Published online Mar 21, 2010. doi: 10.3748/wjg.v16.i11.1321
Revised: February 10, 2010
Accepted: February 17, 2010
Published online: March 21, 2010
Alcoholic liver disease (ALD) is one of the leading causes of liver diseases and liver-related death worldwide. Of the many factors that contribute to the pathogenesis of ALD, gut-derived lipopolysaccharide (LPS) plays a central role in induction of steatosis, inflammation, and fibrosis in the liver. In this review, we discuss the mechanisms by which alcohol contributes to increased gut permeability, the activation of Kupffer cells, and the inflammatory cascade by LPS. The role of the Toll-like receptor 4 (TLR4) complex in LPS recognition and the importance of the TLR4-induced signaling pathways are evaluated in ALD.
- Citation: Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol 2010; 16(11): 1321-1329
- URL: https://www.wjgnet.com/1007-9327/full/v16/i11/1321.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i11.1321
The clinical spectrum of alcoholic liver disease (ALD) includes alcoholic fatty liver, alcoholic steatohepatitis, alcoholic cirrhosis (Laennec’s cirrhosis), and increased risk of hepatocellular carcinoma[1,2]. The pathomechanism of ALD involves complex interactions between the direct effects of alcohol and its toxic metabolites on various cell types in the liver, induction of reactive oxygen species (ROS), upregulation of the inflammatory cascade, and other cell-specific effects in the liver[3,4]. Lipopolysaccharide (LPS), also known as endotoxin, has been identified as a major factor in the pathogenesis of ALD. Indeed, LPS can lead to liver steatosis, as it induces inflammation and contributes to cirrhosis, which are all features of ALD[5,6]. These effects of LPS are manifested in the various cell types in the liver and the source of LPS appears to be the gut in ALD, resulting from alcohol-induced disturbance of gut permeability. At the cellular and molecular level, LPS is recognized by the Toll-like receptor 4 (TLR4) complex and induces specific intracellular activation pathways. This review will focus on the role of LPS in ALD and will summarize the current state of art on alcohol-related changes in the gut-liver axis.
The gut is a habitat for billions of microorganisms and the gut mucosal epithelium serves as a barrier between microbiota and gut lumen[7]. LPS (endotoxins) derived from Gram-negative bacteria in the intestinal microflora normally penetrate the mucosa only in trace amounts, enter the portal circulation, and become cleared in the liver to maintain the control of immune homeostasis. Resident macrophages (Kupffer cells) and hepatocytes both contribute to this process through different LPS recognition systems[3,8-10]. There is a positive correlation between liver dysfunction and the occurrence of bacterial translocation[11-14], and the clearance of LPS from the circulation is decreased in states of hepatic dysfunction, such as cirrhosis[15]. Studies in animals suggest that the liver quickly removes about 40%-50% of an intravenous dose of LPS from the bloodstream[16,17]. Hepatic uptake and detoxification is important for preventing systemic reactions to blood-borne LPS. It has been proposed that LPS initially is taken up by Kupffer cells and then by hepatocytes[18]. LPS is removed via several mechanisms, including molecules that bind LPS and prevent it from activating TLR4, enzymes that degrade the lipid A moiety to decrease its activity, inactivation of LPS following uptake into the liver and spleen, and cellular adaptations that modify target cell responses[19]. Another mechanism for LPS neutralization is by serum lipoproteins, HDL, LDL, VLDL, and chylomicrons, apolipoproteins apoE and apoA-I LPS[20-22]. All of these mechanisms can chaperone endotoxin to hepatocytes, Kupffer cells, or sinusoidal endothelial cells, resulting in clearance of LPS without significant inflammatory cell activation.
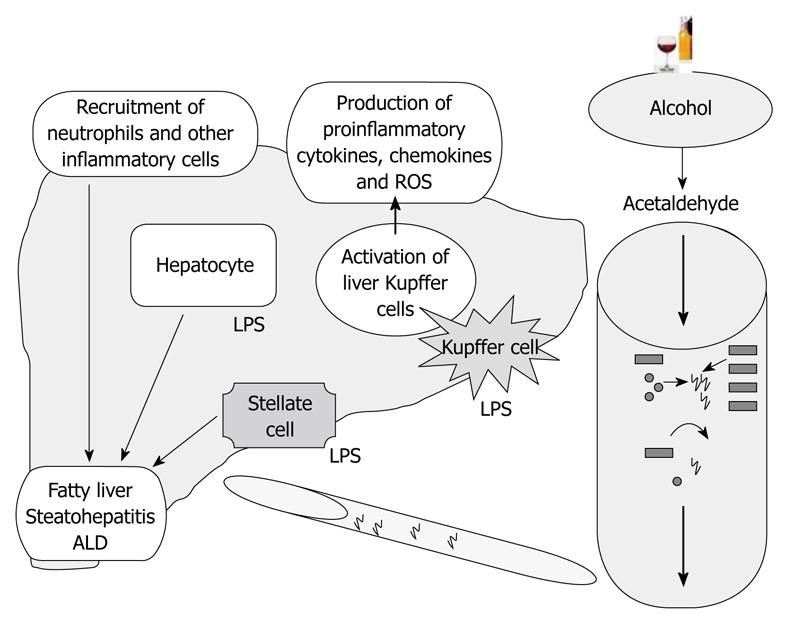
The role of LPS in alcoholic liver injury has been shown in several studies[12,23,24]. The importance of gut-derived endotoxin in ALD was suggested by experiments where treating the animals either with antibiotics or with lactobacilli to remove or reduce the gut microflora provided protection from the features of ALD[12,25,26]. In mice and rats, circulating endotoxin levels were increased after chronic alcohol feeding[27,28] and plasma endotoxin levels were also increased in patients with ALD compared to normal subjects[29]. The persistence of endotoxin not only activates the liver immune cells but also affects the function of liver parenchymal cells.
The progression of ALD is a complex phenomenon, as it not only results from the direct effects of alcohol and its metabolites, but other factors also play an important role in its pathogenesis, such as leaky gut, which results in endotoxemia[30]. Both chronic ethanol- mediated microbial proliferation[31,32] and acetaldehyde-mediated opening of intestinal tight junctions (TJs)[33] enhance the passage or release of endotoxins into the intestinal lumen, which are later transported to liver. However, when excess amounts of endotoxin are not cleared efficiently by the liver and accumulate in blood circulation, innate immune cells, including Kupffer cells, are activated, leading to the release of various pro-inflammatory cytokines, chemokines, and other factors[34,35].
