Published online Nov 14, 2009. doi: 10.3748/wjg.15.5326
Revised: September 23, 2009
Accepted: September 30, 2009
Published online: November 14, 2009
AIM: To ascertain the usefulness of a histological scoring system devised to assist in the interpretation of liver histology in neonatal cholestasis (NC).
METHODS: Liver biopsy specimens obtained from infants with NC referred to a tertiary pediatric unit in Malaysia were prospectively studied. The first author, blinded to the final diagnosis, devised the histological diagnosis based on a 7-feature (portal ductal proliferation, bile plugs in portal ductules, porto-portal bridging, lymphocytic infiltration in portal region, multinucleated hepatocytes, neutrophilic infiltration, hepatocellular swelling), 15-point (0 to 15) scoring system. The author classified the histological diagnosis as either biliary atresia (BA) or neonatal hepatitis (NH, all other diagnoses), and subsequently compared the author’s diagnosis with the final diagnosis.
RESULTS: Eighty-four biopsy specimens obtained from 78 patients were reviewed. Without the scoring system, BA was correctly diagnosed by the author histologically in 30 cases, labelled as NH in 3. For other diagnoses, BA was excluded correctly in 33 cases and mislabeled as BA in 2 cases. The overall sensitivity for BA was 91%, specificity 86% and accuracy 88%. With the scoring system, a score of ≥ 7 had the best diagnostic utility to differentiate BA from other intrahepatic cholestasis histologically (sensitivity 88%, specificity 94%, accuracy 92%). Four patients with a score < 7 had BA, and 3 patients with a score ≥ 7 had NH.
CONCLUSION: A 7-feature, 15-point histological scoring system had good diagnostic accuracy in the interpretation of liver histology in neonatal cholestasis.
- Citation: Lee WS, Looi LM. Usefulness of a scoring system in the interpretation of histology in neonatal cholestasis. World J Gastroenterol 2009; 15(42): 5326-5333
- URL: https://www.wjgnet.com/1007-9327/full/v15/i42/5326.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5326
| Parameter | Histological characterization | Histological grade |
| Portal ductal proliferation | None | 0 |
| Mild | 1 | |
| Moderate | 2 | |
| Marked | 3 | |
| Bile plug in portal ductules | Absent | 0 |
| Present | 2 | |
| Porto-portal bridging | None | 0 |
| < 50% of portal tracts | 1 | |
| > 50% of portal tracts | 2 | |
| Lymphocytic infiltrate in portal region | None | 2 |
| Mild | 1 | |
| Moderate/severe | 0 | |
| Multinucleated hepatocytes | None | 2 |
| Only around central vein | 1 | |
| Diffuse | 0 | |
| Neutrophils in the infiltrate | Absent or mild | 1 |
| Moderate or marked | 0 | |
| Hepatocellular swelling | None | 2 |
| Mild/focal | 1 | |
| Periportal/diffuse | 0 |
| Biliary atresia (n = 33) | Intra-hepatic cholestasis (n = 50) | All (n = 83) | |
| Age at biopsy (d) | |||
| Median (range) | 65 (34-371) | 62 (26-180) | 63 (26-371) |
| Route of biopsy | |||
| Percutaneous | 16 | 37 | 53 |
| Operative | 17 | 13 | 30 |
| Number of portal tracts | |||
| < 5 | 4 | 20 | 24 |
| ≥ 5 | 29 | 30 | 59 |
| Author’s histological diagnoses | ||||
| Biliary atresia | Neonatal hepatitis | Paucity bile | Final clinical diagnosis ducts | |
| Biliary atresia | 33 | 30 | 3 | |
| Idiopathic neonatal hepatitis | 35 | 2 | 33 | |
| Cytomegalovirus hepatitis | 6 | 3 | 3 | |
| PFIC | 4 | 1 | 3 | |
| Alagille syndrome | 1 | 1 | ||
| Parenteral nutrition-associated cholestasis | 1 | 1 | ||
| Congenital hypothyroidism | 1 | 1 | ||
| Caroli disease | 1 | 1 | ||
| Severe asphyxia | 1 | 1 | ||
| Total | 83 | 37 | 45 | 1 |
| Patient | Bilirubin total/conj. (μmol/L) | γGT (IU/L) | RHBS | Liver biopsy | Reasons for exploratory laparotomy | ||
| Days | Route | Major histological findings | |||||
| NKC | 154/120 | 179 | Non-excretory | 34 | Percutaneous | Inadequate number of portal tracts. No portal bile duct and pale stools proliferation. Hepatocytic degeneration and swelling, occasional giant cells formation | Persistent jaundice |
