Published online Sep 7, 2010. doi: 10.3748/wjg.v16.i33.4180
Revised: May 20, 2010
Accepted: May 27, 2010
Published online: September 7, 2010
AIM: To evaluate a new hemostatic method using hemostatic forceps to prevent perforation and perioperative hemorrhage during colonic endoscopic submucosal dissection (ESD).
METHODS: We studied 250 cases, in which ESD for colorectal tumors was performed at the Kyoto Prefectural University of Medicine or Nara City Hospital between 2005 and 2010. We developed a new hemostatic method using hemostatic forceps in December 2008 for the efficient treatment of submucosal thick vessels. ESD was performed on 126 cases after adoption of the new method (the adopted group) and the new method was performed on 102 of these cases. ESD was performed on 124 cases before the adoption of the new method (the unadopted group). The details of the new method are as follows: firstly, a vessel was coagulated using the hemostatic forceps in the soft coagulation mode according to the standard procedure, and the coagulated vessel was removed using the forceps in the “endocut” mode without perioperative hemorrhage. Secondly, the partial surrounding submucosa was dissected using the forceps in the endocut mode. In the current study, we evaluated the efficacy of this method.
RESULTS: Coagulated vessels were successfully removed using the hemostatic forceps in all 102 cases without severe perioperative hemorrhage. Moderate perioperative hemorrhage occurred in five cases (4.9%); however, it was stopped by immediately reuse of the hemostatic forceps. The partial surrounding submucosa was dissected using the forceps in all 102 cases. In the adopted group, the median operation time was 105 min. The proportion of endoscopic en bloc resection was 92.8% (P < 0.01) compared to 80.6% in the unadopted group. The postoperative hemorrhage and perforation rates were 2.3% and 2.3%. The rate of perforation was significantly lower than that in the unadopted group (9.6%, P < 0.01). We evaluated the ease of use of this method by allowing our three trainees to performed ESD on 46 cases, which were accomplished without any severe hemorrhage.
CONCLUSION: The new method effectively treated submucosal thick vessels and shows promise for the prevention of perforation and perioperative hemorrhage in colonic ESD.
- Citation: Yoshida N, Naito Y, Kugai M, Inoue K, Wakabayashi N, Yagi N, Yanagisawa A, Yoshikawa T. Efficient hemostatic method for endoscopic submucosal dissection of colorectal tumors. World J Gastroenterol 2010; 16(33): 4180-4186
- URL: https://www.wjgnet.com/1007-9327/full/v16/i33/4180.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i33.4180
Endoscopic submucosal dissection (ESD) has emerged as a standard therapy for the treatment of large gastric tumors in Japan[1]. However, ESD is not a standard procedure for treating colorectal tumors due to technical difficulties[2-10] that arise from the winding nature and thin wall of the colon. Perioperative hemorrhage is a frequent complication of ESD for gastric and colorectal tumors. Severe hemorrhage hampers the speedy dissection of the submucosa and can increase the risk of perforation. Therefore, submucosal thick vessels detected during ESD are clamped and coagulated using hemostatic forceps to prevent hemorrhage. However, this causes coagulation in the surrounding submucosa. Moreover, coagulation of the submucosal vessel and surrounding submucosa decreases submucosal elevation and hinders speedy submucosal dissection. If the submucosa is not properly elevated, the risk of perforation, which is the most severe complication associated with ESD for colorectal tumors, significantly increases. The frequency of perforation has been reported to be in the range of 1.5%-10.4%[2-10] in ESD for colorectal tumors. In the current study, we developed a new hemostatic method using hemostatic forceps for the treatment of submucosal thick vessels. We assessed the safety and efficacy of this method with respect to the prevention of perforation and perioperative hemorrhage in ESD for colorectal tumors.
We studied 250 colorectal tumor cases that were treated with ESD at the Department of Molecular Gastroenterology and Hepatology, Kyoto Prefectural University of Medicine or at the Center for Digestive and Liver Disease, Nara City Hospital, between April 2005 and March 2010. We developed a new hemostatic method using a Coagrasper (Olympus Medical System Co., Tokyo, Japan) in December 2008 and performed ESD on 126 cases, which was defined as the adopted group. The new method was used to remove submucosal thick vessels and to dissect the partial submucosa in 102 of these cases. We had performed ESD on 124 cases before the adoption of the new method, thus this group was defined as the unadopted group. We used hemostatic forceps in 96 cases to coagulate the thick vessel according to the standard procedure in the unadopted group.
