Published online Feb 14, 2009. doi: 10.3748/wjg.15.717
Revised: January 7, 2009
Accepted: January 14, 2009
Published online: February 14, 2009
AIM: To assess the value of widely used clinical scores in the early identification of acute pancreatitis (AP) patients who are likely to suffer from intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS).
METHODS: Patients (n = 44) with AP recruited in this study were divided into two groups (ACS and non-ACS) according to intra-abdominal pressure (IAP) determined by indirect measurement using the transvesical route via Foley bladder catheter. On admission and at regular intervals, the severity of the AP and presence of organ dysfunction were assessed utilizing different multifactorial prognostic systems: Glasgow-Imrie score, Acute Physiology and Chronic Health Evaluation II (APACHE-II) score, and Multiorgan Dysfunction Score (MODS). The diagnostic performance of scores predicting ACS development, cut-off values and specificity and sensitivity were established using receiver operating characteristic (ROC) curve analysis.
RESULTS: The incidence of ACS in our study population was 19.35%. IAP at admission in the ACS group was 22.0 (18.5-25.0) mmHg and 9.25 (3.0-12.4) mmHg in the non-ACS group (P < 0.01). Univariate statistical analysis revealed that patients in the ACS group had significantly higher multifactorial clinical scores (APACHE II, Glasgow-Imrie and MODS) on admission and higher maximal scores during hospitalization (P < 0.01). ROC curve analysis revealed that APACHE II, Glasgow-Imrie, and MODS are valuable tools for early prediction of ACS with high sensitivity and specificity, and that cut-off values are similar to those used for stratification of patients with severe acute pancreatitis (SAP).
CONCLUSION: IAH and ACS are rare findings in patients with mild AP. Based on the results of our study we recommend measuring the IAP in cases when patients present with SAP (APACHE II > 7; MODS > 2 or Glasgow-Imrie score > 3).
- Citation: Dambrauskas Z, Parseliunas A, Gulbinas A, Pundzius J, Barauskas G. Early recognition of abdominal compartment syndrome in patients with acute pancreatitis. World J Gastroenterol 2009; 15(6): 717-721
- URL: https://www.wjgnet.com/1007-9327/full/v15/i6/717.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.717
Acute pancreatitis (AP) remains a disease with an unpredictable clinical course, and significant associated morbidity and mortality[1]. Recently, the elevated intra-abdominal pressure (IAP) following the onset of AP has attracted growing attention, because it is increasingly recognized as an important risk factor for mortality in the early phase of the disease[2–4]. It was shown that intra-abdominal hypertension (IAH) is associated with higher mortality and morbidity rates, and prolonged ICU stay, in comparison to other patients who had normal IAP[5–8]. IAH has been recognized as a cause of organ dysfunction in critically ill patients, including those suffering from severe acute pancreatitis (SAP)[9–12]. Abdominal compartment syndrome (ACS) is defined as an increase of IAP > 20 mmHg, which is associated with occurrence of a new organ failure. A previously reported incidence of ACS among patients with SAP ranges from 23% to 56%[1113–15]. The mechanisms involved in the development of IAH and ACS include increased capillary permeability, hypoalbuminemia and volume overload, which produce a large retroperitoneal and visceral edema[616].
It has been shown that early recognition and treatment of IAH and ACS result in a significant improvement in patient survival and decreased morbidity. Due to its simplicity and minimal cost, the standard for intermittent IAP measurement is via the urinary bladder with a maximal instillation volume of 25 mL sterile saline[17]. Compared with bladder pressure measurements, clinical abdominal assessment showed poor sensitivity (56%) and accuracy (77%) for identifying elevated IAP[18]. It was shown that the essential approach to diagnosis and management of ACS is a timely IAP measurement. It is still not clear whether early IAP measurement should be routine for all AP patients and which patients would benefit most from the IAP monitoring.
This study aimed to assess the value of Acute Physiology and Chronic Health Evaluation II (APACHE II), Multiorgan Dysfunction Score (MODS) and Glasgow-Imrie clinical scores in early recognition of patients who are likely to suffer from IAH and ACS, and who would benefit from IAP monitoring and management. We also investigated the incidence of ACS, the role of its interventional management and clinical outcomes in patients with AP.
This work has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. The Regional Ethics Committee approved the study (protocols no. BE-2-47 and P1-113/2005) and all patients provided written informed consent.
