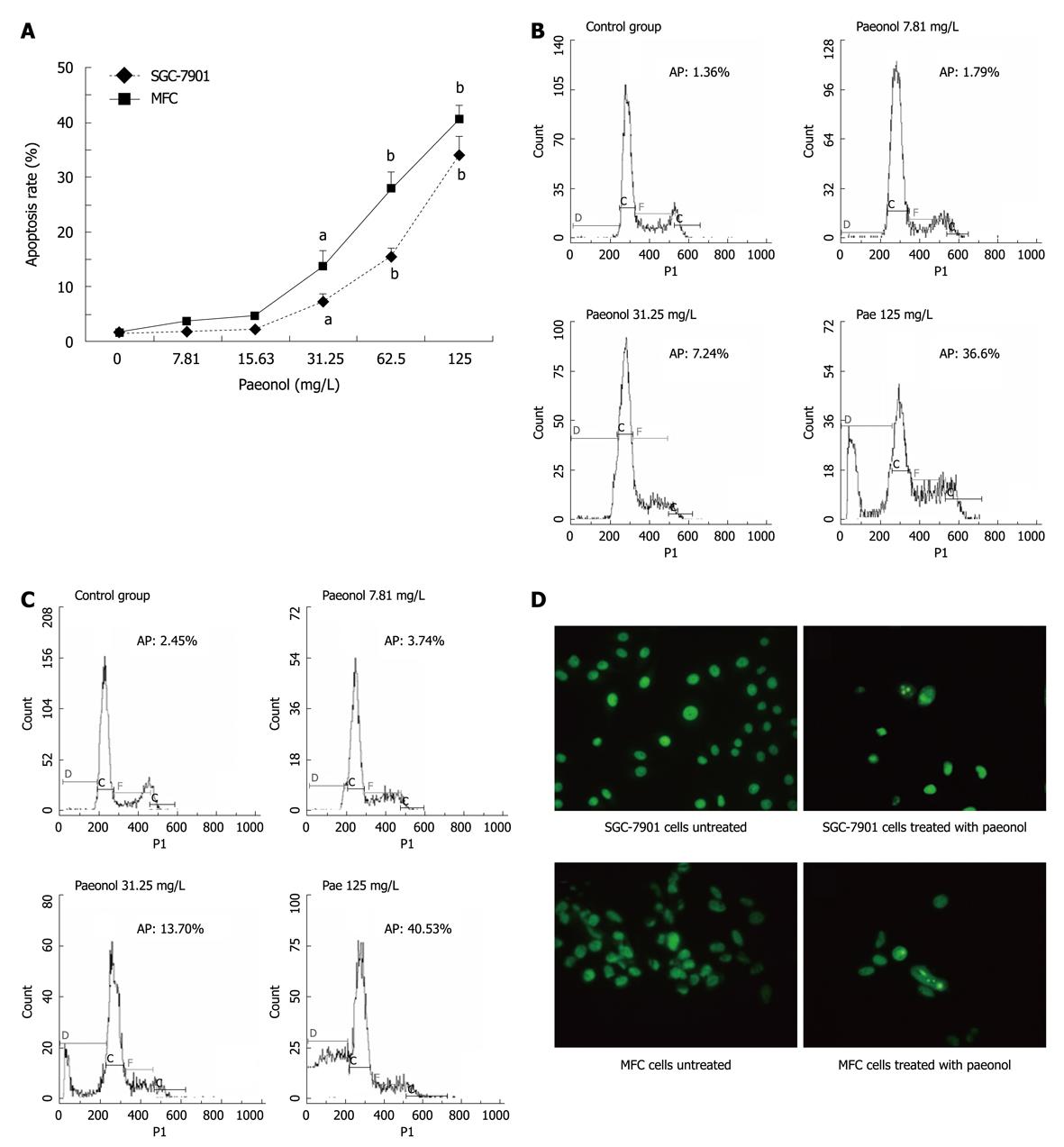
As arrest of cell cycle progression in tumor cells is usually associated with concomitant activation of cell apoptosis pathways, we checked the induction of apoptosis after paeonol-treated gastric cell cultures. By flow cytometric analysis, the number of cells with sub-G1 DNA content, which represents apoptotic cell population, was significantly increased in a concentration-dependent manner in both MFC and SGC-790 cells (Figure 3A-C) after 24 h paeonol treatment. Consistent with this finding, there was an increased number of apoptotic cells stained with acridine orange after paeonol treatment compared with untreated gastric cancer cells. The cells treated with paeonol for 48 h showed typically apoptotic changes, including reduction in cell volume, chromatin condensation and deformed and fragmented nuclei (Figure 3D).