Kupffer cell activation has been identified as one of the key elements in the pathogenesis of alcoholic steatohepatitis. Studies in mice and rats demonstrated that inactivation of Kupffer cells with gadolinium chloride or clodronate injection can almost fully ameliorate alcohol-induced liver disease[36,37]. These observations led to the currently accepted model of ALD, where Kupffer cell activation by gut-derived endotoxin, induction of chemokines such as MCP-1, and upregulation of the inflammatory cascade represent a central component of the pathomechanisms of ALD (Figure 1).
The mechanisms underlying the disruption of the intestinal barrier by alcohol appear to be at multiple levels, including disruption of the gut barrier and changes in microbial flora.
Tight junctions are scaffolds of various transmembrane proteins (e.g. claudins, occludin, JAMs, and tricellulin) and a complex network of adaptors proteins that crosslink junctional membrane proteins (i.e. ZO-1/2/3, PATJ, PAR-3, and PAR-6) to the actin cytoskeleton as well as to different intracellular signaling components. Both alcohol and its metabolites affect the integrity of TJs.
Several studies in the literature suggest the role of acetaldehyde (one of the metabolites of alcohol) in increasing intestinal permeability[30,38,39]. Acetaldehyde causes the redistribution of tight junction proteins (occludin and ZO-1) and adherens junction (E-cadherin and β-catenin) proteins from the intercellular junctions[40,41]. Furthermore, acetaldehyde increases the tyrosine phosphorylation of ZO-1, E-cadherin, and β-catenin, without affecting tyrosine kinase activity[40]. Acetaldehyde also disrupts the interactions between E-cadherin, β-catenin, and PTP1B, which are the vital components of adherens junctions and epithelial cell-cell adhesion[42]. These studies indicate the central role of acetaldehyde in disruption of gut integrity, however, not much is known about the effects of other metabolic products of alcohol on gut permeability.
Increased expression of inducible nitric oxide synthase (iNOS) is another factor by which alcohol disrupts the intestinal barrier function. Increase in iNOS, NO, and superoxide correlates with an increase in nitration and oxidation of tubulin, causing increased levels of disassembled tubulin that subsequently damage the microtubule cytoskeleton and result in disruption of barrier function in alcohol treated CaCo2 cells[43,44]. NF-κB is involved in oxidation-induced upregulation of iNOS as well in nitration and oxidation of cytoskeleton[45]. Interestingly epidermal growth factor has a protective role in intestinal barrier function via downregulation of iNOS activity, which results in the stabilization of cytoskeleton[46-48].
Not only chronic alcohol intake results in the disruption of intestinal barrier, but acute alcohol consumption also damages intestinal mucosal membrane, as reported in a rat model[49]. In a mouse model, a single dose of acute ethanol (6 g/kg) causes injury to the mucosal lining of the small intestine[50].
Another mechanism by which alcohol increases intestinal permeability is by indirectly affecting tight junction proteins through miRs. In particular, a recent study showed the involvement of miRs in gut barrier disruption in alcohol treated cells. miR-212 targets the ZO-1 protein negatively, thus increasing intestinal permeability[51]. Consistent with this in vitro observation, higher levels of miR-212 and lower amounts of ZO-1 protein were found in colon biopsy tissues from patients with ALD[51]. However, more work needs to be done to explore the role of miRs in regulating tight and adherent junction proteins in ALD.
Chronic alcohol abuse not only causes gut leakiness, but also affects the composition of colonic mucosa-associated bacterial microbiota in alcohol-fed rats[52]; however, the latter finding needs to be validated in human subjects. While there is evidence of bacterial overgrowth (Gram negative) in the gut of alcoholics[53], little is known about how alcohol consumption is related to increased intestinal bacterial growth. Interestingly, we do not know whether alcohol consumption affects Gram-positive bacteria, which are the source of peptidoglycan. Nevertheless, increased peptidoglycan levels were found in mice after prolonged administration of alcohol in their drinking water. Interestingly, this mode of alcohol administration does not result in ALD[54].
LPS is a major component of the outer membrane of Gram-negative bacteria and it comprises three distinct parts: a carbohydrate “O-antigen”, the oligosaccharide core region, and a lipid portion “lipid A”. Only the lipid A moiety is toxic and is responsible for the activation of the innate immune response in mammals[55]. LPS and other bacterial cell wall constituents are released during bacterial multiplication or when bacteria die or lyse[56]. As soon the immune system recognizes the presence of microorganisms (bacteremia) or LPS in the blood stream (endotoxemia), various proinflammatory cytokines, chemokines, ROS, and other mediators are released to activate monocytes, macrophages, and to recruit lymphocytes. The liver plays an important role in the body’s defense mechanism against bacteria and bacterial products.
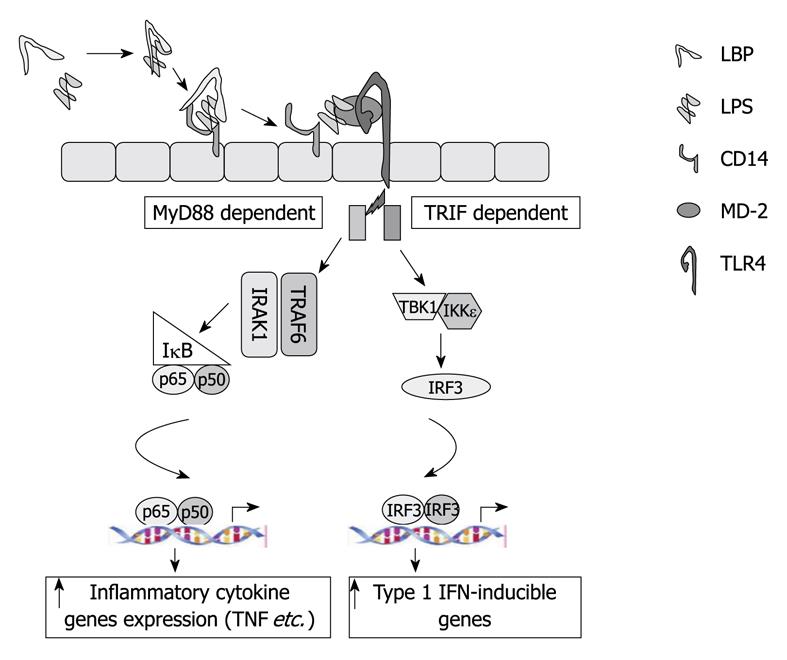
LPS is recognized by various receptors in the cells. CR3 (CD11b/CD18) was the first described LPS receptor[57] in human macrophages. Later on, cluster of differentiation 14 (CD14) and LPS binding protein (LBP) were recognized as receptors for LPS[58]. Recently, myeloid differentiation factor-2 (MD-2) was found as another LPS binding molecule (direct binding)[59]. However, CD14 and MD-2 lack a transmembrane domain and, therefore, a second receptor is required to activate the signaling cascade, which was recently described as TLR4 (indirect binding)[60,61].
