Published online Nov 14, 2009. doi: 10.3748/wjg.15.5300
Revised: September 22, 2009
Accepted: September 29, 2009
Published online: November 14, 2009
AIM: To determine if a nasojejunal tube (NJT) is required for optimal examination of enteroclysis and if patients can be examined only in the supine position.
METHODS: Data were collected from all patients undergoing small bowel (SB) magnetic resonance imaging (MRI) examination over a 32-mo period. Patients either underwent a magnetic resonance (MR) follow-through (MRFT) or a MR enteroclysis (MRE) in the supine position. The quality of proximal and distal SB distension as well as the presence of motion artefact and image quality were assessed by 2 radiologists.
RESULTS: One hundred and fourteen MR studies were undertaken (MRFT-49, MRE-65) in 108 patients in the supine position only. Image artefact was more frequent in MRE than in MRFT (29.2% vs 18.4%), but was not statistically significant (P = 0.30). Adequate distension of the distal SB was obtained in 97.8% of MRFT examinations and in 95.4% of MRE examinations, respectively. Proximal SB distension was, however, less frequently optimal in MRFT than in MRE (P = 0.0036), particularly in patients over the age of 50 years (P = 0.0099). Image quality was good in all examinations.
CONCLUSION: All patients could be successfully imaged in the supine position. MRE and MRFT are equivalent for distal SB distension and artefact effects. Proximal SB distension is frequently less optimal in MRFT than in MRE. MRE is, therefore, the preferred MR examination method of the SB.
- Citation: Lawrance IC, Welman CJ, Shipman P, Murray K. Small bowel MRI enteroclysis or follow through: Which is optimal? World J Gastroenterol 2009; 15(42): 5300-5306
- URL: https://www.wjgnet.com/1007-9327/full/v15/i42/5300.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5300
| Patients | MRFT | MRE | |
| Gender: Male | 41.2% (47/114) | 32.65% (16/49) | 47.7% (31/65) |
| Age (yr): mean (range) ± SE | 40.7 (14-78) ± 16.5 | 39.1 (14-74) ± 15.4 | 43.6 (17-78) ± 17.6 |
| Indication | |||
| Known or suspected CD | 86.0% (98/114) | 93.8% (46/49) | 80.0% (52/65) |
| Iron deficiency anaemia | 7.0% (8/114) | 0% | 12.3% (8/65) |
| Resistant coeliac disease | 3.5% (4/114) | 6.1% (3/49) | 1.5% (1/65) |
| Other | 3.5% (4/114) | 0% | 6.2% (4/65) |
| All Patients | MRFT | MRE | |
| Good proximal bowel distension | 76.3% (87/114) | 65.3% (32/49) | 84.6% (55/65) |
| Good distal bowel distension | 93.9% (107/114) | 97.8% (45/49) | 95.4% (62/65) |
| No artifact | 75.4% (86/114) | 81.6% (40/49) | 70.8% (46/65) |
| Good proximal and distal bowel distension | 75.4% (86/114) | 63.3% (31/49) | 84.6% (55/65) |
| Proximal SB distension | P value | |||
| Good proximal SB distension | Odds ratio | 95% CI | ||
| MRE | 84.6% (55/65) | 4.365 | 1.619-11.772 | 0.004 |
| MRFT | 65.3% (32/49) | Ref | ||
| 30-50 yr | 86.7% (39/45) | 4.882 | 1.463-16.294 | 0.010 |
| < 30 yr | 71.0% (22/31) | 1.561 | 0.495-4.917 | 0.447 |
| > 50 yr | 68.4% (26/38) | Ref | ||
| Female | 77.6% (52/67) | 1.646 | 0.616-4.395 | 0.320 |
| Male | 74.5 % (35/47) | Ref | ||
Investigation of small bowel (SB) pathology can be very difficult. Assessment of the terminal ileum (TI) by endoscopy is not optimal and conventional barium radiology (SB follow-through or SB enteroclysis) has a sensitivity of 23%-80% for the detection of lesions typical of Crohn’s disease (CD)[1-3]. Wireless capsule endoscopy may allow for excellent visualisation of the SB mucosa and any abnormalities, but its specificity is lower than other methods[3] and it often does not clearly localise the area of the small intestine where a lesion is identified. There is also a small but definite capsule retention rate that contraindicates its use in SB strictures[4]. Computed tomography enteroclysis (CTE) has a good sensitivity of 71%-95% and an impressive specificity of 90%-98% and is superior to conventional enteroclysis[5,6]. A single abdominal CT, however, may increase a patient’s life-time risk of malignancy[7], which is even greater in the younger population[8]. The sensitivity and specificity of SB magnetic resonance imaging (MRI) examinations are also higher in the evaluation of CD than CTE[9]. Although MRI cannot provide the consistently good mucosal detail as conventional enteroclysis, it, however, correlates with pathologic findings and does not use ionising radiation[10-12].