ESD was indicated if the colorectal tumor measured more than 20 mm in diameter, had a protruding or superficial, but not pedunculated, morphology, and the carcinoma was suspected to have invaded the superficial submucosa, as determined with endoscopic findings or histopathological examinations as previously reported[8]. The method of ESD was approved by the institutional review boards in our institutions. The patients provided their written informed consent for undergoing ESD. Two endoscopic surgeons specializing in endoscopic colorectal treatment and three trainees performed ESD on all the patients.
We used a lower gastrointestinal endoscope with a single-channel endoscope (PCF-Q260AI; Olympus Medical System Co., Tokyo, Japan or EC-590MP; Fuji Film Medical, Tokyo, Japan) and an automatically controlled high-frequency generator (VIO300D; Erbe Elektromedizin Ltd., Tubingen, Germany). A sodium hyaluronate solution (MucoUp; Johnson & Johnson, Tokyo, Japan) and a glycerin solution (Glycerol; Chugai Pharmaceutical Co., Tokyo, Japan) were injected into the submucosa, according to a previously described method[11,12]. According to standard colonoscopic examinations, an isotonic polyethylene glycol electrolyte solution (Niflec; Ajinomoto Pharma Co., Ltd., Tokyo, Japan) was used to achieve good bowel preparation. We used an obtuse-edged, short-tipped Flush knife (Fuji Film Medical, Tokyo, Japan) that can also be used for injections[9,10].