| SQ | 189/153 | 442 | Non-excretory | 45 | Percutaneous | Biopsy material fragmented. Giant cells transformation and pale stools. Intra-hepatocytic cholestasis and bile plug formation | Persistent jaundice |
| LQY | 124/109 | 144 | Non-excretory | 78 | Operative | Mild ductular proliferation and lymphocytic infiltration of portal tracts | |
| Histological features | Biliary atresia | Intrahepatic cholestasis1 |
| Ductular proliferation | ||
| None | 1 | 31 |
| Mild | 2 | 11 |
| Moderate | 3 | 4 |
| Severe | 27 | 2 |
| Bile plug in bile ductules | ||
| Absent | 10 | 42 |
| Present | 21 | 7 |
| Porto-portal bridging | ||
| None | 1 | 33 |
| < 50% of portal tracts | 8 | 6 |
| > 50% of portal tracts | 22 | 6 |
| Lymphocytic infiltration | ||
| Moderate to severe | 7 | 23 |
| Mild | 16 | 24 |
| Absent or mild | 10 | 3 |
| Neutrophilic infiltration | ||
| Moderate to severe | 3 | 15 |
| Absent to mild | 30 | 35 |
| Giant cell transformation of hepatocytes | ||
| Diffuse | 1 | 16 |
| Only around central vein | 13 | 23 |
| None | 19 | 11 |
| Hepatocytes swelling | ||
| Hepatocytes swelling | 4 | 27 |
| Mild/focal | 13 | 22 |
| None | 16 | 1 |
| Histological features | BA | Non-BA | Sensitivity for BA (%) | Specificity for BA (%) | Positive PV for BA & negative PV for non-BA (%) | Negative PV for BA & positive PV for non-BA (%) |
| Bile ductular proliferation | ||||||
| Moderate or severe | 30 | 6 | 30/33 (91) | 30/33 (91) | 30/36 (83) | 42/45 (93) |
| None or mild | 3 | 42 | ||||
| Bile plug in bile ductules | ||||||
| Present | 21 | 7 | 21/31 (68) | 42/49 (86) | 21/28 (75) | 42/52 (81) |
| Absent | 10 | 42 | ||||
| Porto-portal bridging | ||||||
| > 50% of portal tracts | 22 | 6 | 22/31 (71) | 39/45 (87) | 22/28 (79) | 39/48 (81) |
| None/< 50% of portal tracts | 9 | 39 |
| Histological features | Non-BA | BA | Sensitivity for BA (%) | Specificity for BA (%) | Positive PV for non-BA & negative PV for BA (%) | Negative PV for non-BA & positive PV for BA (%) |
| Lymphocytic infiltration | ||||||
| Moderate to severe | 23 | 7 | 23/50 (46) | 26/33 (79) | 23/30 (77) | 26/53 (49) |
| Absent or mild | 27 | 26 | ||||
| Neutrophilic infiltration | ||||||
| Moderate to severe | 15 | 3 | 15/50 (30) | 30/33 (91) | 15/18 (83) | 30/65 (46) |
| Absent to mild | 35 | 30 | ||||
| Giant cell transformation of hepatocytes | ||||||
| Diffuse | 16 | 1 | 16/50 (32) | 32/33 (97) | 16/17 (94) | 32/66 (48) |
| None or around central vein | 34 | 32 | ||||
| Hepatocytes swelling | ||||||
| Periportal or diffuse | 27 | 4 | 27/50 (54) | 29/33 (88) | 27/31 (87) | 29/52 (56) |
| None or mild/focal | 23 | 29 | ||||
| Total score | Biliary atresia | Non-biliary atresia |
| 0 | 0 | 3 |
| 1 | 0 | 6 |
| 2 | 1 | 6 |
| 3 | 0 | 11 |
| 4 | 2 | 10 |
| 5 | 0 | 8 |
| 6 | 1 | 0 |
| 7 | 0 | 3 |
| 8 | 4 | 0 |
| 9 | 3 | 1 |
| 10 | 4 | 1 |
| 11 | 3 | 0 |
| 12 | 4 | 0 |
| 13 | 7 | 1 |
| 14 | 4 | 0 |
| < 6 | 4 | 44 |
| ≥ 6 | 29 | 7 |
| < 7 | 4 | 47 |
| ≥ 7 | 29 | 3 |
| < 8 | 8 | 47 |
| ≥ 8 | 25 | 3 |
| Total | 33 | 50 |
| Parameters | Sensitivity | Specificity | Diagnostic accuracy |
| Author’s own interpretation-without scoring system | 91 | 86 | 88 |
| Author’s own interpretation-with scoring system | 88 | 94 | 92 |
| Pathologists’ diagnosis | 82 | 80 | 81 |
A priority in the investigation of infants with neonatal cholestasis (NC) is to distinguish non-obstructive from obstructive causes to facilitate timely surgery for infants with biliary atresia (BA)[1,2]. Liver biopsy is one of the most important diagnostic steps in the evaluation of NC and may be performed safely even in the smallest infants with local anaesthesia and sedation[3,4]. Percutaneous liver biopsy has been found to be particularly helpful in infants suspected of having BA in developing countries[5].