Toll receptors were first discovered in Drosophila[62] and later on their human homologs were identified[63]. TLR4 recognizes LPS with the cooperation of its co-receptors, CD14 or MD-2[64,65]. LPS recognition by TLR4 results in recruitment of the adaptor molecules MyD88 and TRIF, which each activate separate downstream signaling cascades (Figure 2). Formation of the TLR4-MyD88 complex activates the IRAK kinases, which turn on the IKK complex to activate NF-κB, which results in increased production of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-1β[64]. Activation of TRIF pathway results in TBK/IKKe phosphorylation and activation of the interferon regulatory factor-3 (IRF3), which leads to induction of Type-I interferons (IFNs)[66]. Activation of both of the pro-inflammatory and Type-I IFN pathways by TLR4-LPS is unique, and evaluation of these specific pathways has recently received attention in ALD.
The TLR4-LPS signaling pathway plays a critical role in alcohol-induced liver injury. Both chronic and acute (or binge) alcohol use affect the various components of TLR4 signaling[67-71]. The effect of alcohol use on TLR4 signaling was recently reviewed in detail[3]. There is increased expression of TLR4 and its co-receptors, as well as other TLRs, in ALD in mice. Early studies in TLR4 mutant mice demonstrated protection from early ALD[72] and more recent reports using TLR4 deficient mice validated the important role of TLR4 in the pathogenesis of ALD[73]. We also investigated the specific role of the MyD88 adapter in ALD and found that MyD88-deficient mice were not protected from alcoholic steatosis and inflammation. Consistent with the hypothesis that MyD88-independent, TLR4-mediated, pathways are involved in ALD, we found protection from ALD in TLR4-deficient, as well as in IRF3-deficient, mice[73]. The role of IRF3 in ALD was also indicated by another study[74].
MD-2 is a type II acute phase protein and is expressed on the surface of myeloid and endothelial lineage cells[75,76]. Although it lacks a transmembrane domain, it attaches to the cell surface through its interaction with TLR4[77,78]. MD-2 also presents in a soluble form (sMD-2) and is secreted by various cells[79,80]. Increased sMD-2 activity is found in plasma of sepsis patients[81,82]. It is postulated that at high concentrations, sMD-2 might inhibit endotoxin induced cell activation in a similar way to LBP and soluble CD14[78,83]. IL-1β regulates the production of MD-2 in hepatocytes and myeloid cells[83]. Chronic alcohol feeding results in an upregulation of MD-2 in the liver[73].
CD14 is expressed in various cell types, including monocytes, macrophages, B cells, liver parenchymal cells, and some fibroblast cells[84,85]. It is absent in early myeloid progenitor cells; however, with maturation, its expression increases. Human CD14 transgenic mice are hypersensitive to LPS[86], whereas CD14 knockout mice are resistant to endotoxin shock[87], indicating its crucial role in LPS signaling. CD14 is also present in soluble forms, as sCD14 α and sCD14 β, and is secreted by macrophages[88] and liver parenchyma cells[89].
Alcohol consumption affects CD14 expression and plays an important role in LPS induced immune activation in alcoholics. Increased expression of CD14 is found in Kupffer cells or whole livers of chronic ethanol-fed animals[25,90,91]. A correlation between CD14 expression and the severity of ALD has been reported in humans and it has been suggested that CD14 is one of risk factor in ethanol-induced pathology[92,93]. Interestingly, acute alcohol treatment also induces CD14 expression in whole liver cells[94] and CD14-deficient mice were protected from alcohol-induced liver steatosis[92].
LBP is an acute phase protein and is induced by LPS, IL-6, and IL-1β[95,96]. Although liver is a major source of LBP production, other organs, such as lungs, kidneys, and heart, also produce LBP[97]. This protein is present in normal serum; however, its levels become elevated during acute phase responses[98,99]. LBP catalyzes the transfer of LPS to CD14, and thus enhances the LPS-induced activation of monocytes, macrophages, and other immune cells[100]. Anti-LBP antibodies, together with LPS, protected the mice from death[101]. Neutralization of LBP protects the host from LPS-induced toxicity, suggesting its critical role in innate immunity[102].
In addition to its pro-inflammatory role, it also acts as an antiinflammatory, where it transfers LPS (Gram negative) or LTA (Gram positive) to HDL and other lipoproteins, and also aids the neutralization of LPS[103]. The antiinflammatory role of this protein is well described in various reports[99,104-107]. It is postulated that low concentrations of LBP enhance the LPS-induced activation of mononuclear cells, whereas the acute-phase rise in LBP concentrations inhibit LPS-induced immune cell activation[108].
Not much is known about the role of LBP in alcoholics, except one report where its role is described in early alcohol-induced liver injury where it enhances the production of cytokines, such as TNF-α. Ethanol fed LBP KO mice showed reduced TNF-α expression and reduced liver damage[109]. There was no change in endotoxin levels of both wild-type and LBP knockout mice; however, decreased steatosis in LBP knockout ethanol-fed mice was observed[109]. A potential antiinflammatory role of the above mentioned LBPs in the pathogenesis of ALD is yet to be explored.
In summary, it appears that LBPs and receptors modulate the LPS response bifunctionally, either by neutralizing or enhancing its response.
There is ample evidence for increased inflammatory cascade activation in ALD[3]. Alcoholic steatohepatitis is characterized by infiltration of various inflammatory cells into the liver, including neutrophils, leukocytes, monocytes, and macrophages and this occurs as a result of chemokine activation (e.g. IL-8, MCP-1, and MIPs)[110-112]. In humans with alcoholic steatohepatitis, serum TNF-α, IL-6, and IL-8 levels are increased and there is also evidence for activation of circulating monocytes based on increased TNF-α production and increased NF-κB activation[113-115]. Serum levels and liver expression of these LPS-inducible pro-inflammatory cytokines are also increased in animal models of ALD[73]. Isolated Kupffer cells from mice and rats show increased production of TNF-α after chronic alcohol feeding[116] and this has been linked to increased TNF-α mRNA stability, as well as to upregulation of Erk, MAPK, and Egr-1 kinases[117]. While LPS has been proposed to play a major role in Kupffer cell and macrophage activation in ALD, in vitro studies in human monocytes/macrophages suggest that chronic alcohol exposure itself can promote a pro-inflammatory phenotype and amplify LPS-induced pro-inflammatory responses[71]. Our laboratory showed that increased LPS responsiveness after chronic alcohol exposure in monocytes is due to reduced expression of IRAK-M, which is a negative regulator of TLR4 activation[71]. Thus, chronic alcohol exposure alone not only results in pro-inflammatory activation of macrophages, but also sensitizes cells to LPS-induced pro-inflammatory signals[71].