The study population included 44 patients with AP admitted to the Department of Surgery, Kaunas University of Medicine Hospital, from May 2007 to February 2008. General inclusion criteria were defined as follows: (1) a time interval between onset of typical abdominal symptoms and study inclusion of 72 h and less; (2) at least 3-fold elevated serum amylase or lipase levels; (3) no previous history of acute or chronic pancreatitis. On the first day of admission, the severity of the AP and presence of organ dysfunction were assessed utilizing three different multifactorial prognostic systems: Glasgow-Imrie score, APACHE-II score, and MODS. Later, the severity of disease and clinical status were repeatedly reassessed using the same prognostic tools every 7 d, and when the deterioration of clinical condition occurred and after interventional treatment of ACS. The contrast-enhanced CT scan was performed on day 4 to 7 after the onset of disease to demonstrate the presence of pancreatic necrosis. According to the clinical course and clinical severity scores (APACHE II > 7; Glasgow-Imrie > 2; MODS > 2; peak C-reactive protein value > 150 mg/L) patients were stratified into mild and severe AP groups. The data were prospectively recorded in a specially created database. All patients were treated according to our standard AP management protocol following the recent international guidelines.
For IAP measurement, we used a standard two-way 16 Fr. Foley catheter inserted into the urinary bladder. The patient was placed in supine position. Twenty-five milliliters of 0.9% sterile NaCl were instilled and the catheter was connected to a tube from the urine collection bag. The pubic symphysis was considered level 0 and IAP was measured in cm H2O, then recalculated in mmHg. IAP was measured every 24 h during a period of 3 d in all patients. For patients that developed IAH (IAP > 12 mmHg), the conservative treatment (according to the recommendations of international experts on IAH and ACS) was initiated and IAP was monitored every 12 h until the normal IAP was reached and sustained at least for 24 h. In cases when IAH > 18 mmHg was recorded, IAP was monitored every 4-6 h until IAP normalized or ACS developed. ACS was defined as an increase of IAP > 20 mmHg, which is associated with occurrence of a new organ failure[1617].
Statistical analysis was performed using SPSS® for Windows release 16.0 (SPSS, Chicago, IL, USA). The quantitative variables are presented as mean ± SD or median (with interquartile range). For comparison between groups, the Mann-Whitney test, Student’s t test or χ2 test was employed where appropriate. The diagnostic performance of scores predicting ACS development, cut-off values and specificity and sensitivity of prognostic tools were established using receiver operating characteristic (ROC) curve analysis. Results with P < 0.05 were considered statistically significant.
A total of 44 patients with AP were included in the study. Demographic and clinical data of these patients are represented in Table 1.
| Demographic and clinical variables | Values |
| Number of patents | 44 |
| Age (years ± SD) | 49 ± 18 |
| Gender male | 70.4% (31/44) |
| SAP | 70.4% (31/44) |
| Presence of necrosis | 49.9% (18/44) |
| Extent (percentage) of necrosis | |
| < 30% | 38.9% (7/18) |
| 30%-50% | 11.1% (2/18) |
| > 50% | 50.0% (9/18) |
| IAH (IAP ≥ 12 mmHg) | 43.2% (19/44) |
| ACS (IAP ≥ 20 mmHg + MOF) | 13.6% (6/44) |
| Mortality | 13.6% (6/44) |
| APACHE II score at admission1 | 6.5 (4.0-10.0) |
| Max APACHE II score during hospitalization1 | 8.0 (5.0-11.0) |
| Glasgow-Imrie score at admission1 | 3.0 (2.0-3.0) |
| Max Glasgow-Imrie score during hospitalization1 | 3.0 (2.0-4.0) |
| MODS score at admission1 | 2.0 (1.0-3.0) |
| Max MODS score during hospitalization1 | 2.0 (1.0-4.0) |
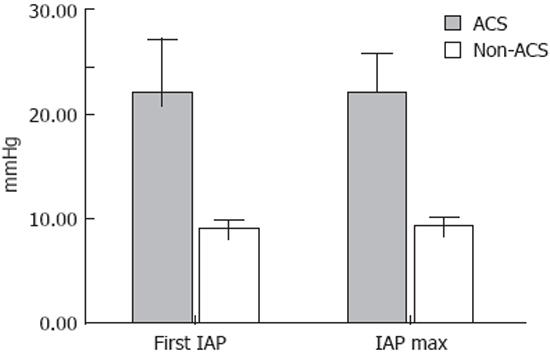
All patients were divided into ACS and non-ACS groups. Median IAP in the ACS group at admission was 22.0 (18.5-25.0) mmHg and 9.25 (3.0-12.4) mmHg in the non-ACS group (P < 0.01) (Figure 1).