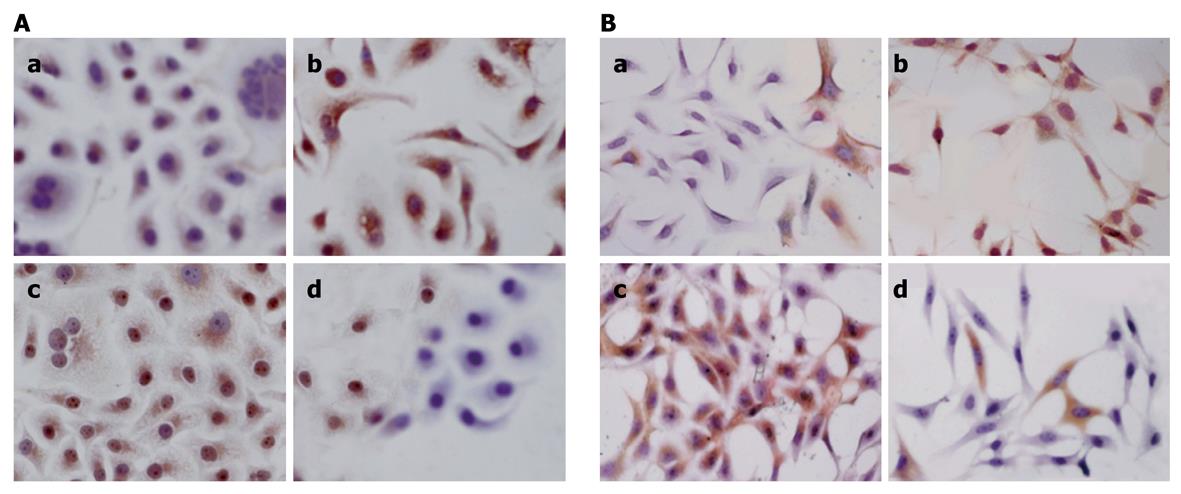
The streptavidin-peroxidase method was used to examine the expression of Bcl-2 and Bax. Positive Bcl-2 and Bax staining was identified by brown and yellow staining mainly in cytoplasm or membrane (Figure 4). Treatment of paeonol in gastric cancer cell line MFC and SGC-790 cells significantly reduced the number of Bcl-2 positive cells, with weak to moderate staining in a concentration-related manner while the number of Bax positive cells was increased in the paeonol-treated group in a concentration-related manner.
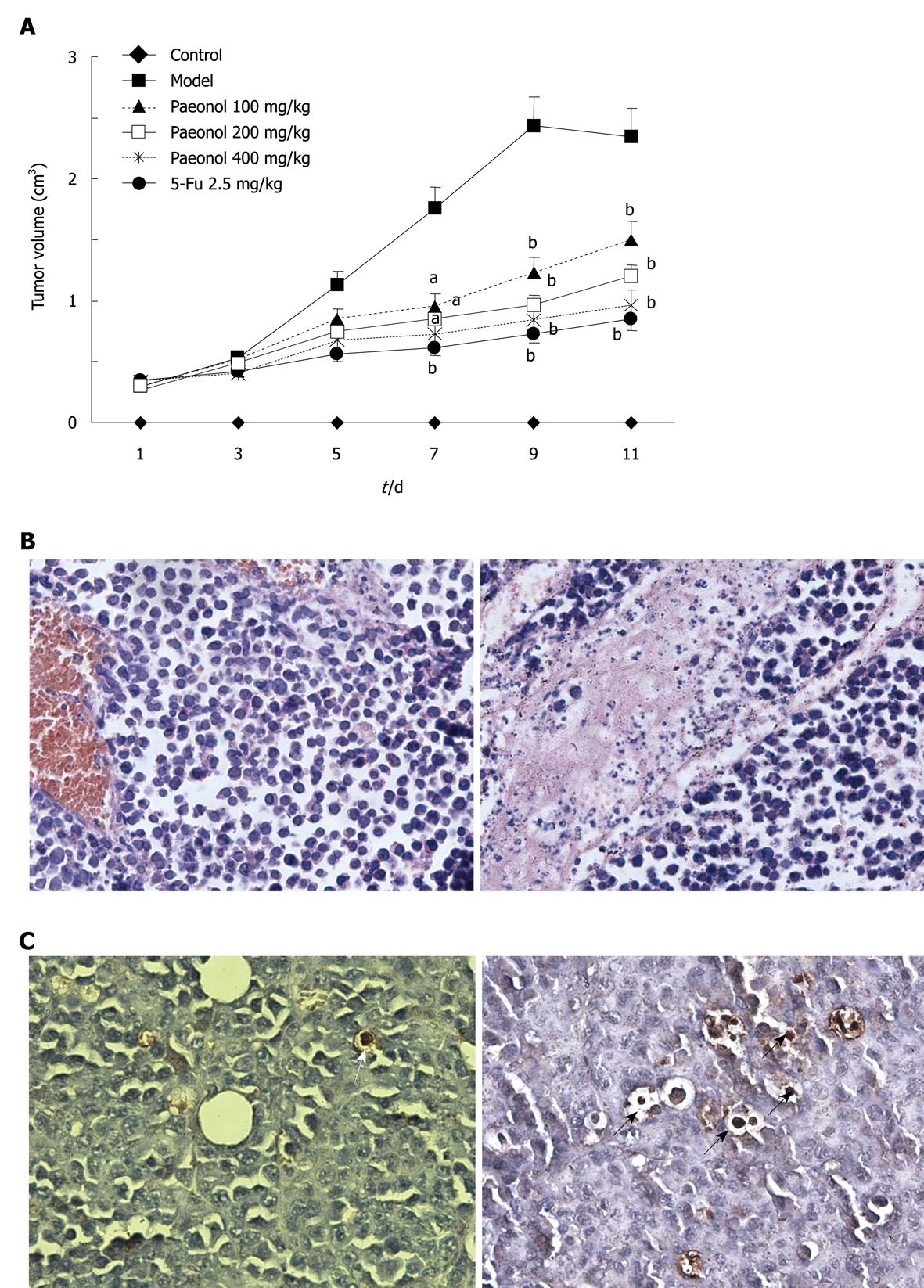
Based on the observed anti-proliferative and proapoptotic effects of paeonol in gastric cancer cell lines in vitro, we next tested whether paeonol treatment could affect growth of gastric cancer cells in vivo. For these studies, we established the experimental model of MFC tumor-bearing mice and then treated these animals with paeonol (100, 200 and 400 mg/kg per day, ip). We also used 5-FU (2.5 mg/kg per day, ip) as positive control. Most of the mice developed visible subcutaneous MFC tumors 7 d after MFC cell injection. The mice with visible subcutaneous MFC tumors were divided randomly into 5 groups. Paeonol or 5-FU treatment was started and lasted 11 d. The volume of tumor was calculated every 2 d (Figure 5A). When the experiment was terminated 11 d later, mice treated with paeonol (100, 200, and 400 mg/kg per day) had reduction in tumor growth by 37.55%, 51.62% and 54.15%, respectively compared with the model controls. The inhibitory rate of positive control group treated with 5-FU injection was 69.68%. Interestingly, 3/20 mice died in the model control group while 0/12 mice died in all paeonol groups, and 2/12 mice died in 5-FU group during the experiment, indicating no severe drug toxicity of paeonol under these doses.