TLR4, the LPS receptor, is expressed in all cell types in the liver; thus, gut-derived endotoxin can modulate the function of all liver cells in ALD[3]. In hepatocytes, LPS can promote apoptosis, particularly in combination with other hepatotoxins[118,119]. TLR4 expression in hepatic stellate cells (HSC) has been shown to mediate inflammatory signaling by LPS and manifests in activation of Jnk kinase and NF-κB[120]. Oxidative stress induced by alcohol and its metabolites has also been shown to sensitize HSC to LPS-induce activation and subsequent induction of hepatic fibrosis[121,122]. Thus, LPS affects hepatocytes as well as HSC, both directly and via inflammatory cell activation.
The balance of gut microbial flora, intestinal permeability, hepatocyte function, and Kupffer cell activation appears to be critical in the maintenance of normal homeostasis (Figure 1). Indeed, increasing evidence suggests an importance for a gut-liver connection in different liver diseases where gut-derived LPS delivered to the liver through the portal circulation might play a role. For example, increased intestinal permeability was detected in patients with intrahepatic cholestasis of pregnancy[123], and in hepatitis C virus (HCV)-induced liver injury in human immunodeficiency virus infected individuals[124]. An increase in serum endotoxin levels was associated with pro-inflammatory activation of circulating monocytes in chronic HCV infection, even in the absence of cirrhosis[125]. These observations underscore the importance of the gut-liver axis in the pathogenesis of ALD, as well as in other types of liver injuries.
The gut-liver axis, particularly gut-derived endotoxin, seems to play a crucial role in the pathogenesis of liver diseases caused by various insults, including alcohol. However, the mechanisms and source of endotoxin in liver diseases are not fully understood. The importance of alcohol-induced alterations in the gut and the role of the liver in elimination of gut-derived pathogen-derived compounds require further investigation. Furthermore, interactions between immune, non-immune, and parenchymal cells, which take place in vivo, contribute and determine the progression of ALD. Understanding the role of TLR signaling and the cell-specific effects of gut-derived microbial products will provide new insights, not only into the pathomechanisms of ALD, but might also reveal new targets for therapeutic interventions.
Peer reviewers: Yuichi Yoshida, MD, PhD, Assistant Professor, Department of Gastroenterology and Hepatology, Osaka University, 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan; Mark J Czaja, MD, Liver Research Center, Albert Einstein College of Medicine, 1300 Morris Park Ave, Bronx, NY 10461, United States
S- Editor Tian L L- Editor Stewart GJ E- Editor Zheng XM
| 1. | Adachi M, Brenner DA. Clinical syndromes of alcoholic liver disease. Dig Dis. 2005;23:255-263. [Cited in This Article: ] |
| 2. | Tilg H, Day CP. Management strategies in alcoholic liver disease. Nat Clin Pract Gastroenterol Hepatol. 2007;4:24-34. [Cited in This Article: ] |
| 3. | Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258-1266. [Cited in This Article: ] |
| 4. | Hritz I, Velayudham A, Dolganiuc A, Kodys K, Mandrekar P, Kurt-Jones E, Szabo G. Bone marrow-derived immune cells mediate sensitization to liver injury in a myeloid differentiation factor 88-dependent fashion. Hepatology. 2008;48:1342-1347. [Cited in This Article: ] |
| 5. | Szabo G. Moderate drinking, inflammation, and liver disease. Ann Epidemiol. 2007;17:S49-S54. [Cited in This Article: ] |
| 6. | Mello T, Polvani S, Galli A. Peroxisome proliferator-activated receptor and retinoic x receptor in alcoholic liver disease. PPAR Res. 2009;2009:748174. [Cited in This Article: ] |
| 7. | Duerkop BA, Vaishnava S, Hooper LV. Immune responses to the microbiota at the intestinal mucosal surface. Immunity. 2009;31:368-376. [Cited in This Article: ] |
| 8. | Wu D, Cederbaum AI. Oxidative stress and alcoholic liver disease. Semin Liver Dis. 2009;29:141-154. [Cited in This Article: ] |
| 9. | Yajima S, Morisaki H, Serita R, Suzuki T, Katori N, Asahara T, Nomoto K, Kobayashi F, Ishizaka A, Takeda J. Tumor necrosis factor-alpha mediates hyperglycemia-augmented gut barrier dysfunction in endotoxemia. Crit Care Med. 2009;37:1024-1030. [Cited in This Article: ] |
| 10. | Bode C, Bode JC. Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol Clin Exp Res. 2005;29:166S-171S. [Cited in This Article: ] |
| 11. | Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20:453-460. [Cited in This Article: ] |
| 12. | Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218-224. [Cited in This Article: ] |
| 13. | Verstak B, Nagpal K, Bottomley SP, Golenbock DT, Hertzog PJ, Mansell A. MyD88 adapter-like (Mal)/TIRAP interaction with TRAF6 is critical for TLR2- and TLR4-mediated NF-kappaB proinflammatory responses. J Biol Chem. 2009;284:24192-24203. [Cited in This Article: ] |
| 14. | Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783-801. [Cited in This Article: ] |
| 15. | Schafer SL, Lin R, Moore PA, Hiscott J, Pitha PM. Regulation of type I interferon gene expression by interferon regulatory factor-3. J Biol Chem. 1998;273:2714-2720. [Cited in This Article: ] |
| 16. | Dolganiuc A, Norkina O, Kodys K, Catalano D, Bakis G, Marshall C, Mandrekar P, Szabo G. Viral and host factors induce macrophage activation and loss of toll-like receptor tolerance in chronic HCV infection. Gastroenterology. 2007;133:1627-1636. [Cited in This Article: ] |
| 17. | Roth J, McClellan JL, Kluger MJ, Zeisberger E. Attenuation of fever and release of cytokines after repeated injections of lipopolysaccharide in guinea-pigs. J Physiol. 1994;477:177-185. [Cited in This Article: ] |