Bile ductular proliferation, bile plugging, multinucleated giant cells, focal necrosis of liver parenchyma, extramedullary haemopoiesis and inflammatory cell infiltrate in the portal area are all well-recognised histological features of BA[3]. Bile ductular proliferation is considered a highly specific and sensitive feature, while the others are less specific and sensitive[6,7]. The histological features of neonatal hepatitis, on the other hand, are more heterogeneous[8,9], with giant-cell hepatitis a prominent feature, but others such as diffuse hepatocyte degeneration, minimal degree of bile ductular proliferation, and portal infiltration of lymphocytes, eosinophils and neutrophils less specific[8,9]. Other authors noted that the only consistent hepatic histological feature of neonatal hepatitis was extramedullary haemopoiesis[10]. Recently, a mathematical approach have been devised to identify the most useful histological feature differentiating BA from other causes of neonatal cholestasis[11]. However, it has not received wide attention due to its complex nature[11].
We devised an objective, 7-feature, 15-point histological scoring system for the interpretation of liver histology to differentiate BA from other causes of NC. Its diagnostic usefulness was tested prospectively in a cohort of infants with NC referred to a tertiary pediatric unit in Malaysia.
This prospective, descriptive study was conducted in the Department of Paediatrics, University of Malaya Medical Centre (UMMC), Kuala Lumpur and was approved by the institutional medical ethics committee.
The first author (Lee WS), blinded to the final clinical diagnosis, conducted the entire histological interpretation process alone and reviewed the histology of liver biopsy specimens obtained from patients with NC. Histological diagnosis based on the biopsy materials available were made, initially without and subsequently with the histological scoring system. The histological diagnosis was then compared with the final clinical diagnosis to ascertain the diagnostic usefulness of the histological scoring system.
All patients with NC referred to the Department of Paediatrics, UMMC from May 2002 to June 2005 were enrolled. NC was defined as the onset of clinically apparent jaundice within the first four months of life, with the conjugated bilirubin greater than 17 μmol/L if the total bilirubin was less than 85 μmol/L, or the conjugated bilirubin more than 20% of the total bilirubin if the total bilirubin was more than 85 μmol/L[12]. All cases of BA were confirmed with operative cholangiogram, or demonstration of atretic gallbladder and/or extrahepatic biliary tree intra-operatively[13,14]. Diagnosis of other causes of NC were made according to standard and acceptable clinical practices and have been described previously[1,15]. The histology review process was conducted in December 2007, more than two years after the final patient in the study cohort was recruited to allow a firm clinical diagnosis to be made in all patients.
All patients with NC who had a liver biopsy were identified. The liver histology slides were traced and were screened for suitability of interpretation by the first author. Any material with faded stain was noted. The original paraffin-embedded tissue block was re-sectioned and re-stained with H&E stain. The biopsy materials were screened for adequacy in size and the number of portal tracts present. All the portal tracts were observed for the number, size and shape of portal bile ductules. The average number of bile ductules present in all the visible portal tracts was noted.
A review of the literature on the histological interpretation of neonatal cholestasis was performed[2,9-11,16-19]. The histological features indicative of BA are bile ductular proliferation, porto-portal bridging, and the presence of bile plugs in the portal ductules[3,10-12,16]. Histological features more indicative of neonatal hepatitis are portal lymphocytic infiltration, neutrophilic infiltration, multinucleated giant cells, and hepatocellular swelling[2,7,16].
For the purpose of histological interpretation, the histological diagnosis was designated as either BA or non-BA. A 15-point scoring system based on histology of liver biopsy, consisting of seven histological criteria regarded as sufficiently specific to differentiate BA from non-BA, was devised (Table 1). Generally a higher score was indicative of BA, while a lower score was less favourable for BA. From the distribution of the score tabulated against the final diagnosis, a cut-off value with highest sensitivity, specificity and diagnostic accuracy differentiating BA from non-BA was determined.
Bile ductular proliferation was considered to be present if the average number of ductules in the portal tract was more than five. The following criteria were used to grade the degree of bile ductular proliferation: no proliferation - average number of bile ductules per portal tract < 5; mild - average number of bile ductules per portal tract between 5 to 9; moderate - average number of bile ductules per portal tract ≥ 10; marked proliferation - elongated, attenuated, angulated bile ductules in addition to proliferation (average number of bile ductules per portal tract ≥ 10).
The author personally interpreted the histology slides blindly without knowledge of the clinical data or the final clinical diagnosis. The route of biopsy was noted by observing the size and shape of the biopsy materials. Generally biopsy materials obtained via the percutaneous route were long and slender, while materials obtained via laparotomy were wedge-shaped. The adequacy of the size of the materials and the number of portal tracts available for interpretation was noted.
