Published online Jul 21, 2010. doi: 10.3748/wjg.v16.i27.3377
Revised: April 23, 2010
Accepted: April 30, 2010
Published online: July 21, 2010
AIM: To investigate the mechanism by which galangin, a polyphenolic compound derived from medicinal herbs, induces apoptosis of hepatocellular carcinoma (HCC) cells.
METHODS: The 3-(4,5-Dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide assay was used to measure cell viability. Apoptosis was evaluated by in situ uptake of propidium iodide and Hoechst 33258 and was then detected by fluorescence microscopy. Protein expressions were detected by Western blotting. To confirm the apoptotic pathway mediated by galangin, cells were transfected by bcl-2 gene to overexpress Bcl-2 or siRNA to down-regulate Bcl-2 expression.
RESULTS: Galangin (46.25-370.0 μmol/L) exerted an anti-proliferative effect, induced apoptosis, and decreased mitochondrial membrane potential in a dose and time-dependent manner. Treatment with galangin induced apoptosis by translocating the pro-apoptotic protein Bax to the mitochondria, which released apoptosis-inducing factor and cytochrome c into the cytosol. Overexpression of Bcl-2 attenuated galangin-induced HepG2 cell apoptosis, while decreasing Bcl-2 expression enhanced galangin-induced cell apoptosis.
CONCLUSION: Our data suggests that galangin mediates apoptosis through a mitochondrial pathway, and may be a potential chemotherapeutic drug for the treatment of HCC.
-
Citation: Zhang HT, Luo H, Wu J, Lan LB, Fan DH, Zhu KD, Chen XY, Wen M, Liu HM. Galangin induces apoptosis of hepatocellular carcinoma cells
via the mitochondrial pathway. World J Gastroenterol 2010; 16(27): 3377-3384 - URL: https://www.wjgnet.com/1007-9327/full/v16/i27/3377.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i27.3377
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide, particularly in China. Surgical therapy, chemotherapy, and radiation have been used for the treatment of HCC. However, HCC remains one of the more difficult cancers to treat. Although chemotherapy is a common therapeutic strategy after surgery, its use has been toxic to normal tissues and limits their use. Therefore, it is important to screen for new anti-cancer drugs.
Recent studies show that a number of dietary compounds possess anti-cancer properties[1], including curcumin, genistein, quercitin, resveratrol, piceatannol and pterostilbene. These dietary compounds may enhance the efficacy of chemotherapeutic agents by modifying the activity of specific targets that control cell proliferation and apoptosis such as Bcl-2, Bcl-xL, X-linked inhibitor of apoptosis protein[2], Akt[3], c-myc, neuronal apoptosis inhibitory protein, c-IAP-2, nuclear factor-κB[4], survivin, p21WAF1 and p53[5].
Galangin (4H-1-Benzopyran-4-one, 3,5,7-trihydroxy-2-phenyl-), a flavonoid, is a polyphenolic compound derived primarily from medicinal herbs, including Alpinia officinarum Hance, Alnus pendula Matsum, Plantago major L, and Scutellaria galericulata L. (S. scrodifolia Fisch.). Eaton et al[6] demonstrated that galangin could inhibit the methoxyresorufin O-demethylase activity of CYP1A2, CYP1A1 and P-form phenolsulfotransferase. These observations suggest galangin may be a potential chemopreventive agent in sulfation-induced carcinogenesis.
Furthermore, Moon has reported that galangin had cancer preventive properties and induced apoptosis in several cancer cell lines[7]. Another study showed that galangin could induce a G0-G1 cell cycle arrest, modulate the expression of cycline/cdk, and decrease Bcl-2 levels, which leads to apoptosis of imatinib-resistant Bcr-Abl expressing chronic myelogenous leukemia cell lines[8].
Based on these previous reports, it was suggested that galangin could inhibit cell proliferation and induce apoptosis. Therefore, galangin may be a potential anti-tumor agent. However, the mechanism by which galangin exert its anti-tumor activity is unknown. In this study, we demonstrate the effects of galangin on HCC cells and elucidate the mechanism by which galangin induces apoptosis.
Three human liver cancer cell lines (HepG2, Hep3B and PLC/PRF/5) were obtained from American type culture collection (Rockville, MD, USA) and kept in Institute of Biochemistry and Molecular Biology, Guangdong Medical College. All cell lines were cultured in Dulbecco’s modified Eagle medium (Gibco BRL, Grand Island, NY, USA) containing penicillin (100 μg/mL) and streptomycin (100 μg/mL) and supplemented with 10% fetal bovine serum (Sijiqing Laboratories, Hangzhou, China) at 37°C in a humidified atmosphere with 5% CO2.
Galangin was purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in dimethyl sulfoxide (DMSO), with the final concentration of DMSO in the culture medium below 0.1% (v/v). 3-(4,5-Dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT), Hoechst 33258, propidium iodide, rhodamine 123 [2-(6-amino-3-imino-3H-xanthen-9-yl) benzoic acid methyl ester] were purchased from Sigma (St. Louis, MO, USA). Rabbit or goat polyclonal antibodies against Bax, caspase-3, caspase-9, cytochrome c, apoptosis-inducing factor (AIF), PAPR, Vox IV, GAPDH and Bcl-2 were from Santa Cruz Biotechnology (Santa Cruz Biotechnology, CA, USA).
HCC cells (5.0 × 103) were seeded and treated with different concentrations of galangin or different times in 96-well plates. The number of viable cells in each well was determined by adding 10 μL 5 mg/mL MTT solution. The cells were dissolved with 100 μL of solution that contained 20% SDS and 50% dimethyl formamide after cells had been incubated for 4 h at 37°C. The optical densities were quantified at a test wavelength of 570 nm and a reference wavelength of 630 nm using a Varioskan Flash Reader spectrophotometer (THERMO, MA, USA)[9].