Bowel contrast is required for SB distension and adequate distension is required for optimal information on mucosal abnormalities, bowel distensibility and passage of the contrast. The difference between magnetic resonance (MR) SB enteroclysis (MRE) and MR SB follow-through (MRFT) is the administration of bowel contrast via a nasojejunal tube (NJT) for MRE, which is ingested orally for MRFT. It has been proposed that SB distension is best obtained with the use of a NJT, but patients often describe that placement of a NJT is very unpleasant. A NJT also reduces a potential benefit of MRI as it requires fluoroscopic radiation exposure to insert the tube[13]. Recent studies also suggest that MRFT is as sensitive as MRE in the diagnosis of ileal CD[14]. Although MRFT and MRI enteroclysis are excellently correlated with disease findings[15], proximal SB distension may not be as optimal as that of MRFT[12,14,15], suggesting that a NJT may still be required.
CD is one of the major indications for investigation of the SB, as approximately 70% of such patients will have inflammatory involvement of the TI. CD is a chronic, relapsing condition with an increasing prevalence[16] and may frequently present with intestinal fibrosis, stenosis and obstruction. Fibrosis and stricture formation are the most common indications for intestinal surgical resection[17]. Patients undergoing SB radiological investigations are usually examined in the prone position, as it has been suggested that this may result in better SB distension. This, however, is an unpopular technique especially in patients with stomas[18], and it has been reported that the prone and supine positions are equivalent in terms of lesion detection and bowel wall feature visualisation[19].
If optimal SB distension can be obtained without insertion of a NJT, then fluoroscopic radiation could be avoided, which is of particular importance because the predilection of IBD to affect younger patients who frequently require multiple investigations over their lifetime. The aims of this study were thus to assess the difference in the quality of imaging between MRE and MRFT in proximal and distal SB distension and the presence of artefact impairing diagnostic assessment, and to confirm whether imaging of patients in the supine position with a surface array coil can provide abdominal compression and good SB distension in quality studies.
All subjects were patients of a 450-bed tertiary institution located in Perth of Western Australia, which is also the site for the “Centre for Inflammatory Bowel Diseases”, a specialist unit for the management of inflammatory bowel diseases. Data were collected from all patients undergoing MRFT or MRE over a 32-mo period.
Patients were randomly investigated by either MRFT or MRE. If a patient refused a NJT, or placement of the NJT failed due to technical reasons, a MRFT was undertaken. All patients drank only clear fluids for 6 h prior to their MRI and were nil by mouth for 2 h. The bowel contrast agent used was a polyethylene glycol-water (PEG) solution (glycoprep-C, Pharmatel Fresenius Kabi, Australia). Patients who were to undergo a MRFT attempted to drink 1000 mL or more of the bowel contrast agent over 20 min. For a MRE, a NJT (Bilbao-Dotter, Cook, Australia) was placed under fluoroscope. PEG was injected manually through the NJT (from 60 mL/min to 120-150 mL/min). A total of 800-2000 mL was usually required to distend the SB to the TI, which varied depending on previous bowel resections, the presence of stenosing disease, and patient tolerance.
Patients were imaged in the supine position using a 1.5T MRI system (Avanto SQ, Siemens Medical Solutions, Erlangen, Germany) with a surface array coil providing compression. In patients having undergone an ileostomy, a sponge was placed between the surface coil and stoma with the stoma bag empty.
A scout image was acquired to ensure adequate coverage. SB filling was dynamically assessed using a coronal 150 mm-thick single slab T2-weighted (HASTE) fat saturated sequence (TR 4500 ms, TE 749 ms, flip angle 180°, bandwidth 150 Hz/Px, FOV 350 mm, averages 1, concatenation 1, distance factor 50%, GRAPPA acceleration factor 2), which was acquired repeatedly without breath-holding to monitor retrograde stomach filling and SB distension. These images, combined into a cine loop, were used to assess stenotic lesions. If there was a doubt as to TI contrast filling, a single breath-hold coronal T2-weighted sequence (HASTE) (TR 2000 ms, TE 118 ms, flip angle 180°, bandwidth 195 Hz/Px, FOV 350 mm, averages 1, concatenations 2, no parallel imaging) with a 5 mm-thick slice was obtained with a 50% gap.