A blinded histological diagnosis was made for each histological specimen and was labelled as BA, non-BA (including other causes of cholestasis) or a paucity of bile ductules. For the purpose of statistical analysis, biopsies obtained via different routes (percutaneous or operative) from the same patient were considered as separate cases.
For the purpose of ascertaining the diagnostic accuracy of the newly devised histological scoring system, a two-step interpretation was performed, firstly without the scoring system and them with the scoring system.
The author (Lee WS) was un-blinded and the initial histological diagnosis was compared with the final diagnosis. The sensitivity, specificity and diagnostic accuracy for various diagnostic methods were calculated using the following formulas where TP = true positive, TN = true negative, FP = false positive, and FN = false negative. Sensitivity = TP/(TP + FN); Specificity = TN/(TN + FP); Positive predictive value = TP/(TP + FP); Negative predictive value = TN/(TN + FN); Diagnostic accuracy = (TN + TP)/(TN + TP + FN + FP).
During the study period, 84 liver biopsy specimens for histology were obtained from 78 patients who were referred for diagnostic workup of NC. Six patients had both percutaneous and surgical liver biopsies (Table 2). One biopsy specimen (percutaneous) was fragmented and was insufficient for histological interpretation. Thus the remaining 83 specimens were analysed. The final diagnosis of these 83 specimens from 78 patients with NC is shown in Table 3.
The median age at biopsy for all patients was 63 d (Table 2). Fifty-three (64%) of the biopsy materials were obtained via the percutaneous route while 30 (36%) were obtained surgically. A review of the original histology slides showed that 55 original specimens were adequate for interpretation, while re-staining was necessary in specimens from 28 patients. All 30 specimens obtained during exploratory laparotomy contained more than 5 portal tracts for interpretation. Of the 53 biopsy specimens obtained via the percutaneous route, 25 (47%) specimens contained less than 5 portal tracts. In two specimens, no portal tracts were noted.
The diagnostic accuracy of the author’s blinded histological interpretation is shown in Table 3. BA was correctly diagnosed histologically in 30 cases, but was labelled as non-BA in three cases. Non-BA was identified correctly in 33 cases, and labelled as BA in 2 cases. The remaining 15 patients had intrahepatic cholestasis. The author’s histological diagnosis was BA in 5 cases and neonatal hepatitis in 9 cases. In the only case with Alagille syndrome, paucity of interlobular bile duct was correctly identified. The overall sensitivity of the author’s personal histological interpretation for BA was 91%, sensitivity 86% and diagnostic accuracy 88%.
Seven cases of neonatal hepatitis were mislabeled as BA while three cases of BA were mislabeled as non-BA during initial histology without the scoring system (Table 4). Of the 3 cases of BA misdiagnosed as non-BA, liver biopsies in 2 patients were performed early in the course of illness via the percutaneous route. The number of portal tracts was inadequate for interpretation in both cases.
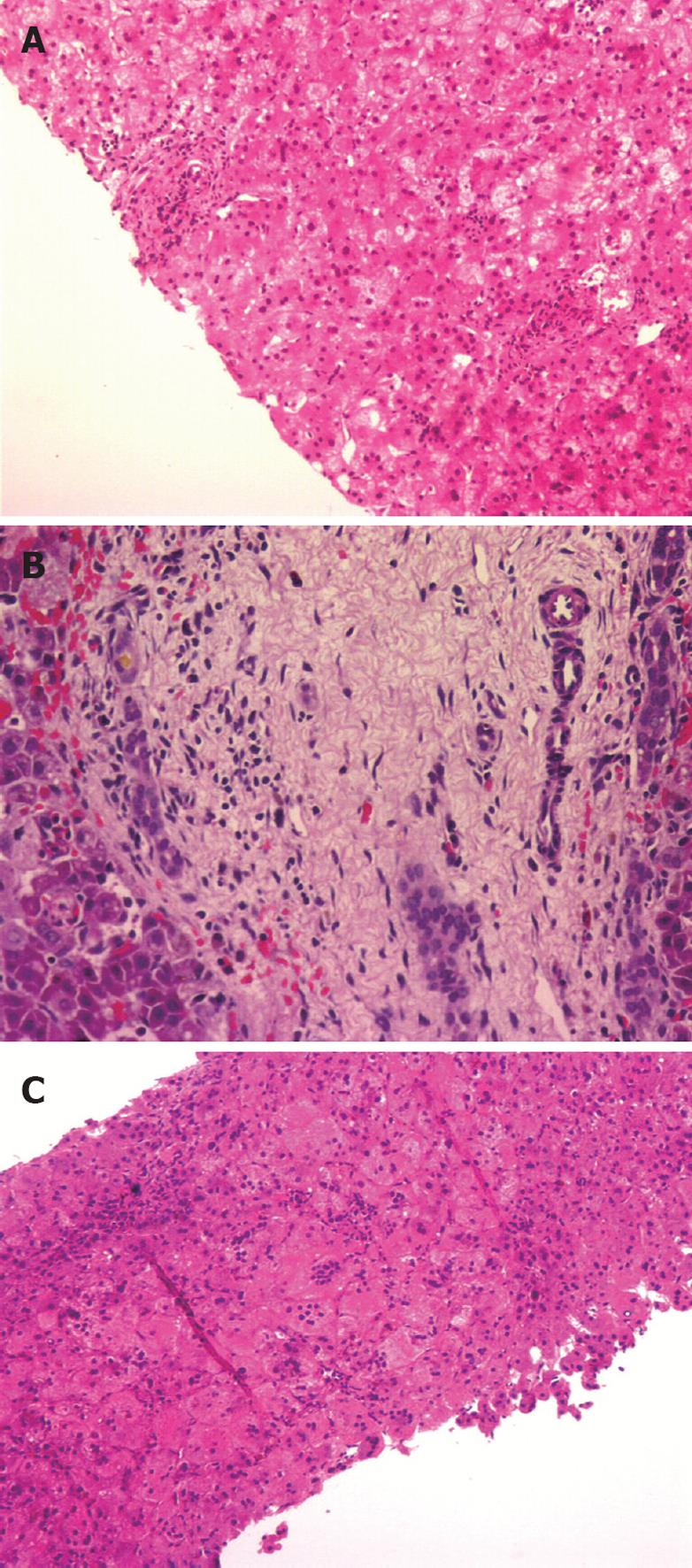