The HCC cells were cultured in 6-well plates (3.0 × 105 cells/well) and treated with different concentrations of galangin at 37°C in a humidified atmosphere with 5% CO2 for 24 h. Cell apoptosis was evaluated by in situ uptake of propidium iodide and Hoechst 33258 as described by McKeague et al[10]. Briefly, galangin-treated cells were washed with phosphate buffered saline (PBS), and incubated in PBS containing 40 μg/mL propidium iodide and 2.5 μg/mL Hoechst 33258 for 10 min. Five hundred microliters of methanol: acetic acid (3: 1) fixative were then added directly to each well. Cells were viewed under fluorescence microscopy (Nikon Eclipse ET2000-E, Japan). The apoptotic index was calculated from the number of apoptotic nuclei vs total number of nuclei at each visual field.
HCC cells were treated with different concentrations of galangin for different times. Cells were then treated with rhodamine 123 with a final dye concentration of 10 μg/mL at 37°C for 15 min, 5% CO2 atmosphere prior to examination. Mitochondrial membrane potential was determined by flow cytometry. The change of fluorescent intensity of rhodamine 123 indicated the change in mitochondrial membrane potential.
The HCC cells were transfected with different plasmids [pcDNA3.1(+)-Bcl-2, pcDNA3.1(+)Vectors] using Lipofectamine 2000 (Invitrogen, CA, USA) according to the manufacturer’s protocol. Bcl-2 siRNA sequence was synthesized according to Fu’s report[12], sense sequence: 5'-CGGAGGCUGGGAUGCCUUUdTdT-3', antisense sequence: 3'-dTdTGCCUCCGACCCUACGGAAA-5'. Before transfection, cells were seeded in 6 well plates or 60 mm tissue culture dishes containing DMEM medium without antibiotics for 24 h. Cells were transfected using lipofectamine 2000 with 2 μg plasmid or 100 pmol siRNA in each well. Bcl-2 protein level was measured by immunoblot analysis 24 h post-transfection. Cells were treated with galangin for another 24 h, and viability was determined by MTT and fluorescence staining methods.
Twenty four hours after treating with different concentrations of galangin, cells were washed twice in ice-cold PBS and resuspended in five volumes of ice-cold extract buffer (20 mmol/L Hepes-KOH, 1.5 mmol/L MgCl2, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L DTT, and 0.1 mmol/L PMSF, pH 7.5). The resuspended cells were homogenized and centrifuged at 750 g for 10 min at 4°C. The supernatants were centrifuged at 13 000 g for 15 min at 4°C to obtain the mitochondrial pellets. The remaining supernatants were centrifuged to obtain the cytosolic fractions. The protein concentrations of the resulting supernatants and mitochondrial fractions were measured.
The cells were loaded with cell decomposition buffer (pH 8.0) that contained 50 mmol/L Tris-HCl, 150 mmol/L NaCl, 5 mmol/L EDTA, 1% NP40, 0.05% phenylmethanesulfonyl fluoride (PMSF), 2 μg/mL aprotinin (Sigma, USA), and 2 μg/mL leupeptin (Sigma, USA). The proteins were determined as described previously by Western blotting[9] using the antibody (Santa Cruz Biotechology, Santa Cruz, CA, USA), and Western blotting luminal reagent (Amersham Biosciences, Uppsala, Sweden).
The values given are presented as mean ± SD. Statistical analysis was performed using one-way analysis of variance with LSD test. In all cases, P < 0.05 was considered as significant.
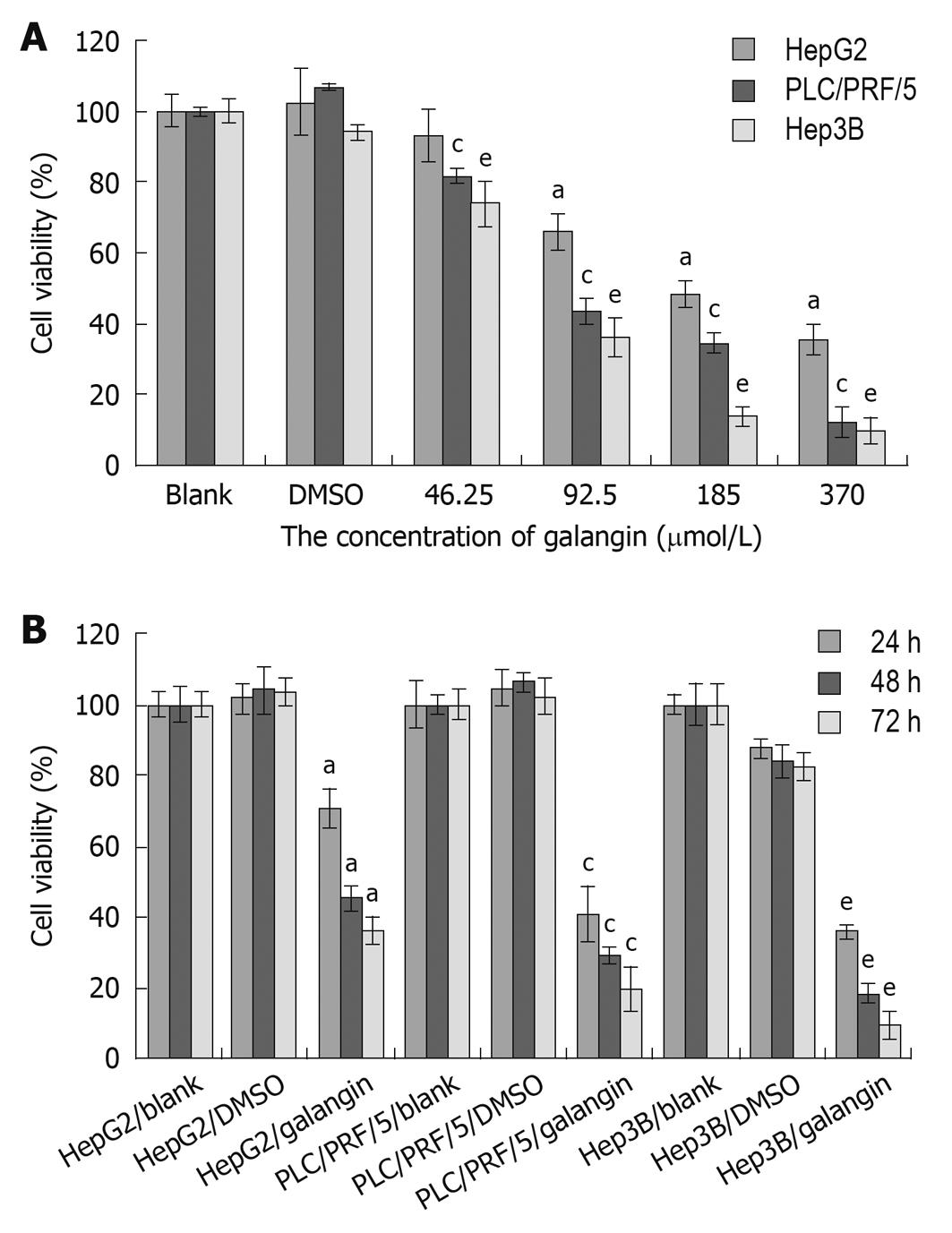
We used the MTT assay to determine the effects of galangin on the proliferation of HCC cells. Using galangin at concentrations of 46.25, 92.5, 185 and 370 μmol/L, we observed an anti-proliferative effect on HCC cells that was dose-dependent (Figure 1A). Additionally, galangin could also inhibit HCC cell proliferation in a time-dependent manner (Figure 1B). The IC50 of galangin to HCC cells (HepG2, Hep3B, and PLC/PRF/5) 24 h post-treatment were 134.0, 87.3 and 79.8 μmol/L, respectively.