Differences of APACHE II, Glasgow-Imrie and MODS median scores on admission and maximal scores (Max) during hospitalization period between ACS and non-ACS groups are presented in Table 2. The study revealed that patients in the ACS group had significantly higher multifactorial clinical scores on admission and higher maximal scores during hospitalization (P < 0.01). There were no significant differences between median admission and maximal scores of APACHE II, Glasgow-Imrie and MODS within ACS or non-ACS groups (P > 0.05). The ACS group was characterized by a markedly higher incidence of severe and necrotizing AP, and by the presence of high volume pancreatic necrosis in comparison to the non-ACS group. Mortality rate in the ACS group was also significantly higher, when compared to the non-ACS group (Table 3).
| Clinical scores | ACS group median (lower & upper quartiles) | Non-ACS group median (lower & upper quartiles) | P |
| APACHE II score on admission | 12.0 (9.0-1.0) | 6.0 (4.0-9.0) | < 0.01 |
| Max APACHE score | 14.0 (11.0-18.0) | 7.0 (4.0-10.0) | < 0.01 |
| Glasgow-Imrie score on admission | 5.0 (4.0-5.0) | 2.0 (2.0-3.0) | < 0.01 |
| Max Glasgow-Imrie score | 5.0 (4.0-5.0) | 2.5 (1.0-3.0) | < 0.01 |
| MODS score on admission | 4.5 (3.0-8.0) | 1.5 (1.0-3.0) | < 0.01 |
| Max MODS score | 4.5 (3.0-8.0) | 2.0 (1.0-3.0) | < 0.01 |
| Clinical characteristics | ACS group (n = 6, %) | Non-ACS group (n = 38, %) | P |
| Severe AP | 6 (100) | 25 (65.8) | NS |
| Necrotizing AP | 5 (83.3) | 13 (34.2) | < 0.05 |
| Necrosis > 30% | 5 (83.3) | 6 (15.8) | < 0.05 |
| Deaths | 4 (66.6) | 2 (5.2) | < 0.01 |
SAP was diagnosed in 70.4% (31/44) of all cases in this study group. We believe such a relatively high incidence of SAP is associated with the concentration of patients with severe disease in our tertiary care center referred from other regional hospitals, and a special focus on the patients with systemic inflammatory response syndrome and multiorgan failure (MOF). Nevertheless, the incidence of ACS in our study population was 19.35% (6/31) and did not exceed the prevalence of IAH and ACS shown in other clinical studies. Interestingly, the prevalence of IAH was significantly lower in the mild AP group, with only 7.69% (1/13) when compared to 58.06% (18/31) in the SAP group (P > 0.01). Specifically, all cases of ACS occurred in the SAP group with an incidence of 19.35% (6/31), while there were no cases of ACS in the mild AP group (0/13).
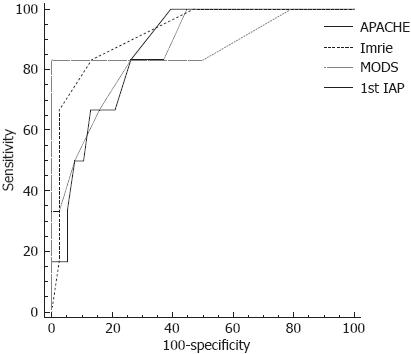
The diagnostic performance of multifactorial clinical scores and the first IAP measurement in predicting development of ACS during the course of AP were assessed using ROC curve analysis. ROC analysis revealed that all the analysed clinical scores are of good prognostic value in determining patients who are likely to further develop ACS (Figure 2). The areas under the ROC curves, cut-off values, specificity and sensitivity of prognostic tools are presented in Tables 4 and 5.