The biopsy specimen of the first patient (NKC, performed at 34 d) contained less than 5 portal tracts (Figure 1A) with no portal bile duct proliferation. There was hepatocytic degeneration and swelling, and occasional giant cell transformation. The patient had persistent pale stools. A radionuclide hepatobiliary scintigraphic study was non-excretory. The child had an exploratory laparotomy at 42 d of age, which confirmed BA. A second liver biopsy obtained surgically showed features of BA with bile ductular proliferation, canalicular bile plugs and intracellular cholestasis (Figure 1B).
The biopsy specimen of the second patient (SQ, at 45 d) was fragmented. The liver parenchyma showed giant cell transformation. There was cholestasis within the hepatocytes and bile plug formation was seen within the canaliculi. The patient has persistent pale stools and had a laparotomy which confirmed BA.
The biopsy specimen of the third patient (LQY), obtained at laparotomy at 78 d of age, showed features of neonatal hepatitis (Figure 1C).
Seven histological features were assessed individually to ascertain their usefulness in differentiating BA from non-BA (Table 5). Three features were found to be present in more than 50% of cases of BA: moderate to severe bile ductular proliferation (91%), presence of bile plugs in bile ductules (70%) and porto-portal bridging involving more than 50% of portal tracts (71%; Tables 6 and 7).
The presence of moderate or severe bile ductular proliferation had the highest sensitivity (91%), specificity (88%) and positive predictive value (83%) for BA (Table 6). In addition to proliferation, the bile ductules were often elongated and tortuous (Figure 1A). The remaining four features, i.e. lymphocytic and neutrophilic infiltrations, giant cell transformation and hepatocytic swelling were uncommon in BA (between 3% and 21%).
Generally, the features of intra-hepatic cholestasis were less sensitive and specific. The only histological feature noted to be present in more than 50% of patients with intrahepatic cholestasis was periportal or diffuse hepatocellular swelling (54%, Table 7). The remaining three features were noted to be present in 25% to 50% of intrahepatic cholestasis. Features indicative of BA (moderate to severe bile ductular proliferation, bile plugs in bile ductules, and porto-portal bridging involving more than 50% of portal tracts, were not common (between 13% and 14%).
Although not sufficiently sensitive (32%), diffuse giant cell transformations of hepatocytes were highly specific (97%) and has a positive predictive value of 94% for intrahepatic cholestasis. The other criterion, periportal or diffuse hepatocyte swelling was also specific (88%) and had a positive predictive value of 87%. The sensitivity was 54%.
With a score of ≥ 6 to differentiate between BA and non-BA histologically, the diagnostic utility of the scoring system was: Sensitivity = 29/(29 + 4) = 88%; Specificity = 44/(44 + 7) = 86%; Positive predictive value = 29/(29 + 3) = 91%; Diagnostic accuracy = (29 + 44)/83 = 88%. With a score of ≥ 7, the diagnostic utility of the scoring system was: Sensitivity = 29/(29 + 4) = 88%; Specificity = 47/(47 + 3) = 94%; Positive predictive value = 29/(29 + 3) = 91%; Diagnostic accuracy = (29 + 47)/83 = 92%; Finally, using a score of ≥ 8, Sensitivity = 25/(25 + 8) = 76%; Specificity= 47/(47 + 3) = 94%; Positive predictive value = 25/(25 + 3) = 89%; Diagnostic accuracy = (25 + 47)/83 = 87%. A score of ≥ 7 had the best diagnostic utility to differentiate between BA and other intrahepatic cholestasis histologically. With this score, four patients with a score < 7 had a final diagnosis of BA while three patients with a score ≥ 7 had neonatal hepatitis.