To reduce bowel peristalsis and prolong SB distension, 10 mg intravenous hyoscine butylbromide (Buscopan, Boehringer Ingleheim, Australia) was given if there were no contraindications. Once there was adequate SB filling, a coronal pre-contrast T1-weighted 3D gradient echo (VIBE) (TR 9.38 ms, TE 4.46 ms, flip angle 20°, bandwidth 630 Hz/Px, FOV 400 mm, averages 1, concatenation 1, phase over-sampling 25%, distance factor 20%, GRAPPA acceleration factor 2) with a 2.5 mm-thick slice was obtained. Gadodiamide (Omniscan, Amersham, Australia) was intravenously injected (0.2 mL/kg) with post contrast imaging commencing at 60 s. Post-contrast VIBE sequences were obtained in the coronal (imaging factors the same as pre-contrast VIBE) and axial (TR 3.37 ms, TE 1.22 ms, flip angle 12°, bandwidth 490 Hz/Px, FOV 320 mm, averages 1, concatenation 1, phase oversampling 0%, distance factor 20%, GRAPPA acceleration factor 2, a 2 mm-thich slice). The axial plane required 2-3 overlapping sections covering the upper and lower abdomen.
Further imaging was obtained in 2-3 blocks of the upper and lower abdomen, including a coronal steady-state free precession sequence (true FISP) (TR 3.65 ms, TE 1.83 ms, flip angle 64°, bandwidth 501 Hz/Px, FOV 380mm, averages 1) with a 6 mm-thick slice and 30% gap obtained with and without fat saturation, an axial steady-state free precession sequence (true FISP) (TR 3.69 ms, TE 1.83 ms, flip angle 64°, bandwidth 501 Hz/Px, FOV 350 mm, averages 1) with a 6 mm-thick slice and 30% gap obtained without fat saturation in 2-3 blocks of the upper and lower abdomen, a coronal T2-weighted half Fourier single shot turbo spin echo (HASTE) sequence (imaging factors as above) with a 5 mm-thick slice and 30% gap, and an axial T2-weighted half Fourier single shot turbo spin