ESD was performed as previously reported[5-7]. In brief, the submucosa below the tumor was injected with a sodium hyaluronate solution and glycerin using a 25 G needle (TOP Co., Tokyo, Japan). Subsequently, a partial mucosal incision was performed on the anal side of the tumor. Thereafter, the submucosa below the tumor was dissected from the anal side. A circumferential mucosal incision was added appropriately through submucosal dissection and finally, en bloc resection of the tumor was accomplished.
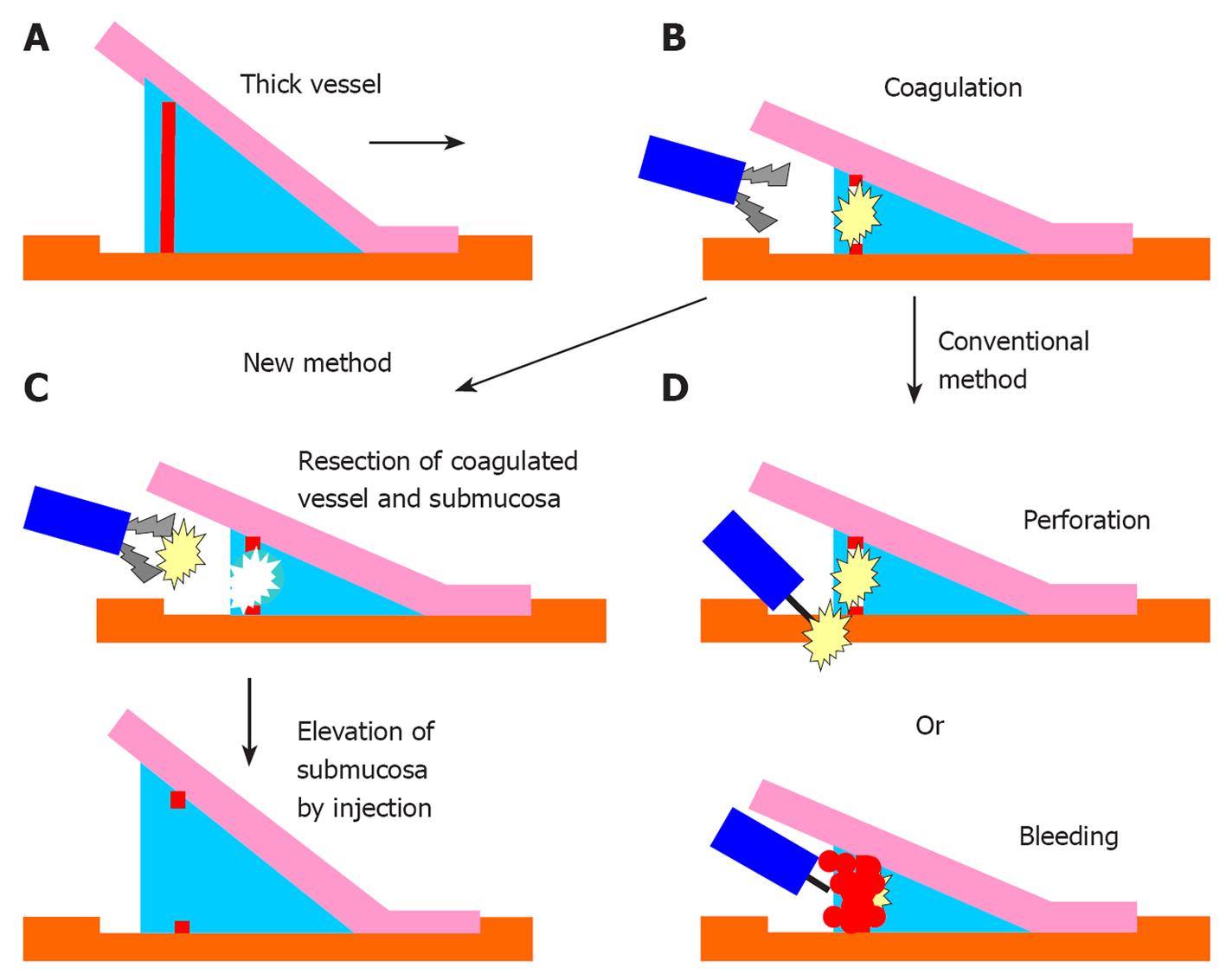
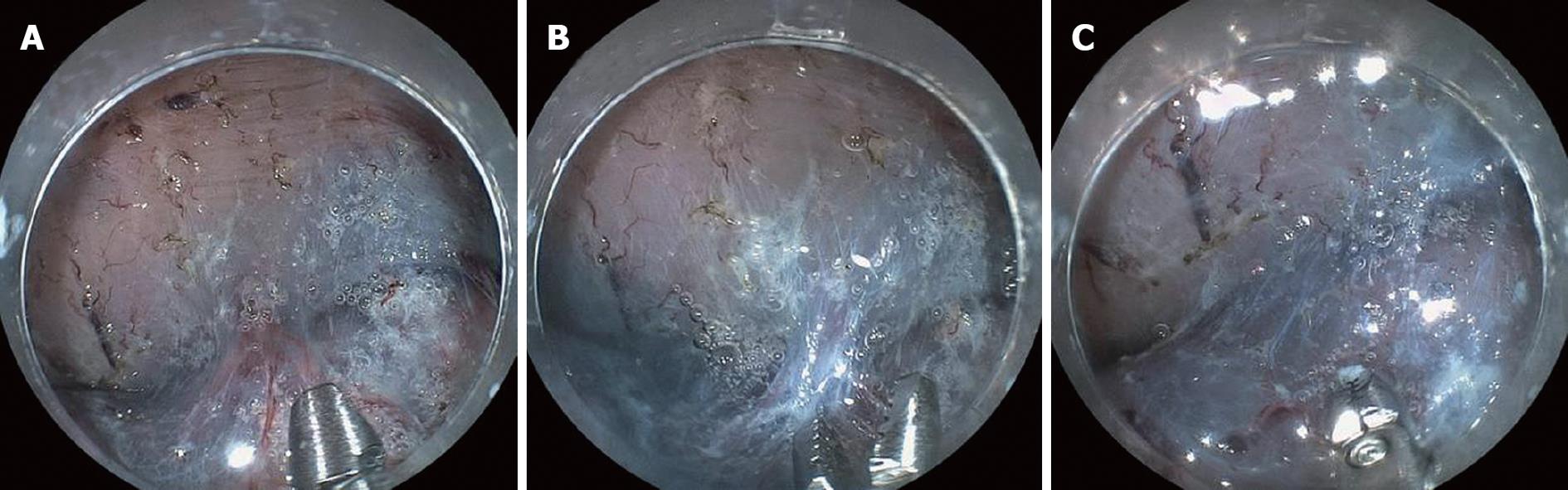
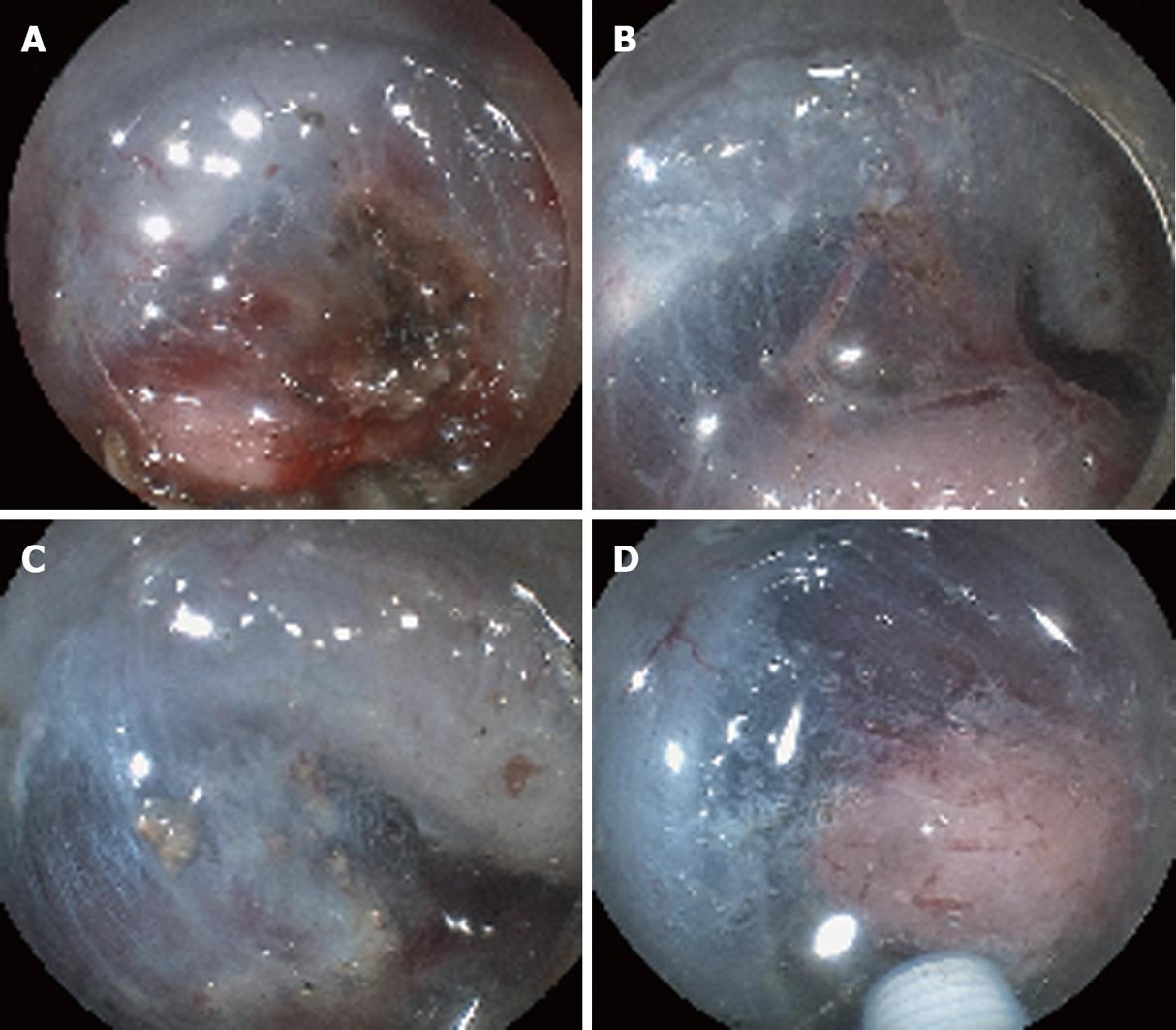
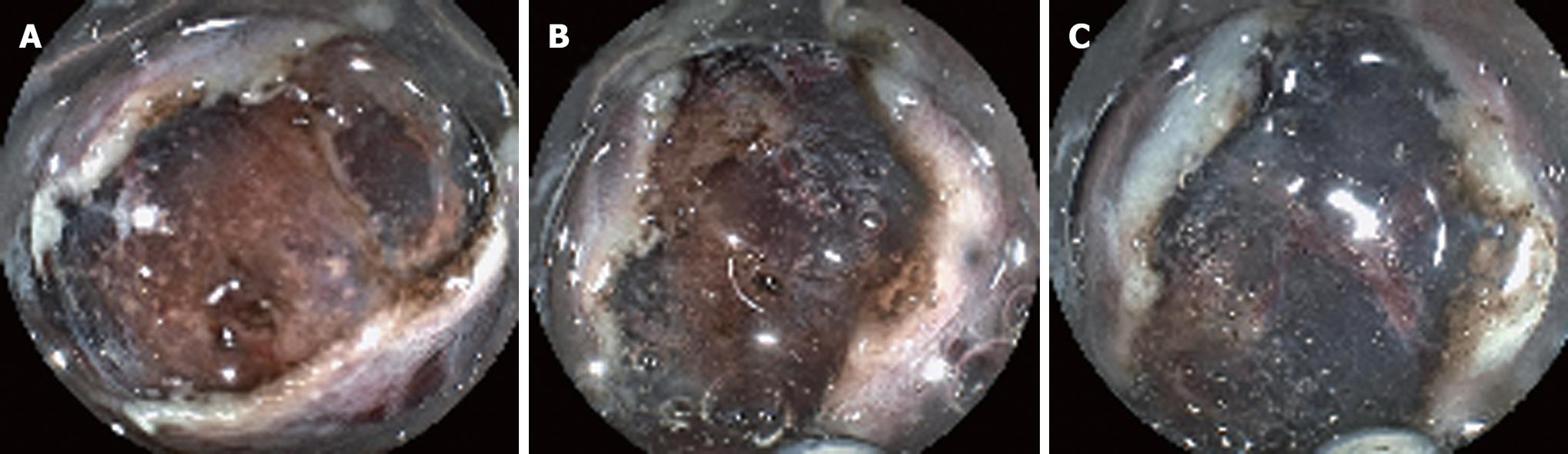
The new hemostatic method using hemostatic forceps was performed to remove submucosal thick vessels and to dissect the partial submucosa (Figure 1A-D). From the many available hemostatic forceps, we used a Coagrasper (FD-410LR or FD-410QR; Olympus, Tokyo, Japan) that has a rotation function and small teeth (Figure 2). The procedure was as follows: when a vessel with a diameter greater than 1 mm was found in the submucosa, it was slightly pulled out using the hemostatic forceps. It was then coagulated with the forceps in the soft coagulation mode (output, 60 W; effect 5) as previously reported[5-7] (Figure 3A). The vessel became whitish, the submucosa became less elevated, and the muscularis propria was close to the coagulated vessel (Figure 3B). The coagulated vessel was once again grasped using the hemostatic forceps and removed in the “endocut” mode (effect 2, duration 2, interval 1) (Figure 3C). Subsequently, the coagulated submucosa surrounding the vessel was dissected using the hemostatic forceps in the endocut mode (effect 2, duration 2, interval 1) (Figure 3C). In the situation of hemorrhage, the brownish coagulated submucosa was dissected using the hemostatic forceps in the endocut mode (effect 2, duration 2, interval 1) (Figure 4A-C and Figure 5).