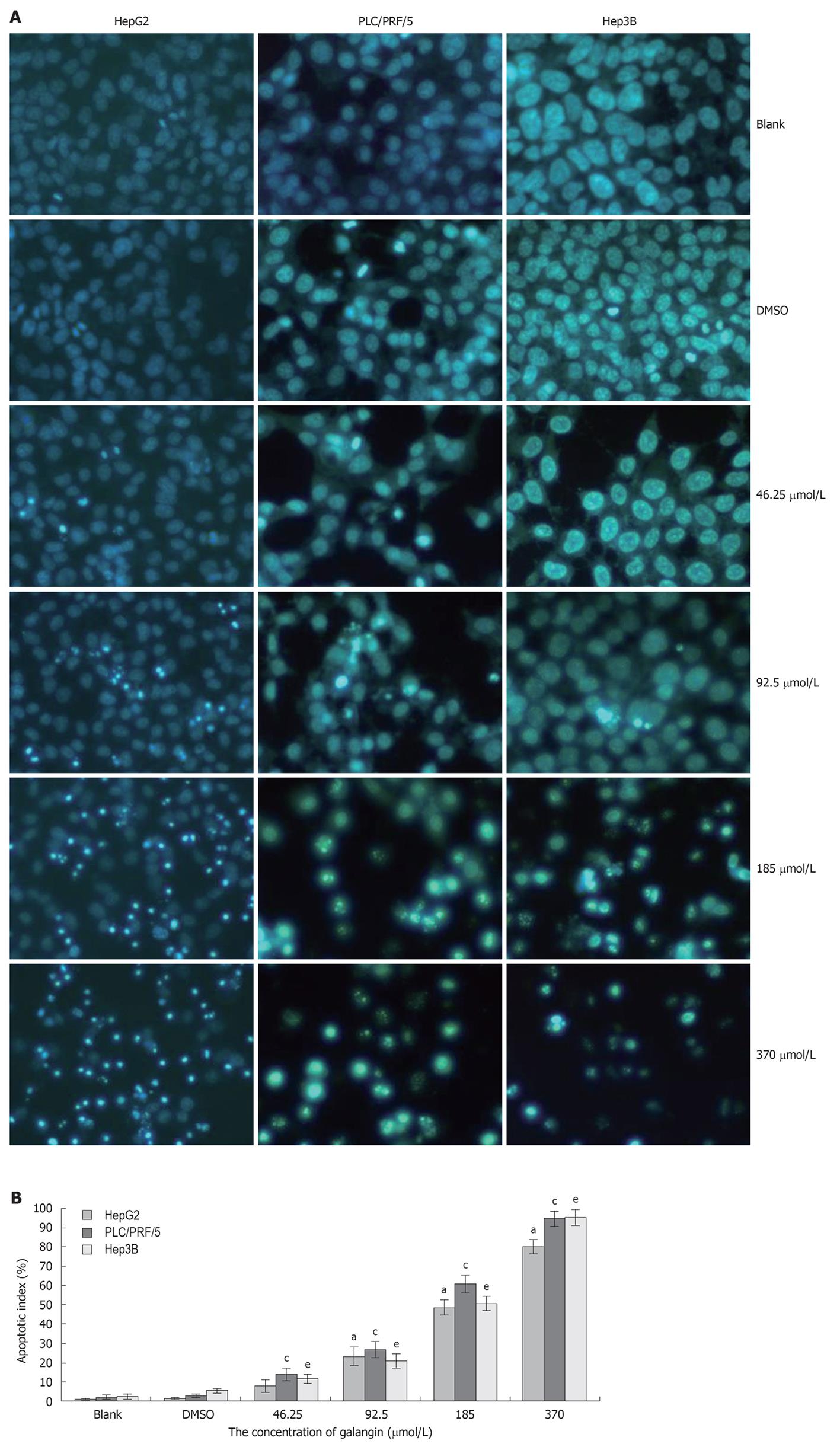
To determine whether galangin-treated HCC cells undergo apoptosis, we stained cells using Hoechst 33258/PI. As shown in Figure 2A, we observed a significant increase in the number of cells that exhibited nuclear condensation when treated with galangin for 24 h. This observation was similarly found in all three HCC cell lines tested. Our data also showed that the apoptotic index of the three HCC cells increased in a dose-dependent manner treated by galangin (Figure 2B).