The results of the present study were compared with histological diagnoses made by the reporting pathologists (Table 9). The diagnostic accuracy of the present study (with scoring system, at 92%) was better than the diagnostic accuracy of 81% made by the reporting pathologists.
The Cholestasis Guideline Committee of NASPGN recommends that a liver biopsy should be performed in most infants with undiagnosed cholestasis and should be interpreted by a pathologist with expertise in paediatric liver disease[12]. A percutaneous liver biopsy is recommended before performing a surgical procedure to diagnose BA. If the biopsy is performed early in the course of the disease (before 6 wk of age), biopsy may have to be repeated if the results are equivocal[12].
In the present study, using conventional H&E staining in 83 liver biopsies obtained from 78 patients with NC, the overall diagnostic accuracy was 88% (Table 4). BA was correctly diagnosed histologically in 30 of 33 cases while non-BA was correctly diagnosed in 33 of 35 cases. This degree of diagnostic accuracy is in keeping with observations made by other authors[3,11,18], but is lower than the degree of diagnostic accuracy reported by Lai et al[4]. Moyer et al[12] reported that with percutaneous liver biopsy, the sensitivity for BA was 89%-99% and specificity was 82.5%-98%.
In the present study, there were 10 histological misdiagnoses with H&E staining without the histological scoring system. Three cases of BA were missed. Of these, the biopsies in 2 cases were obtained early in life (34 and 45 d of age). In all three cases there was no prominent proliferation of bile ductules. In the remaining 7 cases, there was an overdiagnosis of BA in specimens obtained from patients who did not have BA.
With a histological scoring system devised to differentiate BA from other causes of NC using 7 histological criteria, the overall diagnostic accuracy was 92%, which was better than that without the scoring system (88%).
Histological interpretation of hepatic histology in patients with NC requires considerable skills which many general pathologists do not normally possess[11]. The advantage of a histological scoring system, in addition to its higher diagnostic accuracy, is that it provides a more objective and systematic way of assessing liver biopsy materials histologically. This may be the most important reason for the very high diagnostic accuracy achieved with this scoring system. In addition, since more than one criterion was used, it is less influenced by the adequacy of the biopsy materials. The seven histological criteria used in the scoring system during the present study were chosen based on the findings of other authors which showed the greatest discriminatory power between BA and other intrahepatic cholestatic disorders[9,11,16,17].
Another advantage of the scoring system used in the present study was its non-complicated nature. Other authors have also attempted to devise a histological scoring system[10,11]. The scoring system reported by Zerbini et al[11] involved a series of initial complex statistical analyses using a logistic regression method. The accuracy, sensitivity, and specificity were 90.5%, 100% and 75.9%, respectively.
In the present study, the presence of moderate to severe bile ductular proliferation was the most consistent histological feature noted in BA, and was present in 30 of the 33 specimens analysed[11,16,17]. The other two features, i.e. bile plug in bile ductules and porto-portal bridging were not as consistent.
On the other hand, no single histological feature was consistently present in intrahepatic cholestasis. All four features analysed, i.e. lymphocytic and neutrophilic infiltration, giant cell transformation, and hepatocellular swelling, were present in 30% to 54% of cases of intrahepatic cholestasis. This is not surprising as intrahepatic cholestasis is a heterogeneous condition. Parenteral nutrition-associated cholestasis may cause steatosis, bile ductular proliferation and, less commonly bridging fibrosis[20]. CMV hepatitis may cause intense hepatitis, giant cell transformation, intracytoplasmic inclusion bodies, bile stasis, inflammation, fibrosis and bile ductular proliferation[21,22]. On the other hand, conditions such as PFIC and Alagille syndrome caused a paucity of interlobular bile ducts[23].
There are other advantages in using liver biopsies in the investigation of neonatal cholestasis besides its ability of achieving a high degree of diagnostic accuracy. Firstly, there is a shorter time delay of 1 to 3 d, compared to 3 to 5 d for radionuclide hepatobiliary scintigraphy[12]. In addition, percutaneous liver biopsy can be performed safely even in young infants[12].