echo (HASTE) sequence (TR 1000 ms, TE 85 ms, flip angle 150°, bandwidth 391 Hz/Px, FOV 350 mm, averages 1, concatenation 1, GRAPPA acceleration factor 2) with a 6 mm-thick slice and 30% gap. If a site of pathology was identified at an overlap point on the axial images, then a further set of targeted axial true FISP and HASTE images were obtained.
For MRFT, an initial single breath-hold coronal T2-weighted sequence (HASTE) (imaging factors as above) with a 6 mm-thick slice and 30% gap was obtained. If there was adequate filling of the TI, buscopan was injected and routine imaging was obtained as above. With suboptimal filling of the TI, but sufficient bowel contrast proximally, reassessment was performed at 5-min intervals for 15 min. If there was still inadequate filling of SB loops, the patient was removed from the MRI unit and drank a further 500 mL of contrast prior to recommencing imaging.
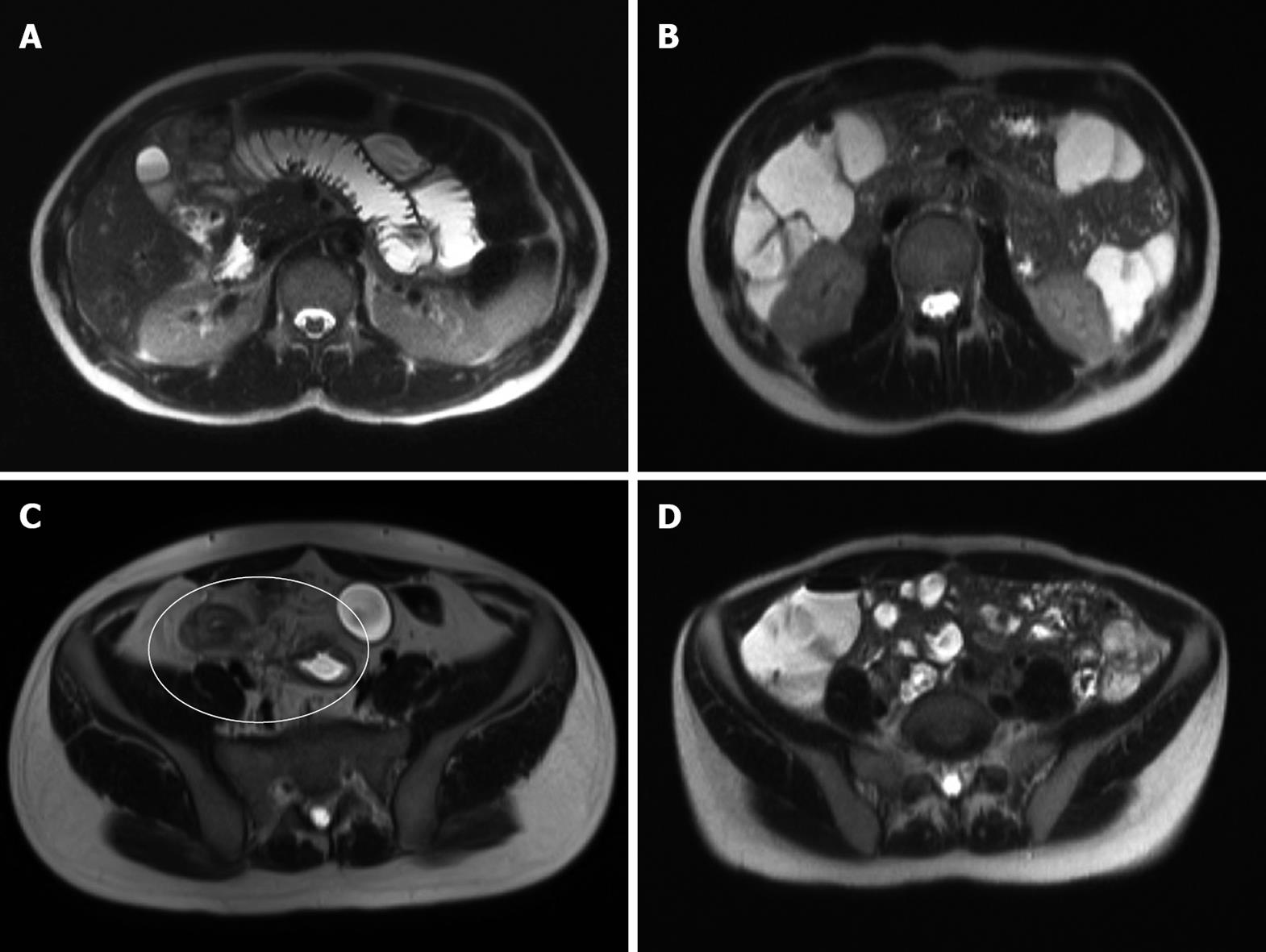
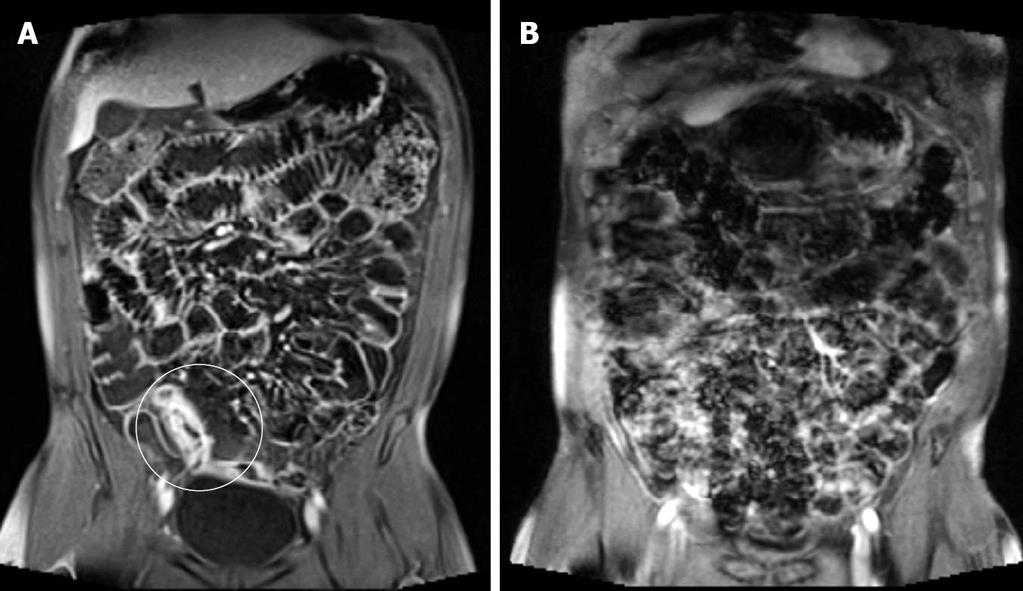
Each MRI was evaluated by consensus of two radiologists (CW and PS) with experience in both gastrointestinal and MR imaging. Image analysis was performed using a standardised worksheet. The quality of proximal and distal SB distension and the presence of artefact were assessed. Good distension was defined as luminal fluid present within the bowel lumen allowing clear visualisation of both endoluminal surfaces. Poor distension was lack of the above (Figure 1). Artefact, generally related to poor breath-holding, was present if it rendered the images diagnostically impaired on both sequence planes through a region (Figure 2).
The Fremantle Hospital Human Research Ethics Committee deemed that patient consent was not required for this study as both MRFT and MRI are considered to be standard examination methods.