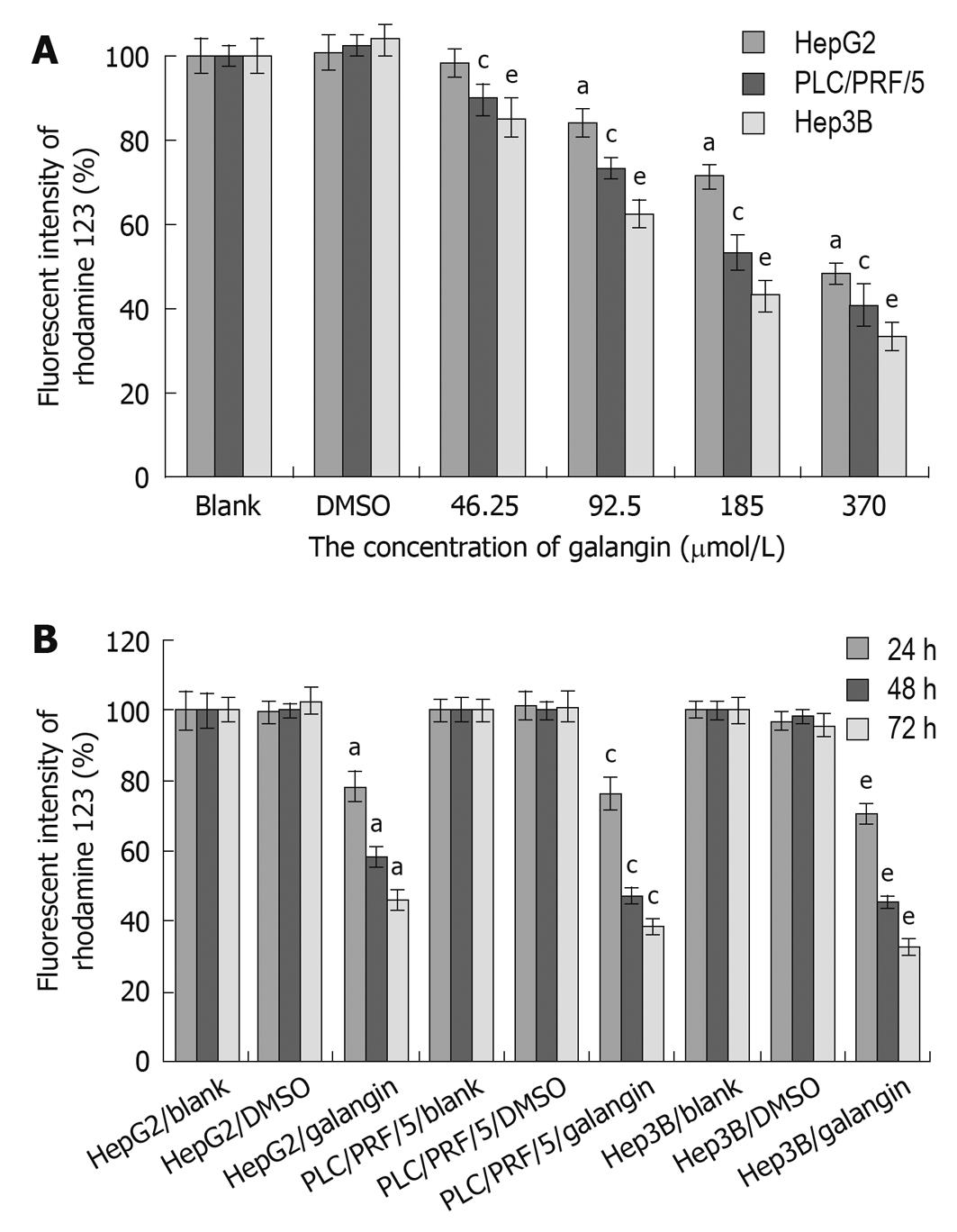
We observed a reduction in the mitochondrial membrane potential of all three HCC cell lines after treating with galangin (Figure 3). The mitochondrial membrane potential of the HCC cells decreased following treatment with galangin and this occurred in a dose- and time-dependent manner.
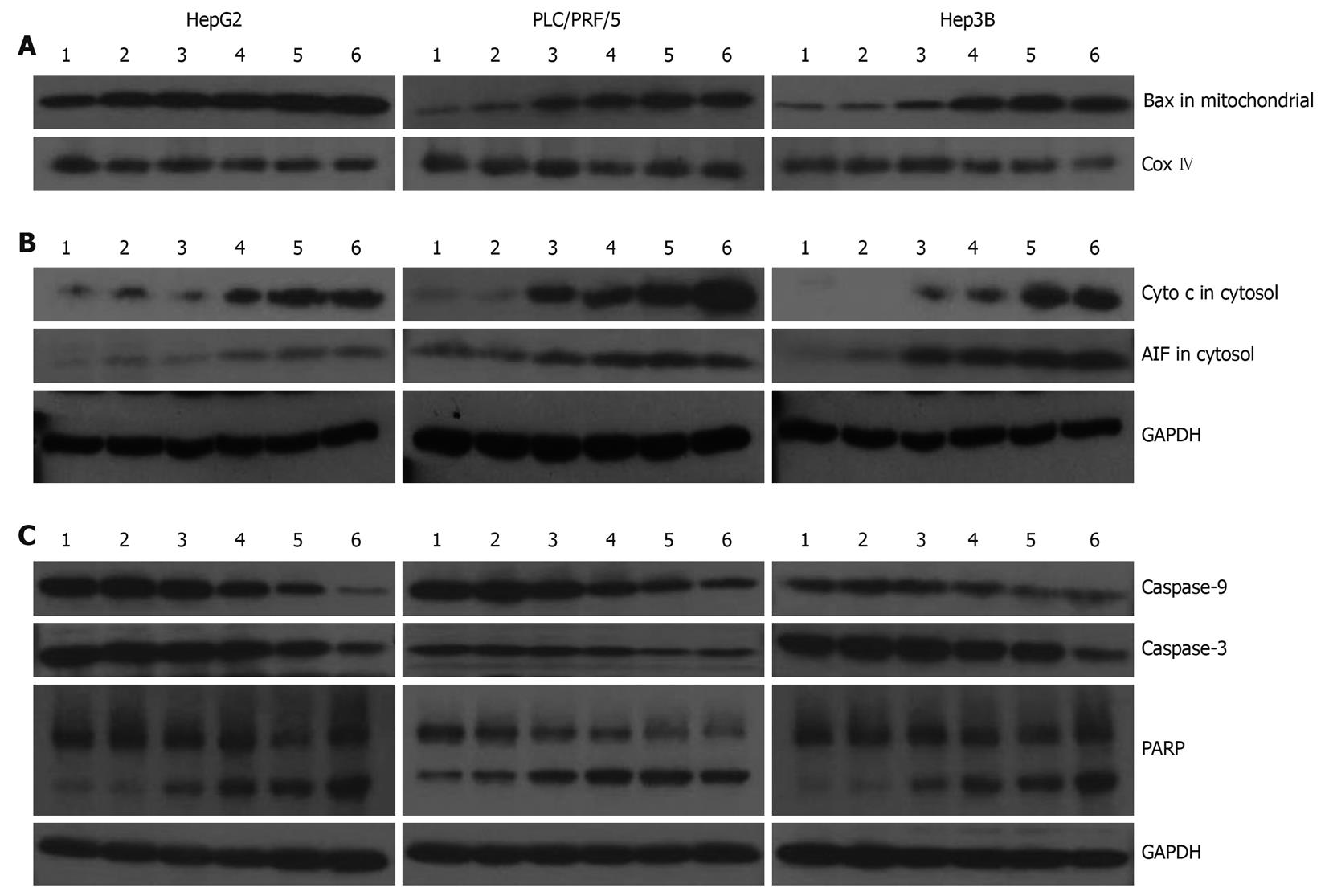
In Figure 4, we evaluated the effects of galangin on protein expression involved in apoptosis of the HCC cell lines. Translocation of Bax to the mitochondria can alter the outer mitochondrial membrane permeability. Some pro-apoptotic proteins, including cytochrome c and AIF, are released into the cytosol from the mitochondria. Our data shows that Bax levels in the mitochondrial fraction of galangin-treated HCC cells increased in a dose-dependent manner, suggesting that the translocation of Bax into the mitochondria was involved in cell death induced by galangin (Figure 4A). Furthermore, we observed that the levels of cytochrome c and AIF in the cytosol of galangin-treated HCC cells increased in a dose-dependent manner. This suggests that the mitochondrial