However, some difficulties remain in the interpretation of hepatic histology in neonatal cholestasis. One of these difficulties is inadequate biopsy materials obtained, especially via the percutaneous route. In the present study, while all the biopsy specimens obtained surgically contained an adequate number of portal tracts, slightly less than half (46%) of the specimens obtained via the percutaneous route contained less than 5 portal tracts for adequate interpretation. In 2 specimens, no portal tracts were observed. Thus, histological features indicative of BA which depend on an adequate number of portal tracts, such as bile ductular proliferation, bile plugs in bile ductules and porto-portal bridging, were not observed for adequate interpretation.
The present study shows that contrary to the claims made by some authors[24,25], liver biopsy is a sensitive and specific method in the investigation of neonatal cholestasis. This study shows that with proper and adequate training in hepatic histology interpretation, the interpretation of liver histology in neonatal cholestasis can be achieved with sufficient sensitivity, specificity and accuracy.
In conclusion, histological interpretation of liver biopsies based on a 7-criteria scoring system had the highest sensitivity, specificity and diagnostic accuracy to differentiate BA from other intrahepatic cholestasis.
One of the most important priorities in the investigations of infants with neonatal cholestasis is to make an accurate diagnosis of obstructive causes such as biliary atresia to allow timely surgery, as early surgery for biliary atresia is one of the most important prognostic factors of successful outcome. However, there is considerable overlap in the clinical, biochemical and histological features of biliary atresia and other causes of neonatal cholestasis.
Making an accurate histological diagnosis for neonatal cholestasis has always been considered a challenge for many pathologists, particularly in the early neonatal period. Many previously reported attempts to histologically differentiate biliary atresia from other causes of neonatal cholestasis using a scoring system have not been widely accepted, mainly due to their complexities.
In this study, the authors examined the diagnostic usefulness of a 7-feature, 15-point histological scoring system to differentiate biliary atresia from other causes of neonatal cholestasis histologically. It was found that when applied to biopsy specimens obtained from infants with neonatal cholestasis and when interpreted without a scoring system, this new histological scoring system offered good diagnostic accuracy. The diagnostic accuracy was better than the histological diagnosis made by reporting pathologists.
By devising a new, objective histological scoring system with high sensitivity, specificity and diagnostic accuracy to differentiate biliary atresia from other non-obstructive causes, practising pathologists can make timely histological diagnoses in infants with neonatal cholestasis to avoid unnecessary surgery in those without biliary atresia, and early surgical correction in those with biliary atresia.
Neonatal cholestasis refers to the response of immature liver cells in the presence of various types of external insults. It is usually seen in newborn infants with somewhat immature liver function, and is characterized physiologically as a measurable decrease of bile flow, pathologically as the histological presence of bile pigment in hepatocytes and bile ducts, and clinically as the accumulation in blood and extrahepatic tissues of substances normally excreted in the bile. Although the insults to the liver may come in many different forms, there is considerable overlap in the clinical, biochemical and histological manifestations of neonatal cholestasis because the response of immature hepatocytes to external injuries is somewhat limited.
The article is a good attempt at making the diagnosis of neonatal hepatitis objectively. The authors have clarified the criteria for selection of the histological features differentiating biliary atresia from other causes of neonatal cholestasis. The basis of allotment of scoring points over each feature has also been clarified. The authors also noted that as compared to the diagnostic accuracy of the reporting histologists (81%), the diagnostic accuracy of the present scoring system (92%) was superior.
Peer reviewer: Dr. Philip Abraham, Professor, Consultant Gastroenterologist & Hepatologist, P. D. Hinduja National Hospital & Medical Research Centre, Veer Savarkar Marg, Mahim, Mumbai 400 016, India
S- Editor Tian L L- Editor Webster JR E- Editor Lin YP
| 1. | Lee WS. Pre-admission consultation and late referral in infants with neonatal cholestasis. J Paediatr Child Health. 2008;44:57-61. [Cited in This Article: ] |