| 18. | Messingham KA, Faunce DE, Kovacs EJ. Alcohol, injury, and cellular immunity. Alcohol. 2002;28:137-149. [Cited in This Article: ] |
| 19. | Szabo G, Mandrekar P, Girouard L, Catalano D. Regulation of human monocyte functions by acute ethanol treatment: decreased tumor necrosis factor-alpha, interleukin-1 beta and elevated interleukin-10, and transforming growth factor-beta production. Alcohol Clin Exp Res. 1996;20:900-907. [Cited in This Article: ] |
| 20. | McClain CJ, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology. 1989;9:349-351. [Cited in This Article: ] |
| 21. | Vreugdenhil AC, Snoek AM, van 't Veer C, Greve JW, Buurman WA. LPS-binding protein circulates in association with apoB-containing lipoproteins and enhances endotoxin-LDL/VLDL interaction. J Clin Invest. 2001;107:225-234. [Cited in This Article: ] |
| 22. | Medvedev AE, Lentschat A, Wahl LM, Golenbock DT, Vogel SN. Dysregulation of LPS-induced Toll-like receptor 4-MyD88 complex formation and IL-1 receptor-associated kinase 1 activation in endotoxin-tolerant cells. J Immunol. 2002;169:5209-5216. [Cited in This Article: ] |
| 23. | Nanji AA, Khettry U, Sadrzadeh SM, Yamanaka T. Severity of liver injury in experimental alcoholic liver disease. Correlation with plasma endotoxin, prostaglandin E2, leukotriene B4, and thromboxane B2. Am J Pathol. 1993;142:367-373. [Cited in This Article: ] |
| 24. | Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet. 1984;1:179-182. [Cited in This Article: ] |
| 25. | Enomoto N, Schemmer P, Ikejima K, Takei Y, Sato N, Brenner DA, Thurman RG. Long-term alcohol exposure changes sensitivity of rat Kupffer cells to lipopolysaccharide. Alcohol Clin Exp Res. 2001;25:1360-1367. [Cited in This Article: ] |
| 26. | Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease). Proc Soc Exp Biol Med. 1994;205:243-247. [Cited in This Article: ] |
| 27. | Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol. 2009;50:538-547. [Cited in This Article: ] |
| 28. | Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32:1008-1017. [Cited in This Article: ] |
| 29. | Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol. 1987;4:8-14. [Cited in This Article: ] |
| 30. | Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G881-G884. [Cited in This Article: ] |
| 31. | Yumuk Z, Ozdemirci S, Erden BF, Dundar V. The effect of long-term ethanol feeding on Brucella melitensis infection of rats. Alcohol Alcohol. 2001;36:314-317. [Cited in This Article: ] |
| 32. | Kavanaugh MJ, Clark C, Goto M, Kovacs EJ, Gamelli RL, Sayeed MM, Choudhry MA. Effect of acute alcohol ingestion prior to burn injury on intestinal bacterial growth and barrier function. Burns. 2005;31:290-296. [Cited in This Article: ] |
| 33. | Rao RK. Acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Alcohol Clin Exp Res. 1998;22:1724-1730. [Cited in This Article: ] |
| 34. | Wheeler MD, Kono H, Yin M, Nakagami M, Uesugi T, Arteel GE, Gäbele E, Rusyn I, Yamashina S, Froh M. The role of Kupffer cell oxidant production in early ethanol-induced liver disease. Free Radic Biol Med. 2001;31:1544-1549. [Cited in This Article: ] |
| 35. | Thakur V, McMullen MR, Pritchard MT, Nagy LE. Regulation of macrophage activation in alcoholic liver disease. J Gastroenterol Hepatol. 2007;22 Suppl 1:S53-S56. [Cited in This Article: ] |
| 36. | Koop DR, Klopfenstein B, Iimuro Y, Thurman RG. Gadolinium chloride blocks alcohol-dependent liver toxicity in rats treated chronically with intragastric alcohol despite the induction of CYP2E1. Mol Pharmacol. 1997;51:944-950. [Cited in This Article: ] |
| 37. | Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605-G611. [Cited in This Article: ] |
| 38. | Rao RK. Acetaldehyde-induced barrier disruption and paracellular permeability in Caco-2 cell monolayer. Methods Mol Biol. 2008;447:171-183. [Cited in This Article: ] |
| 39. | Ferrier L, Bérard F, Debrauwer L, Chabo C, Langella P, Buéno L, Fioramonti J. Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. Am J Pathol. 2006;168:1148-1154. [Cited in This Article: ] |
| 40. | Atkinson KJ, Rao RK. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1280-G1288. [Cited in This Article: ] |
| 41. | Seth A, Basuroy S, Sheth P, Rao RK. L-Glutamine ameliorates acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Am J Physiol Gastrointest Liver Physiol. 2004;287:G510-G517. [Cited in This Article: ] |
| 42. | Sheth P, Seth A, Atkinson KJ, Gheyi T, Kale G, Giorgianni F, Desiderio DM, Li C, Naren A, Rao R. Acetaldehyde dissociates the PTP1B-E-cadherin-beta-catenin complex in Caco-2 cell monolayers by a phosphorylation-dependent mechanism. Biochem J. 2007;402:291-300. [Cited in This Article: ] |
| 43. | Banan A, Fields JZ, Decker H, Zhang Y, Keshavarzian A. Nitric oxide and its metabolites mediate ethanol-induced microtubule disruption and intestinal barrier dysfunction. J Pharmacol Exp Ther. 2000;294:997-1008. [Cited in This Article: ] |
| 44. | Tang Y, Forsyth CB, Farhadi A, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ, Keshavarzian A. Nitric oxide-mediated intestinal injury is required for alcohol-induced gut leakiness and liver damage. Alcohol Clin Exp Res. 2009;33:1220-1230. [Cited in This Article: ] |
| 45. | Banan A, Keshavarzian A, Zhang L, Shaikh M, Forsyth CB, Tang Y, Fields JZ. NF-kappaB activation as a key mechanism in ethanol-induced disruption of the F-actin cytoskeleton and monolayer barrier integrity in intestinal epithelium. Alcohol. 2007;41:447-460. [Cited in This Article: ] |
| 46. | Sheth P, Seth A, Thangavel M, Basuroy S, Rao RK. Epidermal growth factor prevents acetaldehyde-induced paracellular permeability in Caco-2 cell monolayer. Alcohol Clin Exp Res. 2004;28:797-804. [Cited in This Article: ] |