release of cytochrome c and AIF into the cytosol may play a role in induction of cell apoptosis by galangin (Figure 4B). We also analyzed the effects of galangin on caspase-3 and caspase-9 activation, and poly(ADP-ribose) polymerase (PARP) cleavage by Western blotting. We showed caspase-9 activation in a dose-dependent manner, and observed a concomitant increase of caspase-3 activation and PARP cleavage (Figure 4C).
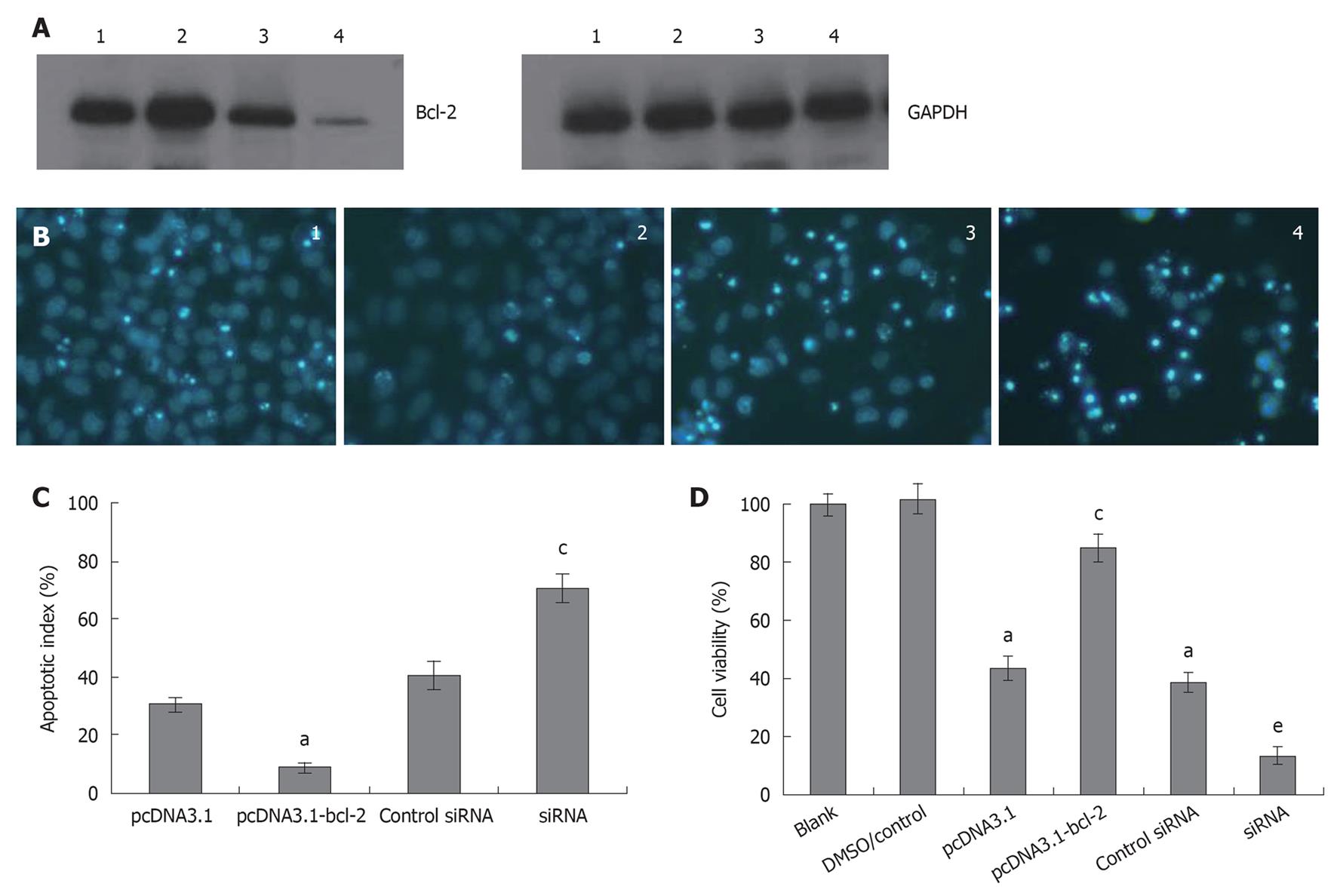
To determine the role of Bcl-2 in galangin-treated HepG2 cells, we overexpressed and downregulated Bcl-2 by transfecting cells with pcDNA3.1-Bcl-2 and siRNA targeting Bcl-2, respectively. In Figure 5A, we show that we were able to overexpress and knockdown Bcl-2 sufficiently. As shown in Figure 5B and C, HepG2 cells overexpressing Bcl-2 were more resistant to galangin-induced apoptosis than control cells (P < 0.05). However, Bcl-2-knockdown HepG2 cells were more sensitive to galangin leading to decreased cell survival (P < 0.05) (Figure 5D). Taken together, these observations suggest that galangin induces apoptosis of HepG2 cell apoptosis via the mitochondrial pathway.