Histological analysis of MFC tumors from untreated mice revealed poorly differentiated, infiltrating gastric cancer with high mitosis rates. In contrast, tumors from mice receiving paeonol showed few mitotic cells (Figure 5B). Compared with the control tumors, mitotic rates were significantly lower in tumors from mice receiving paeonol.
We found that paeonol not only decreased the proliferative rate of subcutaneously implanted gastric cancer, but also significantly increased the rate of apoptosis. Thus, while MFC tumors from untreated mice showed almost no TUNEL staining (Figure 5C), tumor cells from mice receiving paeonol showed frequent condensed nuclei and cytoplasmic shrinkage.
Paeonol is a natural product extracted from the root bark of Paeonia Suffruticosa Andrew[10]. It is a white needle crystal with a relatively low-melting point of 51-52°C. The molecular formula of paeonol is C9H10O3 and the molecular weight is 166.18[11]. Our previous study demonstrated the anti-neoplastic activity of paeonol in various cell lines, such as human erythromyeloid cell line K562, breast cancer gene cell line T6-17, human hepatoma cell line Bel-7404, HepG2 and SMMC-7721, cervical cancer cell line Hela, and human colorectal cancer cell line HT-29[7-9,12,13]. In this study, we found that paeonol inhibited the proliferation of two kinds of gastric cancer cell lines in a dose-dependent manner in vitro. Moreover, treatment of MFC tumor bearing mice with various doses of paeonol (100, 200 and 400 mg/kg per day) significantly suppressed the tumor growth. Our results indicated that the anti-tumor effect of paeonol was related to induction of apoptosis.