| 47. | Basuroy S, Sheth P, Mansbach CM, Rao RK. Acetaldehyde disrupts tight junctions and adherens junctions in human colonic mucosa: protection by EGF and L-glutamine. Am J Physiol Gastrointest Liver Physiol. 2005;289:G367-G375. [Cited in This Article: ] |
| 48. | Banan A, Choudhary S, Zhang Y, Fields JZ, Keshavarzian A. Ethanol-induced barrier dysfunction and its prevention by growth factors in human intestinal monolayers: evidence for oxidative and cytoskeletal mechanisms. J Pharmacol Exp Ther. 1999;291:1075-1085. [Cited in This Article: ] |
| 49. | Tamai H, Kato S, Horie Y, Ohki E, Yokoyama H, Ishii H. Effect of acute ethanol administration on the intestinal absorption of endotoxin in rats. Alcohol Clin Exp Res. 2000;24:390-394. [Cited in This Article: ] |
| 50. | Zhou Z, Wang L, Song Z, Lambert JC, McClain CJ, Kang YJ. A critical involvement of oxidative stress in acute alcohol-induced hepatic TNF-alpha production. Am J Pathol. 2003;163:1137-1146. [Cited in This Article: ] |
| 51. | Tang Y, Banan A, Forsyth CB, Fields JZ, Lau CK, Zhang LJ, Keshavarzian A. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol Clin Exp Res. 2008;32:355-364. [Cited in This Article: ] |
| 52. | Mutlu E, Keshavarzian A, Engen P, Forsyth CB, Sikaroodi M, Gillevet P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res. 2009;33:1836-1846. [Cited in This Article: ] |
| 53. | Bode C, Bode JC. Effect of alcohol consumption on the gut. Best Pract Res Clin Gastroenterol. 2003;17:575-592. [Cited in This Article: ] |
| 54. | Cook RT, Schlueter AJ, Coleman RA, Tygrett L, Ballas ZK, Jerrells TR, Nashelsky MB, Ray NB, Haugen TH, Waldschmidt TJ. Thymocytes, pre-B cells, and organ changes in a mouse model of chronic ethanol ingestion--absence of subset-specific glucocorticoid-induced immune cell loss. Alcohol Clin Exp Res. 2007;31:1746-1758. [Cited in This Article: ] |
| 55. | Raetz CR, Reynolds CM, Trent MS, Bishop RE. Lipid A modification systems in gram-negative bacteria. Annu Rev Biochem. 2007;76:295-329. [Cited in This Article: ] |
| 56. | Hellman J, Loiselle PM, Tehan MM, Allaire JE, Boyle LA, Kurnick JT, Andrews DM, Sik Kim K, Warren HS. Outer membrane protein A, peptidoglycan-associated lipoprotein, and murein lipoprotein are released by Escherichia coli bacteria into serum. Infect Immun. 2000;68:2566-2572. [Cited in This Article: ] |
| 57. | Wright SD, Jong MT. Adhesion-promoting receptors on human macrophages recognize Escherichia coli by binding to lipopolysaccharide. J Exp Med. 1986;164:1876-1888. [Cited in This Article: ] |
| 58. | Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431-1433. [Cited in This Article: ] |
| 59. | Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777-1782. [Cited in This Article: ] |
| 60. | Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085-2088. [Cited in This Article: ] |
| 61. | Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749-3752. [Cited in This Article: ] |
| 62. | Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973-983. [Cited in This Article: ] |
| 63. | Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394-397. [Cited in This Article: ] |
| 64. | Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3-9. [Cited in This Article: ] |
| 65. | da Silva Correia J, Soldau K, Christen U, Tobias PS, Ulevitch RJ. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex. transfer from CD14 to TLR4 and MD-2. J Biol Chem. 2001;276:21129-21135. [Cited in This Article: ] |
| 66. | Kawai T, Takeuchi O, Fujita T, Inoue J, Mühlradt PF, Sato S, Hoshino K, Akira S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol. 2001;167:5887-5894. [Cited in This Article: ] |
| 67. | Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101-108. [Cited in This Article: ] |
| 68. | Dai Q, Pruett SB. Different effects of acute and chronic ethanol on LPS-induced cytokine production and TLR4 receptor behavior in mouse peritoneal macrophages. J Immunotoxicol. 2006;3:217-225. [Cited in This Article: ] |
| 69. | Pruett SB, Fan R. Ethanol inhibits LPS-induced signaling and modulates cytokine production in peritoneal macrophages in vivo in a model for binge drinking. BMC Immunol. 2009;10:49. [Cited in This Article: ] |
| 70. | Szabo G, Dolganiuc A, Dai Q, Pruett SB. TLR4, ethanol, and lipid rafts: a new mechanism of ethanol action with implications for other receptor-mediated effects. J Immunol. 2007;178:1243-1249. [Cited in This Article: ] |
| 71. | Mandrekar P, Bala S, Catalano D, Kodys K, Szabo G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J Immunol. 2009;183:1320-1327. [Cited in This Article: ] |
| 72. | Romics L Jr, Kodys K, Dolganiuc A, Graham L, Velayudham A, Mandrekar P, Szabo G. Diverse regulation of NF-kappaB and peroxisome proliferator-activated receptors in murine nonalcoholic fatty liver. Hepatology. 2004;40:376-385. [Cited in This Article: ] |
| 73. | Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, Kurt-Jones E, Szabo G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224-1231. [Cited in This Article: ] |
| 74. | Zhao XJ, Dong Q, Bindas J, Piganelli JD, Magill A, Reiser J, Kolls JK. TRIF and IRF-3 binding to the TNF promoter results in macrophage TNF dysregulation and steatosis induced by chronic ethanol. J Immunol. 2008;181:3049-3056. [Cited in This Article: ] |
| 75. | Gruber A, Mancek M, Wagner H, Kirschning CJ, Jerala R. Structural model of MD-2 and functional role of its basic amino acid clusters involved in cellular lipopolysaccharide recognition. J Biol Chem. 2004;279:28475-28482. [Cited in This Article: ] |
| 76. | Miyake K, Nagai Y, Akashi S, Nagafuku M, Ogata M, Kosugi A. Essential role of MD-2 in B-cell responses to lipopolysaccharide and Toll-like receptor 4 distribution. J Endotoxin Res. 2002;8:449-452. [Cited in This Article: ] |