Studies have showed that most flavonoids exhibit anti-proliferative effects against tumor derived cell lines including leukemia[13], melanoma[14], colon[15], breast carcinoma[16], lung, and prostate[17]. Some reports have demonstrated that galangin is a naturally occurring non-toxic flavonoid with chemopreventive and anti-proliferative effects[6-8,18,19].
In this study, we demonstrated that galangin inhibited the proliferation of HCC cells and induced apoptosis at concentrations as low as 46.25 μmol/L and in excess of 185 μmol/L, respectively. Galangin-induced apoptosis was characterized by analyzing the effects on caspase-3 activation, PARP cleavage, and DNA condensation in HCC cells.
The mitochondrial pathway is commonly involved in the death stimuli. There are primarily two major events involved in apoptosis via the mitochondrial pathway. The first event is a change in mitochondrial membrane permeability, which leads to decreased mitochondrial membrane potential. Our data demonstrated reduced mitochondrial membrane potential as indicated by rhodamine 123 staining following treatment with galangin at different concentrations. The second event in the mitochondria-induced apoptotic pathway is the release of cytochrome c and AIF from the intermembrane space of the mitochondria into the cytosol. Here, we also showed that galangin increased the release of cytochrome c and AIF in the cytosol. Thus, our data indicates that galangin-induced apoptosis of HCC cells occurs through the mitochondrial pathway.
The Bcl-2 family of proteins is involved in the mitochondrial apoptotic pathway by inducing the release of cytochrome c from the mitochondrial intermembrane space. Cytochrome c cooperates with Apaf-1 (Apoptotic protease activating factor 1) to induce caspase activation, leading to cell apoptosis[20]. The Bcl-2 family proteins are categorized into three groups based on the four Bcl-2 homology domains (BH1-4 domains). Bcl-2, Bcl-w, Bcl-xL and Mcl-1, which contain BH domains 1-4 and are localized to the outer mitochondrial membrane, are anti-apoptotic Bcl-2 proteins[21]. These proteins can directly bind and inhibit the proapoptotic Bcl-2 family in the mitochondria pathway of apoptosis. The proapoptotic proteins of Bcl-2 family members are functionally divided into two classes. One class is the effector molecule, which includes Bak and Bax, and permeabilizes the outer mitochondrial membrane to release cytochrome c into the cytosol. The other class is the BH3-only proteins including Bad, Bid, Bik, Bim, Bmf, bNip3, Hrk, Noxa and Puma, which promote cell apoptosis through protein-protein interactions with other Bcl-2 family members[21].
Our data indicates that galangin causes Bax translocation to the mitochondria in HCC cells. In non-apoptotic cells, Bax is located in the cytosol or loosely bound to the outer membrane of the mitochondria in monomeric forms. However, Bax translocates to the mitochondrial membrane and homodimerizes in the presence of a death signal. As a result, the outer mitochondrial membrane is permeable to release cytochrome c and AIF into the cytosol. The release of cytochrome c, which is an important protein in the electron transfer chain, can lead to reduced mitochondria membrane potential and adenosine triphosphate (ATP) synthesis. The results of our experiments showed that galangin induces cytochrome c release and decreases mitochondrial membrane potential.
In the cytosol, cytochrome c can bind to Apaf-1, which is a cytosolic protein. Apaf-1 undergoes a conformational change upon binding to dATP or ATP, leading to the formation of the apoptosome complex. The apoptosome recruits procaspase-9, resulting in caspase 9-caspase 3 activation. This caspase cascade is responsible for the hydrolysis of key cytoplasmic proteins and for the cleavage of genomic DNA nucleosomes into 180 bp fragments via caspase-activated DNase, such as PARP. Caspase-3 is an executioner of apoptosis that subsequently cleaves many important intracelluar substrates, leading to chromatin condensation, nucleosomal DNA fragmentation, nuclear membrane breakdown, externalization of phosphatidylserine, and formation of apoptotic bodies[22]. Our data also shows galangin-treated HCC cells did indeed cause caspase-9 and caspase-3 activation, and PARP cleavage.