Logistic regression was undertaken by a statistician (KM) and considered significant at the 0.05 level. The significant effects and odds ratios were presented, along with 95% confidence intervals. Logistic regression was used to model separately the three responses: bowel distension proximal (good vs poor), bowel distension distal (good vs poor), and artefact (good vs poor), with potential predictors of treatment (tube or oral), sex, and age group subdivided into low age group under the age of 30 years, medium age group at the age of 30-50 years, and high age group over the age of 50 years.
Data were collected from 114 MR examinations of SB preformed in the supine position on 108 patients (41.7% males, 45/108) over a 32-mo period. A total of 49 MRFT and 65 MRE were assessed. No difference was found in male sex (32.7% vs 47.7%, P = 0.079) or in age (39.1 ± 15.4 years vs 43.6 ± 17.6 years) between the MRE or MRFT patient groups. The primary indication for SB MRI imaging was assessment of CD, either for level of inflammatory activity or for investigation of obstructive symptoms (68.4%, 78/114). The remaining MRI imaging was for suspected CD involving the small intestine (17.5%, 20/114). Of the 114 examinations, 98 (86.0%) were thus for CD or suspected CD. Of the remaining 16 investigations, 8 were for unexplained iron deficiency anaemia, 4 for resistance coeliac disease, 1 for a suspected SB mass seen on wireless capsule endoscopy, 1 for unexplained abdominal pain, and 2 for investigation of SB obstruction, respectively (Table 1).
Proximal and distal SB distension and artefact were assessed (Table 2). Proximal SB distension was more likely to be suboptimal (Figure 1) in MRFT than in MRE (65.3% vs 84.6%). No statistical difference was detected in good distal SB distension (97.8% vs 95.4%, P = 0.77) between MRFT and MRE or between proximal SB distension and patient age or sex. Although the image artefact, which rendered the study diagnostically impaired (Figure 2), was present more frequently in MRE than in MRFT (29.2% vs 18.4%), it was not statistically significant (P = 0.30). Good proximal and distal SB distension was observed in 84.6% (55/65) of the patients undergoing a MRE and in 63.3% (31/49) of the patients undergoing a MRFT, respectively.
Logistic regression findings were correlated with patient age (under 30 years, 30-50 years and over 50 years) and sex. While proximal SB distension was overall more likely to be suboptimal in MRFT than in MRE (OR = 4.365, 95% CI = 1.62-11.77, P = 0.0036), age also had an impact on the presence of good proximal SB distension. Patients aged 30-50 years were more likely to have good proximal SB distension than those aged over 50 years (OR = 4.882, 95% CI = 1.463-16.294, P = 0.0099). No statistically significant difference, however, was found between the patients aged less than 30 years, 30-50 years and over 50 years (Table 3). No statistically significant effect was observed in sex and no significant difference was detected in the diagnostic accuracy of the studies between the various age groups.
All patients were examined in the supine position alone. Nine examinations were undertaken in patients having undergone ileostomy (7.9% of examinations), 4 and 5 of these patients underwent a MRFT and a MRE, respectively. Good proximal and distal bowel distension was achieved in 77.8% (7/9) and in 100% of the patients (9/9), respectively, and image artefact was observed in only 1 patient (11.1%). These findings were very comparable to those in the overall group and no statistically significant difference was found in these parameters between the patients whether they underwent and did not undergo ileostomy.
In our institution, CD or suspected CD is by far the most common indication for SB MR imaging, accounting for more than 85% of cases, which is primarily due to the frequency of involvement of the TI in CD and its transmural nature that may result in obstructive symptoms secondary to intestinal inflammation and/or fibrosis. Radiological investigations have undergone an evolution with both CTE and MRE demonstrating an impressive specificity and sensitivity in the assessment of SB CD. CTE, however, delivers ionising radiation, while MR provides a good soft tissue contrast without radiation, which may potentially differentiate between intestinal inflammation and fibrosis and may thus be superior to CT scanning[12,20,21]. For long-term safety issues regarding cumulative radiation exposure and potentially better diagnostic capabilities, MR examination of the SB appears preferable to CT.
Examination of the SB requires a bowel contrast to achieve adequate SB distension, which may either be taken orally or via a NJT. SB follow-through (SBFT) is dependent on a number of factors, such as the presence or absence of disease, the ability of patients to ingest a sufficient volume of oral contrast over a short period of time, inter-individual variation in bowel transit time, and the type of contrast agent used[22]. Most radiological facilities routinely place a NJT despite a strong patient preference against it due to its discomfort. Our MRI service has paediatric radiology experience in performing MRFT studies with no placement of a NJT, using PEG according to the published techniques[23,24]. Adaptation of this technique for adults was used in this study, but proved difficult due to the significant time variability required for adequate SB filling with tightly scheduled MRI appointments. Since the MR technique used in our facility also requires real-time monitoring, overlapping images can be obtained when needed, thereby enhancing the quality of MR studies.