We analyzed the feasibility and safety of this new method with respect to the removal of coagulated vessels and the dissection of the surrounding submucosa. We also assessed the frequency of perforation and postoperative hemorrhage. We also examined whether this procedure would result in the excess burning of the histopathological findings of the resected specimen. Excess burning was defined as the state in which glands and muscularis mucosae at the margin of the resected specimen were not determined clearly. Furthermore, we evaluated the ease of use this method by allowing our three trainees to perform the method.
We compared the outcome of 126 cases in the adopted group to that of 124 cases in the unadopted group, with respect to tumor size, tumor location, operation time, proportion of en bloc resection, frequency of histopathological curative resection, histopathological diagnosis, and the incidence of complications. Histopathological curative resection was determined as being free of cancer cells in both the vertical and lateral margins. Tumor location was divided into three groups: Right side colon (Cecum to Transverse colon), Left side colon (Descending colon to Sigmoid colon), and Rectum. We used the World Health Organization classification system for histopathological diagnosis[13]. In view of the risk of metastasis of lymph nodes, submucosally invaded cancers were divided into slightly invaded submucosal cancer (sSM) whose invasion length into submucosa was less than 1000 microns) or massively invaded submucosal cancer (mSM) whose invasion length was more than 1000 microns, according to previous report[14]. Complications of perforation and postoperative hemorrhage were assessed. A perforation was defined as a hole in the proper muscle layer that could be detected during ESD and from which released air could be detected using computed tomography. A postoperative hemorrhage was defined as the occurrence of hematochezia requiring endoscopic treatment to stop the hemorrhage and were divided into moderate type and severe type: the former was without significant decrease in hemoglobin (Hb), the latter was with significant decrease in Hb (more than 2 g/dL). Statistical analysis was performed using the chi-square test and the Mann-Whitney U test. A P-value of less than 0.05 was considered statistically significant.
The new hemostatic method using hemostatic forceps was employed in 102 cases. In 97 cases, the whitish coagulated vessel could be successfully removed using the hemostatic forceps without severe perioperative hemorrhage. Moderate perioperative hemorrhage occurred in five cases (4.9%); however, in all 5 cases, hemorrhage was arrested by immediately coagulating the affected vessel by reusing the hemostatic forceps. In all cases, the whitish or brownish coagulated submucosa surrounding the coagulated vessel was safely and easily dissected using the hemostatic forceps. After the dissection of the whitish or brownish coagulated vessel and submucosa, subsequent submucosal dissection using a Flush knife was easily and safely performed because the view of the surrounding submucosa was improved (Figure 4C and Figure 5C).