In our study, overexpression of Bcl-2 could suppress the apoptotic effects of galangin on HCC cells. Bcl-2 is an important anti-apoptotic protein that suppresses different drug-induced activation of the mitochondria-apoptotic pathway, such as etoposide[23], berberine[24], safatoposide[25], epigallocatechin-3-gallate[26], curcumin[27] and anti-inflammatory drugs[28]. The Bcl-2 protein can block the oligomerization of Bax and Bak and inhibit the apoptotic program[29,30]. Moreover, our data also showed that Bcl-2 decrease could enhance HCC cell sensitivity to galangin. These results show that Bcl-2 can modulate the effects of galangin on HCC cells and indicate that galangin induces apoptosis via the mitochondrial pathway.
We demonstrated that AIF is released from mitochondria into the cytosol in HCC cells upon galangin treatment. AIF migrates into the nucleus and induces high-molecular-mass DNA fragmentation and marginal chromatin condensation independent of caspases[31,32]. Therefore, HCC cells undergoing apoptosis may be due to a combination of caspase activation and AIF release.
In summary, we demonstrate that galangin induces HCC cell apoptosis via the mitochondrial pathway. Our data demonstrated that (1) galangin induces HCC cell apoptosis by triggering Bax translocation to the mitochondria; (2) galangin-treated HCC cells causes the release of AIF and cytochrome c into the cytosol from the mitochondria; and (3) overexpression of Bcl-2 attenuated galangin-induced HepG2 cells apoptosis, while down-regulated Bcl-2 expression enhanced galangin to induce cell apoptosis.
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide, particularly in China. However, HCC remains one of the more difficult cancers to treat. It is important to screen for new anti-cancer drugs. Dietary compounds possess anti-cancer properties.
A number of dietary compounds possess anti-cancer properties. These dietary compounds may modify the activity of specific targets that control cell proliferation and apoptosis. Galangin could inhibit the methoxyresorufin O-demethylase activity of CYP1A2, CYP1A1 and P-form phenolsulfotransferase. Galangin induced apoptosis in several cancer cell lines and arrested the cell cycle, modulated the expression of cycline/cdk, and decreased Bcl-2. It was suggested that galangin may be a potential anti-tumor agent. However, the mechanism by which galangin exerts its anti-tumor activity is unknown.
In this study, the authors demonstrate the effects of galangin on HCC cells and elucidate the mechanism by which galangin induces apoptosis. This is the first study to report that galangin mediates apoptosis through a mitochondrial pathway. Galangin may be a potential chemotherapeutic drug for the treatment of hepatocellular carcinoma cells.
Understanding the mechanism by which galangin induces apoptosis may lead to its use as an anti-cancer treatment of HCC. This study may represent a future potential chemotherapeutic drug in the treatment of HCC with galangin.
The mitochondrial pathway is an important apoptotic pathway involved in the mitochondrial membrane permeability change and the release of cytochrome c and apoptosis-inducing factor (AIF) from the intermembrane space of the mitochondria into the cytosol. Bcl-2 is an important anti-apoptotic protein that suppresses different drug-induced activation of the mitochondria-apoptotic pathway.
In this study, Zhang et al demonstrated that galangin, a polyphenolic compound from herbs, induced apoptosis in hepatocellular carcinoma cells through the mitochondrial pathway. Galangin treatment led to inhibition of cell growth, nuclear fragmentation, mitochondrial membrane potential collapse, caspase activation, Bax mitochondrial translocation, and release of cytochrome c and AIF. Modulation of Bcl-2 by overexpression or knockdown altered galangin-induced apoptosis. The results are convincing and the study is informative. The manuscript could be improved by making several changes.
Peer reviewer: Lin Zhang, PhD, Associate Professor, Department of Pharmacology and Chemical Biology, University of Pittsburgh Cancer Institute, University of Pittsburgh School of Medicine, UPCI Research Pavilion, Room 2.42d, Hillman Cancer Center, 5117 Centre Ave., Pittsburgh, PA 15213-1863, United States
S- Editor Wang YR L- Editor O'Neill M E- Editor Lin YP
| 1. | Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768-780. [Cited in This Article: ] |
| 2. | Notarbartolo M, Poma P, Perri D, Dusonchet L, Cervello M, D'Alessandro N. Antitumor effects of curcumin, alone or in combination with cisplatin or doxorubicin, on human hepatic cancer cells. Analysis of their possible relationship to changes in NF-kB activation levels and in IAP gene expression. Cancer Lett. 2005;224:53-65. [Cited in This Article: ] |
| 3. | Li Y, Wang Z, Kong D, Li R, Sarkar SH, Sarkar FH. Regulation of Akt/FOXO3a/GSK-3beta/AR signaling network by isoflavone in prostate cancer cells. J Biol Chem. 2008;283:27707-27716. [Cited in This Article: ] |
| 4. | Solomon LA, Ali S, Banerjee S, Munkarah AR, Morris RT, Sarkar FH. Sensitization of ovarian cancer cells to cisplatin by genistein: the role of NF-kappaB. J Ovarian Res. 2008;1:9. [Cited in This Article: ] |
| 5. | Banerjee S, Zhang Y, Ali S, Bhuiyan M, Wang Z, Chiao PJ, Philip PA, Abbruzzese J, Sarkar FH. Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer. Cancer Res. 2005;65:9064-9072. [Cited in This Article: ] |
| 6. | Eaton EA, Walle UK, Lewis AJ, Hudson T, Wilson AA, Walle T. Flavonoids, potent inhibitors of the human P-form phenolsulfotransferase. Potential role in drug metabolism and chemoprevention. Drug Metab Dispos. 1996;24:232-237. [Cited in This Article: ] |