| Variables | Area | Std. error | Asymptotic sig | Confidence Interval | |
| lower | upper | ||||
| 1st IAP on admission | 0.932 | 0.065 | 0.001 | 0.805 | 1.059 |
| Glasgow-Imrie score | 0.921 | 0.054 | 0.001 | 0.816 | 1.026 |
| APACHE II score | 0.866 | 0.062 | 0.004 | 0.745 | 0.987 |
| MODS score | 0.829 | 0.098 | 0.010 | 0.636 | 1.022 |
| Variable | Cut-off | Sensitivity (%) | Specificity (%) |
| APACHE II score | > 7 | 100.0 (54.1-100.0) | 60.5 (43.4-75.9) |
| Glasgow-Imrie score | > 3 | 83.3 (36.1-97.2) | 86.8 (71.9-95.5) |
| MODS score | > 2 | 83.3 (36.1-97.2) | 73.7 (56.9- 86.6) |
| 1st IAP on admission | > 18 | 83.3 (36.1-97.2) | 100.0 (90.7-100.0) |
Our study confirmed the earlier published observations that AP is a risk factor for development of IAH and ACS[13141920]. Overall, somewhat higher rates of SAP in our institution could be explained by the concentration of the patients, because our hospital is a tertiary care center and many patients with suspected severe disease are referred to it from regional hospitals. However, the incidence of IAH and ACS in our study was similar to that observed in other studies, and as expected, it was associated with a higher incidence of MOF and higher mortality rates.
An early diagnosis of ACS and its adequate management is crucial[181517]. The measurement and monitoring of IAP via urinary bladder catheter is a simple procedure, which requires virtually no technical skills and little resources. However, this procedure is invasive and is associated with significant discomfort for the patient[2122]. It has also been shown that indwelling urinary catheters are associated with a higher incidence of infectious complications and prevalence of nosocomial pathogens[23–25]. Clearly, placement of a urinary catheter should not be routinely recommended for all patients, especially not for those who are unlikely to develop ACS. Therefore clinical assessment in selecting the patients that are likely to develop ACS is of particular importance. Our study demonstrated that development of IAH and ACS during the AP could be predicted by the use of clinical multifactorial scoring systems (APACHE II, MODS, Glasgow-Imrie score), thus allowing a timely and appropriate selection of patients for this invasive procedure during the first hours and days of the disease. Clinical scores of patients who eventually suffered from IAH were higher during the first days in comparison to the group of patients with normal IAP. These findings are in accord with the observations of other groups[242026]. The ROC analysis disclosed that APACHE II, MODS, and Glasgow-Imrie scores have similar cut-off values to those used for the prediction of SAP. IAP > 18 mmHg on admission is also a valuable indicator that the patient has a higher risk for persistent IAH and development of ACS during the course of AP. All these prognostic markers had a good sensitivity, specificity and large area under the curve. Furthermore, the use of a clinical scoring system in combination with the first IAP measurement (eg. APACHE II + first IAP on admission) allows us to identify nearly 100% of patients who are likely to develop SAP and suffer from ACS.
Previously published studies do not provide us with any useful recommendations or criteria for the selection of the AP patients for the IAP measurement and monitoring, although it would be unnecessary in the majority of cases when patients have a mild and self-limiting disease.
Based on the results of our study we recommend measuring the IAP only in cases when patients present with SAP (i.e. APACHE II > 7; MODS > 2 or Glasgow-Imrie score > 3). We advocate a continuous monitoring of IAP in all cases when the patient suffers from SAP and has an IAP > 18 mmHg on first measurement. We would recommend utilizing the simpler Glasgow-Imrie or MODS scores in daily clinical practice and a more complex APACHE II score in the clinical trial setting.
Placement of a urinary catheter for the monitoring of IAP would be unnecessary in the majority of AP cases, when patients have a mild and self-limiting disease.
We recommend measuring the IAP only in cases when patients present with SAP (i.e. APACHE II > 7; MODS > 2 or Glasgow-Imrie score > 3). We advocate a continuous monitoring of IAP in all cases when the patient suffers from SAP and has an IAP > 18 mmHg on first measurement.
Acute pancreatitis (AP) remains a disease with an unpredictable clinical course, and significant associated morbidity and mortality. Recently, the elevated intra-abdominal pressure (IAP) after onset of AP has gained growing attention, because it is increasingly recognized as an important risk factor for mortality in the early phase of the disease. Intra-abdominal hypertension (IAH) has been recognized as a cause of organ dysfunction in critically ill patients, including those suffering from severe acute pancreatitis (SAP).