The patient characteristics in the two groups, the adopted group and unadopted group, are summarized in Table 1. Tumors in the Right side colon in the adopted group were significantly higher than that in the unadopted group. In the adopted group, the median operation time was 106 min and the frequency of endoscopic en bloc resection was 92.8%. Any lesions after EMR were not performed in the unadopted group. On the other hand, three lesions were resected in the adopted group. Histopathological complete resection was achieved in 89.6% of the adopted group. There were no significant differences between two groups in histopathological diagnosis. In sSM cancer, all eight cases were well-differentiated adenocarcinoma; venous infiltration was not detected in any cases of both groups. However, lymphatic infiltration was detected in one case in the adopted group and the patient received an additional surgical operation. In mSM cancer, nine cases were well-differentiated adenocarcinoma, and one case was moderately differentiated adenocarcinoma. Venous infiltration was detected in one case in the adopted group and in one case in the unadopted group. Lymphatic infiltration was detected in one case in the unadopted group. One case of sSM cancer and eight cases of 10 mSM cancer received additional surgical operations and there were no metastasis of the lymph nodes in any of these cases. The frequencies of postoperative hemorrhage in the adopted group and the unadopted group were 2.3 % and 2.4%, respectively. All cases were moderate hemorrhage. The frequencies of perforation in the adopted group and the unadopted group were 2.3% and 9.6% (P < 0.01). One case in the unadopted case received an urgent surgical operation and was discharged in 14 d. The other 14 cases of perforation were cured with endoscopic clipping and fast. They discharged 5 to 10 d after ESD.
| The adopted group | The unadopted group | P-value | |
| No. of cases | 126 | 124 | |
| Median age (yr, range) | 68.4 (55-87) | 64.9 (45-85) | NS |
| Tumor size (mm, range) | 29.6 (15-130) | 28.6 (12-70) | NS |
| Location: Right side colon/left side colon/rectum | 72/26/28 | 52/21/51 | < 0.01 |
| En bloc resection, n (%) | 117 (92.8) | 100 (80.6) | < 0.01 |
| Histopathological complete resection, n (%) | 113 (89.6) | 90 (72.5) | < 0.01 |
| Operation time (min, range) | 106 (40-330) | 105 (20-270) | NS |
| Histopathological diagnosis adenoma/M/sSM/mSM/MP | 57/60/4/5/0 | 55/59/3/5/2 | NS |
| Perforation, n (%) | 3 (2.3) | 12 (9.6) | < 0.01 |
| Postoperative hemorrhage, n (%) | 3 (2.3) | 3 (2.4) | NS |
Excess burning of the vertical margin associated with histopathological diagnosis was detected in three out of 102 cases (2.9%) using the new method. Conversely, it was detected in three out of 96 cases (3.1%) in the group using hemostatic forceps according to the conventional approach. There was no significant difference between these two groups.
In relation to the ease of the new method, three trainees performed ESD in 62 cases in the adopted group. The new method was performed on 46 cases. The characteristics of these cases were described as follows: average tumor size: 28.2 mm, Location: Right side colon 25 cases: Left side colon: 6 cases Rectum 15 cases. ESD was successfully accomplished in all cases without severe hemorrhage and perforation. Their average operation time was 110 min.
In the current study, we developed and assessed the efficacy of a new hemostatic method using hemostatic forceps for the treatment of submucosal thick vessels. Using the new method, coagulated vessels were successfully removed without severe hemorrhage. The dissection of the surrounding partial submucosa improved the view of the submucosa, whose subsequent dissection with a knife could be performed safely. This method enables us to perform the safe dissection of the submucosa without hemorrhage, and to prevent perforation and perioperative hemorrhage during ESD of colorectal tumors.
Moderate hemorrhage was experienced using this method, because of the inadequate coagulation of the vessel; however, the immediate reuse of the hemostatic forceps stopped the hemorrhage. When vessels that have been coagulated using the hemostatic forceps are dissected with a knife following the conventional approach, severe hemorrhage sometimes occurs because of inadequate coagulation. In such a case, the knife needs to be replaced with hemostatic forceps to arrest the hemorrhage; however, continued hemorrhage during this replacement might obscure the view from the endoscope. In our new method, the need for switching instruments is eliminated: the hemostatic forceps can be immediately reused in the event of hemorrhage during vessel removal. Moreover, this method could also shorten the operation time required for ESD.
The cases in the adopted group showed a significant bias towards tumors in the right side colon. This seemed to be caused by the increase of difficult cases that were introduced by other institutions. The proportion of en bloc resection in the adopted group (92.8%) was significantly better than that in the unadopted group (80.6%). Moreover, the rate of perforation was significantly lower in the adopted group (2.4%) than in the unadopted group (9.6%). These improvements are probably due to easier and speedy submucosal dissection using the new method. This new method provided efficient at removing vessels and gave better view of the submucosa during ESD. However, a learning curve was also associated with these improvements. Increasing experience will lead to the choice of a safe strategy for ESD and a suitable choice of knife, which are important in preventing perforation[6].