| 7. | Moon YJ, Wang X, Morris ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol In Vitro. 2006;20:187-210. [Cited in This Article: ] |
| 8. | Tolomeo M, Grimaudo S, Di Cristina A, Pipitone RM, Dusonchet L, Meli M, Crosta L, Gebbia N, Invidiata FP, Titone L. Galangin increases the cytotoxic activity of imatinib mesylate in imatinib-sensitive and imatinib-resistant Bcr-Abl expressing leukemia cells. Cancer Lett. 2008;265:289-297. [Cited in This Article: ] |
| 9. | Zhang HT, Feng ZL, Wu J, Wang YJ, Guo X, Liang NC, Zhu ZY, Ma JQ. Sodium butyrate-induced death-associated protein kinase expression promote Raji cell morphological change and apoptosis by reducing FAK protein levels. Acta Pharmacol Sin. 2007;28:1783-1790. [Cited in This Article: ] |
| 10. | McKeague AL, Wilson DJ, Nelson J. Staurosporine-induced apoptosis and hydrogen peroxide-induced necrosis in two human breast cell lines. Br J Cancer. 2003;88:125-131. [Cited in This Article: ] |
| 11. | Zhang HT, Wu J, Zhang HF, Zhu QF. Efflux of potassium ion is an important reason of HL-60 cells apoptosis induced by tachyplesin. Acta Pharmacol Sin. 2006;27:1367-1374. [Cited in This Article: ] |
| 12. | Fu GF, Lin XH, Han QW, Fan YR, Xu YF, Guo D, Xu GX, Hou YY. RNA interference remarkably suppresses bcl-2 gene expression in cancer cells in vitro and in vivo. Cancer Biol Ther. 2005;4:822-829. [Cited in This Article: ] |
| 13. | Liesveld JL, Abboud CN, Lu C, McNair C, Menon A, Smith A, Rosell K, Rapoport AP. Flavonoid effects on normal and leukemic cells. Leuk Res. 2003;27:517-527. [Cited in This Article: ] |
| 14. | Casagrande F, Darbon JM. Effects of structurally related flavonoids on cell cycle progression of human melanoma cells: regulation of cyclin-dependent kinases CDK2 and CDK1. Biochem Pharmacol. 2001;61:1205-1215. [Cited in This Article: ] |
| 15. | Kuo SM. Antiproliferative potency of structurally distinct dietary flavonoids on human colon cancer cells. Cancer Lett. 1996;110:41-48. [Cited in This Article: ] |
| 16. | So FV, Guthrie N, Chambers AF, Carroll KK. Inhibition of proliferation of estrogen receptor-positive MCF-7 human breast cancer cells by flavonoids in the presence and absence of excess estrogen. Cancer Lett. 1997;112:127-133. [Cited in This Article: ] |
| 17. | Fotsis T, Pepper MS, Aktas E, Breit S, Rasku S, Adlercreutz H, Wähälä K, Montesano R, Schweigerer L. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997;57:2916-2921. [Cited in This Article: ] |
| 18. | Murray TJ, Yang X, Sherr DH. Growth of a human mammary tumor cell line is blocked by galangin, a naturally occurring bioflavonoid, and is accompanied by down-regulation of cyclins D3, E, and A. Breast Cancer Res. 2006;8:R17. [Cited in This Article: ] |
| 19. | Bestwick CS, Milne L. Influence of galangin on HL-60 cell proliferation and survival. Cancer Lett. 2006;243:80-89. [Cited in This Article: ] |
| 20. | Zou H, Li Y, Liu X, Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549-11556. [Cited in This Article: ] |
| 21. | Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157-164. [Cited in This Article: ] |
| 22. | Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770-776. [Cited in This Article: ] |
| 23. | Shawgo ME, Shelton SN, Robertson JD. Caspase-mediated Bak activation and cytochrome c release during intrinsic apoptotic cell death in Jurkat cells. J Biol Chem. 2008;283:35532-35538. [Cited in This Article: ] |
| 24. | Ho YT, Lu CC, Yang JS, Chiang JH, Li TC, Ip SW, Hsia TC, Liao CL, Lin JG, Wood WG. Berberine induced apoptosis via promoting the expression of caspase-8, -9 and -3, apoptosis-inducing factor and endonuclease G in SCC-4 human tongue squamous carcinoma cancer cells. Anticancer Res. 2009;29:4063-4070. [Cited in This Article: ] |
| 25. | Day TW, Wu CH, Safa AR. Etoposide induces protein kinase Cdelta- and caspase-3-dependent apoptosis in neuroblastoma cancer cells. Mol Pharmacol. 2009;76:632-640. [Cited in This Article: ] |
| 26. | Ran ZH, Xu Q, Tong JL, Xiao SD. Apoptotic effect of Epigallocatechin-3-gallate on the human gastric cancer cell line MKN45 via activation of the mitochondrial pathway. World J Gastroenterol. 2007;13:4255-4259. [Cited in This Article: ] |
| 27. | Chen QY, Lu GH, Wu YQ, Zheng Y, Xu K, Wu LJ, Jiang ZY, Feng R, Zhou JY. Curcumin induces mitochondria pathway mediated cell apoptosis in A549 lung adenocarcinoma cells. Oncol Rep. 2010;23:1285-1292. [Cited in This Article: ] |
| 28. | Huang RH, Chai J, Tarnawski AS. Identification of specific genes and pathways involved in NSAIDs-induced apoptosis of human colon cancer cells. World J Gastroenterol. 2006;12:6446-6452. [Cited in This Article: ] |
| 29. | Haraguchi M, Torii S, Matsuzawa S, Xie Z, Kitada S, Krajewski S, Yoshida H, Mak TW, Reed JC. Apoptotic protease activating factor 1 (Apaf-1)-independent cell death suppression by Bcl-2. J Exp Med. 2000;191:1709-1720. [Cited in This Article: ] |
| 30. | Smoot RL, Blechacz BR, Werneburg NW, Bronk SF, Sinicrope FA, Sirica AE, Gores GJ. A Bax-mediated mechanism for obatoclax-induced apoptosis of cholangiocarcinoma cells. Cancer Res. 2010;70:1960-1969. [Cited in This Article: ] |
| 31. | Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441-446. [Cited in This Article: ] |
| 32. | Singh MH, Brooke SM, Zemlyak I, Sapolsky RM. Evidence for caspase effects on release of cytochrome c and AIF in a model of ischemia in cortical neurons. Neurosci Lett. 2010;469:179-183. [Cited in This Article: ] |