It has been shown that early recognition and treatment of IAH and abdominal compartment syndrome (ACS) result in a significant improvement in patient survival and decreased morbidity, however, clinical abdominal assessment showed poor sensitivity and accuracy for identifying the elevated IAP. The essential approach to the diagnosis and management of ACS is a timely IAP measurement. It is still not clear whether early IAP measurement should be a routine for all AP patients and which patients would benefit most from the IAP monitoring.
An early diagnosis of ACS and its adequate management is crucial. The measurement and monitoring of IAP via urinary bladder catheter is a simple procedure, which requires virtually no technical skills and little resources. However, this procedure is invasive and is associated with significant discomfort for the patient. It has also been shown that indwelling urinary catheters are associated with a higher incidence of infectious complications and prevalence of nosocomial pathogens. Clearly, placement of a urinary catheter should not be routinely recommended for all patients, especially not for those who are unlikely to develop ACS. For the first time, our study demonstrated that development of IAH and ACS during the AP could be predicted by the use of clinical multifactorial scoring systems [Acute Physiology and Chronic Health Evaluation II (APACHE-II), Multiorgan Dysfunction Score (MODS), Glasgow-Imrie score], thus allowing a timely and appropriate selection of patients for this invasive procedure during the first hours and days of the disease.
Based on the results of our study, we recommend measuring the IAP only in cases when patients present with SAP (i.e. APACHE II > 7; MODS > 2 or Glasgow-Imrie score > 3). They advocate a continuous monitoring of IAP in all cases when the patient suffers from SAP and has an IAP > 18 mmHg on first measurement.
ACS is a severe increase in the pressure within the abdomen (IAP) such that a patient’s internal organs begin to fail and malfunction. This is a medical emergency. Untreated, ACS has a high mortality rate. There are a number of different methods that your doctor may use to treat the ACS. These may include giving medications to sedate or temporarily paralyze you or your loved one, placing tubes through the nose and into the stomach to remove fluid and air, placing tubes into the abdomen to remove fluid or blood, or opening the abdomen to release the increased pressure. Most patients with IAH and/or ACS will be cared for in an ICU where doctors and nurses constantly monitor for signs of illness and treat patients to keep their heart, lungs, kidneys, liver, and intestines functioning as normally as possible.
An important aspect of AP has been addressed in this paper, as not much has been written about IAP and ACS in relation to AP. The study design is simple and clear.
| 1. | Wilmer A. ICU management of severe acute pancreatitis. Eur J Intern Med. 2004;15:274-280. [Cited in This Article: ] |
| 2. | Buter A, Imrie CW, Carter CR, Evans S, McKay CJ. Dynamic nature of early organ dysfunction determines outcome in acute pancreatitis. Br J Surg. 2002;89:298-302. [Cited in This Article: ] |
| 3. | Dugernier T, Reynaert M, Laterre PF. Early multi-system organ failure associated with acute pancreatitis: a plea for a conservative therapeutic strategy. Acta Gastroenterol Belg. 2003;66:177-183. [Cited in This Article: ] |
| 4. | Khan AA, Parekh D, Cho Y, Ruiz R, Selby RR, Jabbour N, Genyk YS, Mateo R. Improved prediction of outcome in patients with severe acute pancreatitis by the APACHE II score at 48 hours after hospital admission compared with the APACHE II score at admission. Acute Physiology and Chronic Health Evaluation. Arch Surg. 2002;137:1136-1140. [Cited in This Article: ] |
| 5. | Burch JM, Moore EE, Moore FA, Franciose R. The abdominal compartment syndrome. Surg Clin North Am. 1996;76:833-842. [Cited in This Article: ] |
| 6. | Ivatury RR, Diebel L, Porter JM, Simon RJ. Intra-abdominal hypertension and the abdominal compartment syndrome. Surg Clin North Am. 1997;77:783-800. [Cited in This Article: ] |