| 77. | Re F, Strominger JL. Separate functional domains of human MD-2 mediate Toll-like receptor 4-binding and lipopolysaccharide responsiveness. J Immunol. 2003;171:5272-5276. [Cited in This Article: ] |
| 78. | Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191-1195. [Cited in This Article: ] |
| 79. | da Silva Correia J, Ulevitch RJ. MD-2 and TLR4 N-linked glycosylations are important for a functional lipopolysaccharide receptor. J Biol Chem. 2002;277:1845-1854. [Cited in This Article: ] |
| 80. | Visintin A, Iliev DB, Monks BG, Halmen KA, Golenbock DT. MD-2. Immunobiology. 2006;211:437-447. [Cited in This Article: ] |
| 81. | Pugin J, Stern-Voeffray S, Daubeuf B, Matthay MA, Elson G, Dunn-Siegrist I. Soluble MD-2 activity in plasma from patients with severe sepsis and septic shock. Blood. 2004;104:4071-4079. [Cited in This Article: ] |
| 82. | Viriyakosol S, McCray PB, Ashbaugh ME, Chu J, Jia HP, Weiss J, Kirkland TN. Characterization of monoclonal antibodies to human soluble MD-2 protein. Hybridoma (Larchmt). 2006;25:349-357. [Cited in This Article: ] |
| 83. | Tissières P, Araud T, Ochoda A, Drifte G, Dunn-Siegrist I, Pugin J. Cooperation between PU.1 and CAAT/enhancer-binding protein beta is necessary to induce the expression of the MD-2 gene. J Biol Chem. 2009;284:26261-26272. [Cited in This Article: ] |
| 84. | Antal-Szalmas P, Strijp JA, Weersink AJ, Verhoef J, Van Kessel KP. Quantitation of surface CD14 on human monocytes and neutrophils. J Leukoc Biol. 1997;61:721-728. [Cited in This Article: ] |
| 85. | Liu S, Khemlani LS, Shapiro RA, Johnson ML, Liu K, Geller DA, Watkins SC, Goyert SM, Billiar TR. Expression of CD14 by hepatocytes: upregulation by cytokines during endotoxemia. Infect Immun. 1998;66:5089-5098. [Cited in This Article: ] |
| 86. | Ferrero E, Jiao D, Tsuberi BZ, Tesio L, Rong GW, Haziot A, Goyert SM. Transgenic mice expressing human CD14 are hypersensitive to lipopolysaccharide. Proc Natl Acad Sci USA. 1993;90:2380-2384. [Cited in This Article: ] |
| 87. | Haziot A, Ferrero E, Köntgen F, Hijiya N, Yamamoto S, Silver J, Stewart CL, Goyert SM. Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity. 1996;4:407-414. [Cited in This Article: ] |
| 88. | Durieux JJ, Vita N, Popescu O, Guette F, Calzada-Wack J, Munker R, Schmidt RE, Lupker J, Ferrara P, Ziegler-Heitbrock HW. The two soluble forms of the lipopolysaccharide receptor, CD14: characterization and release by normal human monocytes. Eur J Immunol. 1994;24:2006-2012. [Cited in This Article: ] |
| 89. | Pan Z, Zhou L, Hetherington CJ, Zhang DE. Hepatocytes contribute to soluble CD14 production, and CD14 expression is differentially regulated in hepatocytes and monocytes. J Biol Chem. 2000;275:36430-36435. [Cited in This Article: ] |
| 90. | Dai LL, Gong JP, Zuo GQ, Wu CX, Shi YJ, Li XH, Peng Y, Deng W, Li SW, Liu CA. Synthesis of endotoxin receptor CD14 protein in Kupffer cells and its role in alcohol-induced liver disease. World J Gastroenterol. 2003;9:622-626. [Cited in This Article: ] |
| 91. | Kishore R, Hill JR, McMullen MR, Frenkel J, Nagy LE. ERK1/2 and Egr-1 contribute to increased TNF-alpha production in rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol. 2002;282:G6-G15. [Cited in This Article: ] |
| 92. | Yin M, Bradford BU, Wheeler MD, Uesugi T, Froh M, Goyert SM, Thurman RG. Reduced early alcohol-induced liver injury in CD14-deficient mice. J Immunol. 2001;166:4737-4742. [Cited in This Article: ] |
| 93. | Järveläinen HA, Orpana A, Perola M, Savolainen VT, Karhunen PJ, Lindros KO. Promoter polymorphism of the CD14 endotoxin receptor gene as a risk factor for alcoholic liver disease. Hepatology. 2001;33:1148-1153. [Cited in This Article: ] |
| 94. | Wheeler MD, Thurman RG. Up-regulation of CD14 in liver caused by acute ethanol involves oxidant-dependent AP-1 pathway. J Biol Chem. 2003;278:8435-8441. [Cited in This Article: ] |
| 95. | Wan Y, Freeswick PD, Khemlani LS, Kispert PH, Wang SC, Su GL, Billiar TR. Role of lipopolysaccharide (LPS), interleukin-1, interleukin-6, tumor necrosis factor, and dexamethasone in regulation of LPS-binding protein expression in normal hepatocytes and hepatocytes from LPS-treated rats. Infect Immun. 1995;63:2435-2442. [Cited in This Article: ] |
| 96. | Schumann RR, Zweigner J. A novel acute-phase marker: lipopolysaccharide binding protein (LBP). Clin Chem Lab Med. 1999;37:271-274. [Cited in This Article: ] |
| 97. | Su GL, Freeswick PD, Geller DA, Wang Q, Shapiro RA, Wan YH, Billiar TR, Tweardy DJ, Simmons RL, Wang SC. Molecular cloning, characterization, and tissue distribution of rat lipopolysaccharide binding protein. Evidence for extrahepatic expression. J Immunol. 1994;153:743-752. [Cited in This Article: ] |
| 98. | Gallay P, Heumann D, Le Roy D, Barras C, Glauser MP. Lipopolysaccharide-binding protein as a major plasma protein responsible for endotoxemic shock. Proc Natl Acad Sci USA. 1993;90:9935-9938. [Cited in This Article: ] |
| 99. | Zweigner J, Gramm HJ, Singer OC, Wegscheider K, Schumann RR. High concentrations of lipopolysaccharide-binding protein in serum of patients with severe sepsis or septic shock inhibit the lipopolysaccharide response in human monocytes. Blood. 2001;98:3800-3808. [Cited in This Article: ] |
| 100. | Schumann RR, Leong SR, Flaggs GW, Gray PW, Wright SD, Mathison JC, Tobias PS, Ulevitch RJ. Structure and function of lipopolysaccharide binding protein. Science. 1990;249:1429-1431. [Cited in This Article: ] |
| 101. | Gallay P, Heumann D, Le Roy D, Barras C, Glauser MP. Mode of action of anti-lipopolysaccharide-binding protein antibodies for prevention of endotoxemic shock in mice. Proc Natl Acad Sci USA. 1994;91:7922-7926. [Cited in This Article: ] |