We analyzed the effects of paeonol in two gastric cancer cell lines in vitro. In both MFC and SGC-7901 cells, paeonol caused dose-dependent growth inhibition and arrest, and similar results have been observed in other cancer cell lines in our previous studies[8,9]. Flow cytometric analysis of the effects of paeonol on the cell cycle in treated gastric cancer cells revealed a dose-dependent decrease in cell proliferation, a concomitant and proportionate accumulation of cells in phase S, and an increase of cells in the sub-G1 fraction. The latter finding indicates increased apoptosis, a finding confirmed by acridine orange fluorescence staining. These results in vitro suggested that paeonol might have anti-proliferative effects and arrest the cell cycle, leading to cancer cell apoptosis.
We also investigated the underlying mechanisms for the apoptosis induction of gastric cancer cells. Apoptosis is a physiological process in controlling cell number and proliferation that helps maintain the homeostasis of multi-cellular organisms[14,15]. The hypothesis that failure to undergo apoptosis contributes to the development of resistance to anticancer agents has been the subject of extensive research[16]. Therefore, agents that facilitate apoptosis should improve therapeutic efficacy. In the present study, the cells treated with paeonol showed morphological alterations typical of the apoptotic process, including reduction in cell volume, chromatin condensation, deformed and fragmented nuclei, and so on. Similarly, apoptotic peak appeared before G1 phase after paeonol treatment, which resulted from the internucleosomal degradation of DNA. Although understanding of the detailed signaling pathways that trigger apoptosis is incomplete, this process is controlled by a number of complex proteins. The Bcl-2 family proteins are key regulators of apoptosis and are over-expressed in many malignancies[17]. To examine the mechanism of apoptosis, we examined the expression of Bcl-2 protein family, which is an important regulator of apoptosis. We found that paeonol decreased the expression of Bcl-2 and increased the expression of Bax, the ratio of Bcl-2/Bax decreased correspondingly, which could be an important mechanism contributing to the induction of tumor cell apoptosis by paeonol.
Having observed substantial suppression of gastric cancer cell growth by paeonol treatment in vitro, we conducted experiments designed to test the potential for paeonol to exert protective effects against gastric cancer in vivo. It was recognized that the model used in this study, i.e. mice injected with gastric cancer cells subcutaneously, is an artificial one and is not necessarily equivalent to the stepwise development of gastric cancer in human. Nonetheless, the results here showed that treatment with different doses of paeonol inhibited tumor growth in the tumor-bearing mice.
In conclusion, the results obtained in the present study indicate that paeonol has significantly growth-inhibitory and apoptosis-inducing effects in gastric cancer cells both in vitro and in vivo. These promising data provide a rationale for further exploration of paeonol as a therapeutic agent for gastric cancer.
Gastric cancer is a common tumor which seriously threatens the human health worldwide. Currently, a variety of cytotoxic and anti-proliferative agents have been tested in gastric cancer. However, the high rate of severe side effects of these drugs limits their therapeutic results. Thus, there is a critical need to develop more strategies for chemotherapy of gastric cancer.
Chinese herbal medicines are now attracting great attention in the world, which also show promising effects in gastric cancer treatment. Paeonol, a natural product extracted from the root of Paeonia Suffruticosa Andrew, has shown anti-neoplastic activities in both cell lines and animal models.
This is the first report on the anti-proliferation, induction of apoptosis by paeonol in gastric cancer in vitro and in vivo.
Paeonol might be useful as a new agent in gastric cancer.
Paeonol is isolated from the herb Pycnostelma paniculatum (Bunge) K.S., and the root of the plant Paeonia Suffruticosa Andrew. It is a white needle crystal with a relatively low melting point of 51-52°C. The molecular formula of paeonol is C9H10O3 and the molecular weight is 166.18.
The paper describes the effects of paenol on cell lines and a mouse model of gastric tumor. The data have a potential interest in the field of cancer therapy. It would be interesting to have some pieces of information on the chemical structure of paeonol and to discuss the possible molecular mechanism of the action of this substance.