| 7. | Meldrum DR, Moore FA, Moore EE, Franciose RJ, Sauaia A, Burch JM. Prospective characterization and selective management of the abdominal compartment syndrome. Am J Surg. 1997;174:667-672; discussion 672-673. [Cited in This Article: ] |
| 8. | Sugrue M, Jones F, Deane SA, Bishop G, Bauman A, Hillman K. Intra-abdominal hypertension is an independent cause of postoperative renal impairment. Arch Surg. 1999;134:1082-1085. [Cited in This Article: ] |
| 9. | Balogh Z, McKinley BA, Cocanour CS, Kozar RA, Holcomb JB, Ware DN, Moore FA. Secondary abdominal compartment syndrome is an elusive early complication of traumatic shock resuscitation. Am J Surg. 2002;184:538-543; discussion 543-544. [Cited in This Article: ] |
| 10. | Malbrain ML, Chiumello D, Pelosi P, Wilmer A, Brienza N, Malcangi V, Bihari D, Innes R, Cohen J, Singer P. Prevalence of intra-abdominal hypertension in critically ill patients: a multicentre epidemiological study. Intensive Care Med. 2004;30:822-829. [Cited in This Article: ] |
| 11. | Malbrain ML, Chiumello D, Pelosi P, Bihari D, Innes R, Ranieri VM, Del Turco M, Wilmer A, Brienza N, Malcangi V. Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: a multiple-center epidemiological study. Crit Care Med. 2005;33:315-322. [Cited in This Article: ] |
| 12. | Tao HQ, Zhang JX, Zou SC. Clinical characteristics and management of patients with early acute severe pancreatitis: experience from a medical center in China. World J Gastroenterol. 2004;10:919-921. [Cited in This Article: ] |
| 13. | Al-Bahrani AZ, Abid GH, Holt A, McCloy RF, Benson J, Eddleston J, Ammori BJ. Clinical relevance of intra-abdominal hypertension in patients with severe acute pancreatitis. Pancreas. 2008;36:39-43. [Cited in This Article: ] |
| 14. | De Waele JJ, Hoste E, Blot SI, Decruyenaere J, Colardyn F. Intra-abdominal hypertension in patients with severe acute pancreatitis. Crit Care. 2005;9:R452-R457. [Cited in This Article: ] |
| 15. | Pupelis G, Austrums E, Snippe K, Berzins M. Clinical significance of increased intraabdominal pressure in severe acute pancreatitis. Acta Chir Belg. 2002;102:71-74. [Cited in This Article: ] |
| 16. | Malbrain ML, Cheatham ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, Balogh Z, Leppaniemi A, Olvera C, Ivatury R. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med. 2006;32:1722-1732. [Cited in This Article: ] |
| 17. | Cheatham ML, Malbrain ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, Balogh Z, Leppaniemi A, Olvera C, Ivatury R. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. II. Recommendations. Intensive Care Med. 2007;33:951-962. [Cited in This Article: ] |
| 18. | Kirkpatrick AW, Brenneman FD, McLean RF, Rapanos T, Boulanger BR. Is clinical examination an accurate indicator of raised intra-abdominal pressure in critically injured patients? Can J Surg. 2000;43:207-211. [Cited in This Article: ] |
| 19. | Leppaniemi A, Johansson K, De Waele JJ. Abdominal compartment syndrome and acute pancreatitis. Acta Clin Belg Suppl. 2007;43:131-135. [Cited in This Article: ] |
| 20. | Rosas JM, Soto SN, Aracil JS, Cladera PR, Borlan RH, Sanchez AV, Ros FB, Posa LG. Intra-abdominal pressure as a marker of severity in acute pancreatitis. Surgery. 2007;141:173-178. [Cited in This Article: ] |
| 21. | Niel-Weise BS, van den Broek PJ. Urinary catheter policies for short-term bladder drainage in adults. Cochrane Database Syst Rev. 2005;141:CD004203. [Cited in This Article: ] |
| 22. | Saint S, Lipsky BA, Baker PD, McDonald LL, Ossenkop K. Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47:1453-1457. [Cited in This Article: ] |
| 23. | Hatt JK, Rather PN. Role of bacterial biofilms in urinary tract infections. Curr Top Microbiol Immunol. 2008;322:163-192. [Cited in This Article: ] |
| 24. | Nazarko L. Effective evidence based catheter management. Br J Community Nurs. 2008;13:110, 112-110, 114. [Cited in This Article: ] |
| 25. | Sheng WH, Wang JT, Lin MS, Chang SC. Risk factors affecting in-hospital mortality in patients with nosocomial infections. J Formos Med Assoc. 2007;106:110-118. [Cited in This Article: ] |
| 26. | Zhang WF, Ni YL, Cai L, Li T, Fang XL, Zhang YT. Intra-abdominal pressure monitoring in predicting outcome of patients with severe acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2007;6:420-423. [Cited in This Article: ] |