Our experience is similar to the published studies where the total investigation time (18-27 min, average 22.4 min) was achieved with NJT placement[11], but it could vary as many as 15-240 min (average 65 min) when performed as a follow-through study[25]. It should be noted that the imaging time within a MRI unit is approximately the same for both MRFT and MRE. The extra time frequently required for a MRFT is due to the movement of patients in and out of the MRI unit more than once in order to determine SB filling and the need for further oral contrast, which, however, must be weighed against the extra time and radiologist skills required for NJT placement, the need for fluoroscopic radiation, and the strong patient preference against a NJT. Other methods, described to streamline the MRFT technique, however, still require 20-96.6 min of the study time and recurrent periods in and out of the magnet[22], although it is potentially possible to reduce the imaging time to 15-20 min following oral ingestion of PEG over 30-45 min[26].
Our findings regarding the image quality of MRFT and MRE are consistent with previous reports that oral contrast is as sensitive as NJT for distal ileal CD[27]. Our results, however, indicate that MRFT is inferior to MRE for proximal SB distension, particularly in the older age group (over 50 years), which may primarily be due to the fact that many of these patients have difficulty in consuming a sufficient volume of oral contrast over a short period of time. We did not record or assess the volume of bowel contrast used. It has been suggested that 900 mL of either an osmotic or a nonosmotic solution is sufficient to obtain duodenal distension and 1350 mL is sufficient to distend the distal jejunum and ileum via mouth[28]. We strongly encouraged our patients to drink at least 1000 mL of the oral contrast agent. There is often, however, a limit to how much a patient with obstructive symptoms can ingest orally and we have to accept that the finally ingested volume is the best possible for the individual. A recent paper has addressed this specific concern and identified that the SB could be reliably analyzed in healthy volunteers with a volume of 450 mL, and not unexpectedly, less reliably with a volume of 300 mL and 150 mL, respectively[29].
The unpopular technique of imaging in the prone position, could be avoided in our cohort of patients, especially in patients with stomas[18], by putting the patient in the supine position and using a large surface coil, and sponge over the stoma if present, to apply abdominal pressure. This technique delivered similar SB distension in the patients having undergone ileostomy as in those who did not undergo ileostomy. Our findings in 15.4% of supine MRE studies having either poor proximal or distal SB distension are consistent with SBFT/enterography showing poor SB distension in 13.8% of patients examined in the prone position, and 16.1% of patients examined in the supine position[19]. In our study, and as has been observed by other investigators[26], the image quality was still considered good despite the poor SB distension, and was not considered to have reduced the diagnostic accuracy of the examination. Our findings, and studies comparing SB distension examined in the prone and supine positions[19], suggest that the two methods are equivalent in lesion detection and bowel wall feature visualisation.
The findings presented here suggest that both MRE and MRFT are comparable investigations with regard to distal SB bowel distension, image artefact and quality. We also observed no difference in the quality of studies performed in the limited number of patients having undergone ileostomy. Proximal SB distension, however, is frequently less optimal in MRFT than in MRE, but the examination of patients in the supine position is a viable option. Due to reduction in study duration and improved proximal SB distension, the authors prefer to primarily perform all studies in adult patients in the supine position with a NJT. In patients who are unable to tolerate a NJT, or the study is requested to examine the distal SB, a MRFT with oral contrast is an acceptable alternative, particularly in patients under the age of 50 years.
If optimal small bowel (SB) distension can be obtained without insertion of a nasojejunal tube (NJT), then fluoroscopic radiation could be avoided. This study was to assess magnetic resonance (MR) SB enteroclysis (MRE) and MR SB follow-through (MRFT) for proximal and distal SB distension, the presence of movement artefact, and whether imaging in the supine position provides quality studies with good SB distension.
Patients often describe that placement of a NJT is very unpleasant. A NJT also requires fluoroscopic radiation exposure to insert the tube. Recent studies suggest that MRFT is as sensitive as MRE in the diagnosis of ileal disease. This study assessed the optimal method of SB examination by MR and if patients could be examined in the supine position only.
The findings demonstrate that the image quality of MRFT and MRE is equivalent. The results, however, indicate that MRFT is inferior to MRE for proximal SB distension, particularly in patients over the age of 50 years. The unpopular technique of imaging in the prone position, especially in patients with stomas, can be avoided by putting the patient in the supine position and using a large surface coil and sponge over a stoma if present to apply abdominal pressure.
The findings demonstrate that MRE and MRFT are comparable investigations with regard to distal SB bowel distension, image artefact and quality, and examination of patients in the supine position is a viable option. Proximal SB distension, however, is less optimal in MRFT. Due to reduction in study duration and improved proximal SB distension, the authors prefer to perform all studies in the supine position with a NJT. In patients who are unable to tolerate a NJT, or the study is requested to examine the distal SB, a MRFT with oral contrast is an acceptable alternative, particularly in patients under the age of 50 years.