| 102. | Le Roy D, Di Padova F, Tees R, Lengacher S, Landmann R, Glauser MP, Calandra T, Heumann D. Monoclonal antibodies to murine lipopolysaccharide (LPS)-binding protein (LBP) protect mice from lethal endotoxemia by blocking either the binding of LPS to LBP or the presentation of LPS/LBP complexes to CD14. J Immunol. 1999;162:7454-7460. [Cited in This Article: ] |
| 103. | Wurfel MM, Kunitake ST, Lichenstein H, Kane JP, Wright SD. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. J Exp Med. 1994;180:1025-1035. [Cited in This Article: ] |
| 104. | Grunfeld C, Marshall M, Shigenaga JK, Moser AH, Tobias P, Feingold KR. Lipoproteins inhibit macrophage activation by lipoteichoic acid. J Lipid Res. 1999;40:245-252. [Cited in This Article: ] |
| 105. | Hamann L, Stamme C, Ulmer AJ, Schumann RR. Inhibition of LPS-induced activation of alveolar macrophages by high concentrations of LPS-binding protein. Biochem Biophys Res Commun. 2002;295:553-560. [Cited in This Article: ] |
| 106. | Hamann L, Alexander C, Stamme C, Zähringer U, Schumann RR. Acute-phase concentrations of lipopolysaccharide (LPS)-binding protein inhibit innate immune cell activation by different LPS chemotypes via different mechanisms. Infect Immun. 2005;73:193-200. [Cited in This Article: ] |
| 107. | Zweigner J, Schumann RR, Weber JR. The role of lipopolysaccharide-binding protein in modulating the innate immune response. Microbes Infect. 2006;8:946-952. [Cited in This Article: ] |
| 108. | Gutsmann T, Müller M, Carroll SF, MacKenzie RC, Wiese A, Seydel U. Dual role of lipopolysaccharide (LPS)-binding protein in neutralization of LPS and enhancement of LPS-induced activation of mononuclear cells. Infect Immun. 2001;69:6942-6950. [Cited in This Article: ] |
| 109. | Uesugi T, Froh M, Arteel GE, Bradford BU, Wheeler MD, Gäbele E, Isayama F, Thurman RG. Role of lipopolysaccharide-binding protein in early alcohol-induced liver injury in mice. J Immunol. 2002;168:2963-2969. [Cited in This Article: ] |
| 110. | Apte UM, Banerjee A, McRee R, Wellberg E, Ramaiah SK. Role of osteopontin in hepatic neutrophil infiltration during alcoholic steatohepatitis. Toxicol Appl Pharmacol. 2005;207:25-38. [Cited in This Article: ] |
| 111. | Ramaiah SK, Jaeschke H. Hepatic neutrophil infiltration in the pathogenesis of alcohol-induced liver injury. Toxicol Mech Methods. 2007;17:431-440. [Cited in This Article: ] |
| 112. | Purohit V, Russo D. Cellular and molecular mechanisms of alcoholic hepatitis: introduction and summary of the symposium. Alcohol. 2002;27:3-6. [Cited in This Article: ] |
| 113. | Gobejishvili L, Barve S, Joshi-Barve S, Uriarte S, Song Z, McClain C. Chronic ethanol-mediated decrease in cAMP primes macrophages to enhanced LPS-inducible NF-kappaB activity and TNF expression: relevance to alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2006;291:G681-G688. [Cited in This Article: ] |
| 114. | Latvala J, Hietala J, Koivisto H, Järvi K, Anttila P, Niemelä O. Immune Responses to Ethanol Metabolites and Cytokine Profiles Differentiate Alcoholics with or without Liver Disease. Am J Gastroenterol. 2005;100:1303-1310. [Cited in This Article: ] |
| 115. | McClain CJ, Hill DB, Song Z, Deaciuc I, Barve S. Monocyte activation in alcoholic liver disease. Alcohol. 2002;27:53-61. [Cited in This Article: ] |
| 116. | Kishore R, McMullen MR, Cocuzzi E, Nagy LE. Lipopolysaccharide-mediated signal transduction: Stabilization of TNF-alpha mRNA contributes to increased lipopolysaccharide-stimulated TNF-alpha production by Kupffer cells after chronic ethanol feeding. Comp Hepatol. 2004;3 Suppl 1:S31. [Cited in This Article: ] |
| 117. | Thakur V, Pritchard MT, McMullen MR, Wang Q, Nagy LE. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production. J Leukoc Biol. 2006;79:1348-1356. [Cited in This Article: ] |
| 118. | Kudo H, Takahara T, Yata Y, Kawai K, Zhang W, Sugiyama T. Lipopolysaccharide triggered TNF-alpha-induced hepatocyte apoptosis in a murine non-alcoholic steatohepatitis model. J Hepatol. 2009;51:168-175. [Cited in This Article: ] |
| 119. | Nagaki M, Sugiyama A, Osawa Y, Naiki T, Nakashima S, Nozawa Y, Moriwaki H. Lethal hepatic apoptosis mediated by tumor necrosis factor receptor, unlike Fas-mediated apoptosis, requires hepatocyte sensitization in mice. J Hepatol. 1999;31:997-1005. [Cited in This Article: ] |
| 120. | Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37:1043-1055. [Cited in This Article: ] |
| 121. | Karaa A, Thompson KJ, McKillop IH, Clemens MG, Schrum LW. S-adenosyl-L-methionine attenuates oxidative stress and hepatic stellate cell activation in an ethanol-LPS-induced fibrotic rat model. Shock. 2008;30:197-205. [Cited in This Article: ] |
| 122. | Quiroz SC, Bucio L, Souza V, Hernández E, González E, Gómez-Quiroz L, Kershenobich D, Vargas-Vorackova F, Gutiérrez-Ruiz MC. Effect of endotoxin pretreatment on hepatic stellate cell response to ethanol and acetaldehyde. J Gastroenterol Hepatol. 2001;16:1267-1273. [Cited in This Article: ] |
| 123. | Piao W, Song C, Chen H, Diaz MA, Wahl LM, Fitzgerald KA, Li L, Medvedev AE. Endotoxin tolerance dysregulates MyD88- and Toll/IL-1R domain-containing adapter inducing IFN-beta-dependent pathways and increases expression of negative regulators of TLR signaling. J Leukoc Biol. 2009;86:863-875. [Cited in This Article: ] |
| 124. | De Nardo D, Nguyen T, Hamilton JA, Scholz GM. Down-regulation of IRAK-4 is a component of LPS- and CpG DNA-induced tolerance in macrophages. Cell Signal. 2009;21:246-252. [Cited in This Article: ] |
| 125. | Huang Y, Blatt LM, Taylor MW. Type 1 interferon as an antiinflammatory agent: inhibition of lipopolysaccharide-induced interleukin-1 beta and induction of interleukin-1 receptor antagonist. J Interferon Cytokine Res. 1995;15:317-321. [Cited in This Article: ] |