Three trainees performed ESD with this new method in 46 cases. Although a lack of experience with ESD for colorectal tumors is associated with an increased risk of perforation[5-7], perforation did not occur in these 46 cases. The new method decreased the occurrence of difficult submucosal dissections that can increase the risk of perforation. With regards to the ease of use this new method, the trainees could easily perform this new method without any difficulties; thus, we consider that the new method can be safely and effectively performed in other institutions.
It was possible that this new method could cause excess burning of the colorectum, because the head of the hemostatic forceps was larger than the Flush knife; however, histopathological analysis revealed that the rate of excess burning in the resected specimens using the new method was similar to the rate observed in the cases that underwent the standard procedure. Moreover, delayed perforation due to excess burning was not detected in any cases using the new method. The location of the vessel grasped by the forceps was important. When it was located on the near side of the muscularis propria, the effect of burning might invade the muscularis propria. Conversely, when it was located on the near side of the mucosa, the effect of burning might invade the resected specimen. This made histopathological diagnosis difficult. Therefore, we recommend that the vessel grasped by the forceps should be in the middle of the elevated submucosa. A wide range of hemostatic forceps manufactured by various medical companies is currently available. In our new method, we used the Coagrasper that has a rotation function and small teeth. To safely remove vessels, the hemostatic forceps should be positioned parallel to the short axis of the vessel. The teeth on these forceps enabled us to firmly grasp the affected vessel; it is tremendously difficult to grasp the coagulated vessel using toothless forceps. However, care should be taken during the procedure to avoid grasping the muscularis propria.
This new method was also associated with certain cost benefits. In our institution, hemostatic forceps were used in 96 of 124 cases (77.4%) for the coagulation of the submucosal vessel in the unadopted group. In such cases, difficult submucosal dissections could be made using this method. This implies that other knives such as the Hook knife (Olympus Medical Systems, Tokyo, Japan), which are sometimes used for difficult submucosal dissections, are not needed. Therefore, it is expected that there will be a decrease in cases requiring multiple knives using the new method and this will reduce the cost of performing ESD.
Standardization of the ESD procedure for colorectal tumors has not been possible because of technical difficulties. New medical equipment has been developed to overcome the technical difficulties associated with this procedure[15-17]; however, perforation still represents a considerable problem. Perforation might result in a difficult submucosal dissection. Difficult submucosal dissections can sometimes occur due to perioperative hemorrhage. Our method was effective at preventing perioperative hemorrhage and, thus, makes the dissection of the submucosa easier. This new method is likely to be useful for the standardization of ESD for colorectal tumors.
Endoscopic submucosal dissection (ESD) is not a standard procedure for treating colorectal tumors because of technical difficulties. The frequency of perforation has been reported to be in the range of 5%-14% in ESD for colorectal tumors.
In general, perioperative severe hemorrhage hampers the speedy dissection of the submucosa and can increase the risk of perforation. Therefore, submucosal thick vessels detected during ESD are clamped and coagulated using hemostatic forceps to prevent hemorrhage. However, this sometimes hinders speedy submucosal dissection. In the current study, the authors developed a new hemostatic method using hemostatic forceps for the treatment of submucosal thick vessels.
The new method was effective for the treatment of submucosal thick vessels. Moreover, this method showed promise for the prevention of perforation and perioperative hemorrhage in colonic ESD.
This new method was effective at preventing perioperative hemorrhage and, thus, makes the dissection of the submucosa easier. This new method is likely to be useful for the standardization of ESD for colorectal tumors.
ESD was developed as a method using special knife for en bloc resection for large epithelial gastric tumors and has become a standard therapy for large gastric tumors in Japan. On the other hand, endoscopic mucosal resection (EMR) has been generally performed for colorectal tumors. However, it is difficult to perform en bloc resection by EMR for a colorectal tumor whose size is larger than 20 mm. ESD for colorectal tumor was an expected procedure for large colorectal tumor.
The authors described a new technique using hemostatic forceps to coagulate submucosal vessels and to divide these vessels during ESD for colon mucosal lesions. The manuscript would be of interest to endoscopists performing ESD. It can, however, be substantial improved before its final publication.