Magnetic resonance imaging (MRI) is a cross sectional imaging technique that does not utilize radiation and provides excellent tissue differentiation. MRE has the administration of bowel contrast via a NJT and patients undergoing a MRFT drink the bowel contrast. Good bowel distension is defined as luminal fluid present within the bowel lumen allowing clear visualisation of both endoluminal surfaces. Artefact, generally related to poor breath-holding, is present if it renders the images diagnostically impaired on both sequence planes through a region.
The paper is a large study assessing the quality of MRIs with the bowel contrast administered through a tube (ME enterolysis) and contrast injected orally (MR follow-through) and whether or not MRI examination of patients in the supine position is adequate. The authors show that tube administration is the best and that patients in the supine position are OK.
Peer reviewer: Paul E Sijens, PhD, Associate Professor, Radiology, UMCG, Hanzeplein 1, 9713GZ Groningen, The Netherlands
S- Editor Tian L L- Editor Wang XL E- Editor Ma WH
| 1. | Marmo R, Rotondano G, Piscopo R, Bianco MA, Siani A, Catalano O, Cipolletta L. Capsule endoscopy versus enteroclysis in the detection of small-bowel involvement in Crohn’s disease: a prospective trial. Clin Gastroenterol Hepatol. 2005;3:772-776. [Cited in This Article: ] |
| 2. | Triester SL, Leighton JA, Leontiadis GI, Gurudu SR, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with non-stricturing small bowel Crohn’s disease. Am J Gastroenterol. 2006;101:954-964. [Cited in This Article: ] |
| 3. | Solem CA, Loftus EV Jr, Fletcher JG, Baron TH, Gostout CJ, Petersen BT, Tremaine WJ, Egan LJ, Faubion WA, Schroeder KW. Small-bowel imaging in Crohn’s disease: a prospective, blinded, 4-way comparison trial. Gastrointest Endosc. 2008;68:255-266. [Cited in This Article: ] |
| 4. | Cheon JH, Kim YS, Lee IS, Chang DK, Ryu JK, Lee KJ, Moon JS, Park CH, Kim JO, Shim KN. Can we predict spontaneous capsule passage after retention? A nationwide study to evaluate the incidence and clinical outcomes of capsule retention. Endoscopy. 2007;39:1046-1052. [Cited in This Article: ] |
| 5. | Gore RM, Balthazar EJ, Ghahremani GG, Miller FH. CT features of ulcerative colitis and Crohn’s disease. AJR Am J Roentgenol. 1996;167:3-15. [Cited in This Article: ] |
| 6. | Doerfler OC, Ruppert-Kohlmayr AJ, Reittner P, Hinterleitner T, Petritsch W, Szolar DH. Helical CT of the small bowel with an alternative oral contrast material in patients with Crohn disease. Abdom Imaging. 2003;28:313-318. [Cited in This Article: ] |
| 7. | Brenner DJ, Elliston CD. Estimated radiation risks potentially associated with full-body CT screening. Radiology. 2004;232:735-738. [Cited in This Article: ] |
| 8. | Brenner D, Elliston C, Hall E, Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001;176:289-296. [Cited in This Article: ] |
| 9. | Mackalski BA, Bernstein CN. New diagnostic imaging tools for inflammatory bowel disease. Gut. 2006;55:733-741. [Cited in This Article: ] |
| 10. | Gourtsoyiannis N, Papanikolaou N, Grammatikakis J, Papamastorakis G, Prassopoulos P, Roussomoustakaki M. Assessment of Crohn’s disease activity in the small bowel with MR and conventional enteroclysis: preliminary results. Eur Radiol. 2004;14:1017-1024. [Cited in This Article: ] |
| 11. | Gourtsoyiannis NC, Grammatikakis J, Papamastorakis G, Koutroumbakis J, Prassopoulos P, Rousomoustakaki M, Papanikolaou N. Imaging of small intestinal Crohn’s disease: comparison between MR enteroclysis and conventional enteroclysis. Eur Radiol. 2006;16:1915-1925. [Cited in This Article: ] |
| 12. | Masselli G, Casciani E, Polettini E, Lanciotti S, Bertini L, Gualdi G. Assessment of Crohn’s disease in the small bowel: Prospective comparison of magnetic resonance enteroclysis with conventional enteroclysis. Eur Radiol. 2006;16:2817-2827. [Cited in This Article: ] |
| 13. | Martin DR, Danrad R, Herrmann K, Semelka RC, Hussain SM. Magnetic resonance imaging of the gastrointestinal tract. Top Magn Reson Imaging. 2005;16:77-98. [Cited in This Article: ] |