Peer reviewers: Shingo Tsuji, MD, PhD, AGAF, Professor, Department of Internal Medicine and Therapeutics, Osaka University Graduate School of Medicine(A8), 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan; Salvatore Auricchio, MD, PhD, Professor, Scientific Director of European Laboratory for the Investigation of Food-Induced Diseases, University Federico II, Via S. Pansini 5, I-80131 Naples, Italy; Dr. Annie Schmid-Alliana, French Institute of Health and Medical Research Unit 576, Hôpital de l’Archet 1, 151 Route de aint Antoine de Ginestière, BP3079, 06202 Nice cedex 3, France
S- Editor Wang JL L- Editor Ma JY E- Editor Lin YP
| 1. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96. [Cited in This Article: ] |
| 2. | Khushalani N. Cancer of the esophagus and stomach. Mayo Clin Proc. 2008;83:712-722. [Cited in This Article: ] |
| 3. | Li H, Dai M, Jia W. Paeonol attenuates high-fat-diet-induced atherosclerosis in rabbits by anti-inflammatory activity. Planta Med. 2009;75:7-11. [Cited in This Article: ] |
| 4. | Chunhu Z, Suiyu H, Meiqun C, Guilin X, Yunhui L. Antiproliferative and apoptotic effects of paeonol on human hepatocellular carcinoma cells. Anticancer Drugs. 2008;19:401-409. [Cited in This Article: ] |
| 5. | Tsai HY, Lin HY, Fong YC, Wu JB, Chen YF, Tsuzuki M, Tang CH. Paeonol inhibits RANKL-induced osteoclastogenesis by inhibiting ERK, p38 and NF-kappaB pathway. Eur J Pharmacol. 2008;588:124-133. [Cited in This Article: ] |
| 6. | Zhang LH, Xiao PG, Huang Y. [Recent progresses in pharmacological and clinical studies of paeonol]. Zhongguo Zhongxiyi Jiehe Zazhi. 1996;16:187-190. [Cited in This Article: ] |
| 7. | Wan XA, Sun GP, Wang H, Xu SP, Wang ZG, Liu SH. Synergistic effect of paeonol and cisplatin on oesophageal cancer cell lines. Dig Liver Dis. 2008;40:531-539. [Cited in This Article: ] |
| 8. | Sun GP, Wang H, Xu SP, Shen YX, Wu Q, Chen ZD, Wei W. Anti-tumor effects of paeonol in a HepA-hepatoma bearing mouse model via induction of tumor cell apoptosis and stimulation of IL-2 and TNF-alpha production. Eur J Pharmacol. 2008;584:246-252. [Cited in This Article: ] |
| 9. | Sun GP, Wan X, Xu SP, Wang H, Liu SH, Wang ZG. Antiproliferation and apoptosis induction of paeonol in human esophageal cancer cell lines. Dis Esophagus. 2008;21:723-729. [Cited in This Article: ] |
| 10. | Riley CM, Ren TC. Simple method for the determination of paeonol in human and rabbit plasma by high-performance liquid chromatography using solid-phase extraction and ultraviolet detection. J Chromatogr. 1989;489:432-437. [Cited in This Article: ] |
| 11. | Mimura K, Baba S. Determination of paeonol metabolites in man by the use of stable isotopes. Chem Pharm Bull (Tokyo). 1981;29:2043-2050. [Cited in This Article: ] |
| 12. | Xu SP, Sun GP, Shen YX, Peng WR, Wang H, Wei W. Synergistic effect of combining paeonol and cisplatin on apoptotic induction of human hepatoma cell lines. Acta Pharmacol Sin. 2007;28:869-878. [Cited in This Article: ] |
| 13. | Xu SP, Sun GP, Shen YX, Wei W, Peng WR, Wang H. Antiproliferation and apoptosis induction of paeonol in HepG2 cells. World J Gastroenterol. 2007;13:250-256. [Cited in This Article: ] |
| 14. | LaCasse EC, Mahoney DJ, Cheung HH, Plenchette S, Baird S, Korneluk RG. IAP-targeted therapies for cancer. Oncogene. 2008;27:6252-6275. [Cited in This Article: ] |
| 15. | Wang S. The promise of cancer therapeutics targeting the TNF-related apoptosis-inducing ligand and TRAIL receptor pathway. Oncogene. 2008;27:6207-6215. [Cited in This Article: ] |
| 16. | Jagani Z, Khosravi-Far R. Cancer stem cells and impaired apoptosis. Adv Exp Med Biol. 2008;615:331-344. [Cited in This Article: ] |
| 17. | Thomadaki H, Scorilas A. BCL2 family of apoptosis-related genes: functions and clinical implications in cancer. Crit Rev Clin Lab Sci. 2006;43:1-67. [Cited in This Article: ] |