Peer reviewers: Dr. Hajime Isomoto, Basic Research Center for Digestive Diseases, Division of Gastroenterology and Hepatology, Mayo Clinic, 200 First Street, Rochester 55905, United States; Abdellah Essaid, Professor, Hospital Ibn Sina, Rabat 10100, Morocco; James YW Lau, Department of Surgery, Prince of Wales Hospital, the Chinese University of Hong Kong, Hong Kong, China
S- Editor Wang YR L- Editor Stewart GJ E- Editor Lin YP
| 1. | Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929-942. [Cited in This Article: ] |
| 2. | Tanaka S, Oka S, Kaneko I, Hirata M, Mouri R, Kanao H, Yoshida S, Chayama K. Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc. 2007;66:100-107. [Cited in This Article: ] |
| 3. | Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Fu KI, Sano Y, Saito D. Endoscopic treatment of large superficial colorectal tumors: a case series of 200 endoscopic submucosal dissections (with video). Gastrointest Endosc. 2007;66:966-973. [Cited in This Article: ] |
| 4. | Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Oka M, Ogura K. Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clin Gastroenterol Hepatol. 2007;5:678-683; quiz 645. [Cited in This Article: ] |
| 5. | Yoshida N, Wakabayashi N, Kanemasa K, Sumida Y, Hasegawa D, Inoue K, Morimoto Y, Kashiwa A, Konishi H, Yagi N. Endoscopic submucosal dissection for colorectal tumors: technical difficulties and rate of perforation. Endoscopy. 2009;41:758-761. [Cited in This Article: ] |
| 6. | Yoshida N, Yagi N, Naito Y, Yoshikawa T. Safe procedure in endoscopic submucosal dissection for colorectal tumors focused on preventing complications. World J Gastroenterol. 2010;16:1688-1695. [Cited in This Article: ] |
| 7. | Yoshida N, Naito Y, Sakai K, Sumida Y, Kanemasa K, Inoue K, Morimoto Y, Konishi H, Wakabayashi N, Kokura S. Outcome of endoscopic submucosal dissection for colorectal tumors in elderly people. Int J Colorectal Dis. 2010;25:455-461. [Cited in This Article: ] |
| 8. | Tanaka S, Oka S, Chayama K. Colorectal endoscopic submucosal dissection: present status and future perspective, including its differentiation from endoscopic mucosal resection. J Gastroenterol. 2008;43:641-651. [Cited in This Article: ] |
| 9. | Toyonaga T, Man-I M, Morita Y, Sanuki T, Yoshida M, Kutsumi H, Inokuchi H, Azuma T. The new resources of treatment for early stage colorectal tumors: EMR with small incision and simplified endoscopic submucosal dissection. Dig Endosc. 2009;21 Suppl 1:S31-S37. [Cited in This Article: ] |
| 10. | Takeuchi Y, Uedo N, Ishihara R, Iishi H, Kizu T, Inoue T, Chatani R, Hanaoka N, Taniguchi T, Kawada N. Efficacy of an endo-knife with a water-jet function (Flushknife) for endoscopic submucosal dissection of superficial colorectal neoplasms. Am J Gastroenterol. 2010;105:314-322. [Cited in This Article: ] |
| 11. | Yamamoto H, Kawata H, Sunada K, Sasaki A, Nakazawa K, Miyata T, Sekine Y, Yano T, Satoh K, Ido K. Successful en-bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy. 2003;35:690-694. [Cited in This Article: ] |
| 12. | Fujishiro M, Yahagi N, Kashimura K, Mizushima Y, Oka M, Matsuura T, Enomoto S, Kakushima N, Imagawa A, Kobayashi K. Different mixtures of sodium hyaluronate and their ability to create submucosal fluid cushions for endoscopic mucosal resection. Endoscopy. 2004;36:584-589. [Cited in This Article: ] |
| 13. | Hamilton SR, Aaltonen LA. World Health Organization classification of tumours. Pathology and genetics of tumours of the digestive system. Lyon: IARC Press 2000; 104-109. [Cited in This Article: ] |
| 14. | Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, Kawamata H, Kumamoto T, Ishiguro S, Kato Y, Shimoda T. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. 2004;39:534-543. [Cited in This Article: ] |
| 15. | Fujishiro M, Kodashima S, Goto O, Ono S, Muraki Y, Kakushima N, Omata M. Technical feasibility of endoscopic submucosal dissection of gastrointestinal epithelial neoplasms with a splash-needle. Surg Laparosc Endosc Percutan Tech. 2008;18:592-597. [Cited in This Article: ] |
| 16. | Sakamoto N, Osada T, Shibuya T, Beppu K, Matsumoto K, Mori H, Kawabe M, Nagahara A, Otaka M, Ogihara T. Endoscopic submucosal dissection of large colorectal tumors by using a novel spring-action S-O clip for traction (with video). Gastrointest Endosc. 2009;69:1370-1374. [Cited in This Article: ] |