| 14. | Negaard A, Paulsen V, Sandvik L, Berstad AE, Borthne A, Try K, Lygren I, Storaas T, Klow NE. A prospective randomized comparison between two MRI studies of the small bowel in Crohn’s disease, the oral contrast method and MR enteroclysis. Eur Radiol. 2007;17:2294-2301. [Cited in This Article: ] |
| 15. | Schreyer AG, Geissler A, Albrich H, Scholmerich J, Feuerbach S, Rogler G, Volk M, Herfarth H. Abdominal MRI after enteroclysis or with oral contrast in patients with suspected or proven Crohn's disease. Clin Gastroenterol Hepatol. 2004;2:491-497. [Cited in This Article: ] |
| 16. | Munkholm P, Langholz E, Nielsen OH, Kreiner S, Binder V. Incidence and prevalence of Crohn's disease in the county of Copenhagen, 1962-87: a sixfold increase in incidence. Scand J Gastroenterol. 1992;27:609-614. [Cited in This Article: ] |
| 17. | Harper PH, Fazio VW, Lavery IC, Jagelman DG, Weakley FL, Farmer RG, Easley KA. The long-term outcome in Crohn’s disease. Dis Colon Rectum. 1987;30:174-179. [Cited in This Article: ] |
| 18. | Prassopoulos P, Papanikolaou N, Grammatikakis J, Rousomoustakaki M, Maris T, Gourtsoyiannis N. MR enteroclysis imaging of Crohn disease. Radiographics. 2001;21 Spec No:S161-S172. [Cited in This Article: ] |
| 19. | Cronin CG, Lohan DG, Mhuircheartaigh JN, McKenna D, Alhajeri N, Roche C, Murphy JM. MRI small-bowel follow-through: prone versus supine patient positioning for best small-bowel distention and lesion detection. AJR Am J Roentgenol. 2008;191:502-506. [Cited in This Article: ] |
| 20. | Bernstein CN, Greenberg H, Boult I, Chubey S, Leblanc C, Ryner L. A prospective comparison study of MRI versus small bowel follow-through in recurrent Crohn’s disease. Am J Gastroenterol. 2005;100:2493-2502. [Cited in This Article: ] |
| 21. | Lawrance IC, Welman CJ, Shipman P, Murray K. Correlation of MRI-determined small bowel Crohn's disease categories with medical response and surgical pathology. World J Gastroenterol. 2009;15:3367-3375. [Cited in This Article: ] |
| 22. | Lohan D, Cronin C, Meehan C, Alhajeri AN, Roche C, Murphy J. MR small bowel enterography: optimization of imaging timing. Clin Radiol. 2007;62:804-807. [Cited in This Article: ] |
| 23. | Laghi A, Carbone I, Catalano C, Iannaccone R, Paolantonio P, Baeli I, Trenna S, Passariello R. Polyethylene glycol solution as an oral contrast agent for MR imaging of the small bowel. AJR Am J Roentgenol. 2001;177:1333-1334. [Cited in This Article: ] |
| 24. | Laghi A, Borrelli O, Paolantonio P, Dito L, Buena de Mesquita M, Falconieri P, Passariello R, Cucchiara S. Contrast enhanced magnetic resonance imaging of the terminal ileum in children with Crohn’s disease. Gut. 2003;52:393-397. [Cited in This Article: ] |
| 25. | McKenna DA, Roche CJ, Murphy JM, McCarthy PA. Polyethylene glycol solution as an oral contrast agent for MRI of the small bowel in a patient population. Clin Radiol. 2006;61:966-970. [Cited in This Article: ] |
| 26. | Malago R, Manfredi R, Benini L, D'Alpaos G, Mucelli RP. Assessment of Crohn's disease activity in the small bowel with MR-enteroclysis: clinico-radiological correlations. Abdom Imaging. 2008;33:669-675. [Cited in This Article: ] |
| 27. | Masselli G, Casciani E, Polettini E, Gualdi G. Comparison of MR enteroclysis with MR enterography and conventional enteroclysis in patients with Crohn’s disease. Eur Radiol. 2008;18:438-447. [Cited in This Article: ] |
| 28. | Kuehle CA, Ajaj W, Ladd SC, Massing S, Barkhausen J, Lauenstein TC. Hydro-MRI of the small bowel: effect of contrast volume, timing of contrast administration, and data acquisition on bowel distention. AJR Am J Roentgenol. 2006;187:W375-W385. [Cited in This Article: ] |
| 29. | Kinner S, Kuehle CA, Herbig S, Haag S, Ladd SC, Barkhausen J, Lauenstein TC. MRI of the small bowel: can sufficient bowel distension be achieved with small volumes of oral contrast? Eur Radiol. 2008;18:2542-2548. [Cited in This Article: ] |