| 2. | Suchy FJ. Approach to the infant with cholestasis. Liver Disease in Children. New York: Cambridge University Press 2007; 179-189. [Cited in This Article: ] |
| 3. | Hays DM, Woolley MM, Snyder WH Jr, Reed GB GWINN JL, Landing BH. Diagnosis of biliary atresia: relative accuracy of percutaneous liver biopsy, open liver biopsy, and operative cholangiography. J Pediatr. 1967;71:598-607. [Cited in This Article: ] |
| 4. | Lai MW, Chang MH, Hsu SC, Hsu HC, Su CT, Kao CL, Lee CY. Differential diagnosis of extrahepatic biliary atresia from neonatal hepatitis: a prospective study. J Pediatr Gastroenterol Nutr. 1994;18:121-127. [Cited in This Article: ] |
| 5. | Rastogi A, Krishnani N, Yachha SK, Khanna V, Poddar U, Lal R. Histopathological features and accuracy for diagnosing biliary atresia by prelaparotomy liver biopsy in developing countries. J Gastroenterol Hepatol. 2009;24:97-102. [Cited in This Article: ] |
| 6. | Balistreri WF, Bezerra JA, Ryckman FC. Biliary atresia and other disorders of the extrahepatic bile ducts. Liver disease in children. 3rd ed. New York: Cambridge University Press 2007; 247-269. [Cited in This Article: ] |
| 7. | Ferry GD, Selby ML, Udall J, Finegold M, Nichols B. Guide to early diagnosis of biliary obstruction in infancy. Review of 143 cases. Clin Pediatr (Phila). 1985;24:305-311. [Cited in This Article: ] |
| 8. | Craig JM, Landing BH. Form of hepatitis in neonatal period simulating biliary atresia. AMA Arch Pathol. 1952;54:321-333. [Cited in This Article: ] |
| 9. | Brough AJ, Bernstein J. Liver biopsy in the diagnosis of infantile obstructive jaundice. Pediatrics. 1969;43:519-526. [Cited in This Article: ] |
| 10. | Santos JL, Almeida H, Cerski CT, Silveira TR. Histopathological diagnosis of intra- and extrahepatic neonatal cholestasis. Braz J Med Biol Res. 1998;31:911-919. [Cited in This Article: ] |
| 11. | Zerbini MC, Gallucci SD, Maezono R, Ueno CM, Porta G, Maksoud JG, Gayotto LC. Liver biopsy in neonatal cholestasis: a review on statistical grounds. Mod Pathol. 1997;10:793-799. [Cited in This Article: ] |
| 12. | Moyer V, Freese DK, Whitington PF, Olson AD, Brewer F, Colletti RB, Heyman MB. Guideline for the evaluation of cholestatic jaundice in infants: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2004;39:115-128. [Cited in This Article: ] |
| 13. | McKiernan PJ, Baker AJ, Kelly DA. The frequency and outcome of biliary atresia in the UK and Ireland. Lancet. 2000;355:25-29. [Cited in This Article: ] |
| 14. | Fischler B, Papadogiannakis N, Nemeth A. Clinical aspects on neonatal cholestasis based on observations at a Swedish tertiary referral centre. Acta Paediatr. 2001;90:171-178. [Cited in This Article: ] |
| 15. | Lee WS, Chai PF, Lim KS, Lim LH, Looi LM, Ramanujam TM. Outcome of biliary atresia in Malaysia: a single-centre study. J Paediatr Child Health. 2009;45:279-285. [Cited in This Article: ] |
| 16. | Brough AJ, Bernstein J. Conjugated hyperbilirubinemia in early infancy. A reassessment of liver biopsy. Hum Pathol. 1974;5:507-516. [Cited in This Article: ] |
| 17. | Kasai M, Yakovac WC, Koop CE. Liver in congenital biliary atresia and neonatal hepatitis. A histopathologic study. Arch Pathol. 1962;74:152-162. [Cited in This Article: ] |
| 18. | Landing BH, Wells TR, Ramicone E. Time course of the intrahepatic lesion of extrahepatic biliary atresia: a morphometric study. Pediatr Pathol. 1985;4:309-319. [Cited in This Article: ] |
| 19. | Yachha SK, Khanduri A, Kumar M, Sikora SS, Saxena R, Gupta RK, Kishore J. Neonatal cholestasis syndrome: an appraisal at a tertiary center. Indian Pediatr. 1996;33:729-734. [Cited in This Article: ] |
| 20. | Bines JE. Cholestasis associated with parenteral nutrition therapy. Pediatric Gastrointestinal Disease: pathophysiology, diagnosis, management. Ontario: BC Decker 2004; 1455-1465. [Cited in This Article: ] |
| 21. | McCracken GH Jr, Shinefield HM, Cobb K, Rausen AR, Dische R, Eichenwald HF. Congenital cytomegalic inclusion disease. A longitudinal study of 20 patients. Am J Dis Child. 1969;117:522-539. [Cited in This Article: ] |
| 22. | Rosenthal P. Neonatal hepatitis and congenital infections. Liver disease in children. New York: Cambridge University Press 2007; 232-246. [Cited in This Article: ] |
| 23. | Whitington PF, Freese DK, Alonso EM, Schwarzenberg SJ, Sharp HL. Clinical and biochemical findings in progressive familial intrahepatic cholestasis. J Pediatr Gastroenterol Nutr. 1994;18:134-141. [Cited in This Article: ] |
| 24. | Machnik G. [Histological changes in liver tissue in cholestasis]. Z Gastroenterol. 1993;31 Suppl 2:7-10. [Cited in This Article: ] |
| 25. | Vecchione R, Terracciano LM, D'Armiento M, D'Armiento FP. [Neonatal cholestasis: the viewpoint of the pathologist]. Pediatr Med Chir. 1993;15:229-237. [Cited in This Article: ] |