Published online Jun 21, 2009. doi: 10.3748/wjg.15.2862
Revised: April 15, 2009
Accepted: April 22, 2009
Published online: June 21, 2009
AIM: To observe the protective effect of Radix Astragali injection on immune organs (lymph nodes, spleen and thymus) of rats with obstructive jaundice (OJ) and its mechanism.
METHODS: SD rats were randomly divided into sham-operation group, model control group and Radix Astragali treatment group. On days 7, 14, 21 and 28 after operation, mortality rate of rats, pathological changes in immune organs, expression levels of Bax and nuclear factor (NF)-κB p65 proteins, apoptosis indexes and serum tumor necrosis factor (TNF)-α level in spleen and thymus were observed, respectively.
RESULTS: Compared to model control group, the number of dead OJ rats in Radix Astragali treatment group decreased (P > 0.05). The TNF-α level (27.62 ± 12.61 vs 29.55 ± 18.02, 24.61 ± 9.09 vs 31.52 ± 10.95) on days 7 and 21, the pathological severity score for spleen [0.0 (0.0) vs 0.0 (2.0) on days 7 and 14 and for lymph nodes [0.0 (1.0) vs 1.0 (2.0), 1.0 (0.0) vs 2.0 (1.0)] on days 21 and 28, the product staining intensity and positive rate of Bax protein in spleen [0.0 (0.0) vs 1.0 (2.0), 0.0 (1.0) vs 2.0 (1.5) and thymus [0.0 (0.0) vs 1.0 (2.0), 0.0 (1.0) vs 2.0 (1.5)] on days 14 and 28, the apoptotic indexes [0.0 (0.0) vs 0.0 (0.01)] in spleen and thymus [0.0 (0.0) vs 0.0 (0.01) on days 14 and 21 were significantly lower in Radix Astragali treatment group than in model control group (P < 0.05).
CONCLUSION: Radix Astragali has protective effects on immune organs of OJ rats by relieving the pathological changes in immune organs, reducing TNF-α level and inhibiting Bax expression and apoptosis in spleen and thymus.
-
Citation: Zhang RP, Zhang XP, Ruan YF, Ye SY, Zhao HC, Cheng QH, Wu DJ. Protective effect of
Radix Astragali injection on immune organs of rats with obstructive jaundice and its mechanism. World J Gastroenterol 2009; 15(23): 2862-2869 - URL: https://www.wjgnet.com/1007-9327/full/v15/i23/2862.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2862
Obstructive jaundice (OJ) is a kind of common clinical manifestation. The pathogenesis and treatment of OJ have been a hot topic in medical field for a long time[1–3]. Since systemic inflammatory response syndrome and multiple organ dysfunction syndrome were studied in recent years, immune function impairment concomitant with OJ has gradually attracted wide attention and is considered as a cause of death in OJ patients[4–7]. Therefore, one of the important approaches to treatment of OJ is to restore the functions of immune organs[8–10].
Development and utilization of traditional Chinese medicine have good prospects in therapy for OJ since it has a lower cost, more extensive pharmacological effects and fewer side effects. Radix Astragali, dried root of Astragalus membranaceus, is sweet in taste with a warm property and mainly produced in Inner Mongolia Autonomous Region, Shanxi, Gansu and Heilongjiang Provinces of China. The raw Radix Astragali can be used to consolidate the exterior of body. Radix Astragali can regulate sweating, warm muscles, strengthen striae, invigorate Qi (vital energy), and alleviate heat in muscles due to Qi deficiency. Radix Astragali can also be used in treatment of chicken pox or other diseases with vesicular-papules due to inadequate “dispersing of the evils”. Radix Astragali invigorates Qi, ascends the Yang-Qi, protects Qi and consolidates the exterior of body, promotes diuresis, relieves edema (generalized swelling of the body), supports Qi to promote skin wound/ulcer healing and tissue/muscle regeneration. Astragalus injection is made of extraction from Radix Astragali. Since Astragalus injection contains polysaccharide, saponin, flavone and trace elements, it has a variety of pharmacological effects and increases the immunity and protects the liver and kidneys[11–15]. It has been shown that cellular immune function decreases in OJ rats[16], which can be successfully treated with Astragalus injection.
At present, studies about the effects of Astragalus injection on immune organs during OJ are not available. This study was to investigate the protective effect of Astragalus injection on immune organs of OJ rats and its mechanism. The results may provide an experimental basis for its application in clinical practice.
Healthy male SD rats of clean grade, weighing 270-330 g, were provided by Laboratory Animal Research Center, Zhejiang University of Traditional Chinese Medicine (China). Sodium pentobarbital was purchased from Sigma Corporation (USA). Radix Astragali injection (10 mL vial contains active components equivalent to 20 g of the original medicine) was purchased from Chiatai Qingchunbao Pharmaceutical Co, Ltd (China). Serum tumor necrosis factor (TNF)-α ELISA kits were purchased from Shanghai Senxiong Technological Company (China). Anti-nuclear factor (NF)-κB P65 and anti-Bax antibodies were purchased from Santa Cruz Biotechnology, Inc (USA). TUNEL assay kits were purchased from Takara Bio Inc (Jingdu, Japan).
One hundred and eighty OJ rats, enrolled in this study, were randomly divided into sham-operation group, model control group, and treatment group (n = 60), which were further subdivided into 7, 14, 21 and 28 d groups (n = 15) according to the time after operation. After the rats were anesthetized with intraperitoneal injection of 2.5% sodium pentobarbital (0.2 mL/100 g), their abdominal cavity was opened to identify and dissociate the common bile duct along the hepatoduodenal ligament. The proximal end of the common bile duct of rats in the model control and treatment groups was double-ligated with surgical threads, the common bile duct was cut off, and a layered suture of the abdominal wall was performed to close the abdominal cavity. The common bile duct of rats in the sham-operation group was dissociated but not ligated, and a layered suture of the abdominal wall was also performed to close the abdominal cavity. Rats in the treatment group received intraperitoneal Radix Astragali injection at a dose of 0.75 mL/100 g per day, while those in the sham-operation and model control groups received an equal volume of physiological saline solution until the end of 7-, 14-, 21- and 28-d observation periods in the corresponding groups.
Mortality rates of rats in different groups were recorded. Rats were killed after anesthesia with sodium pentobarbital in batches, serum was collected to measure TNF-α level by ELISA, and pathological changes in immune organs (lymph nodes, spleen and thymus) were observed. Pathological severity of immune organs was scored according to related standards. Tissues of spleen, thymus and lymph nodes were cut into sections, but the sections of lymph node were not stained. Changes in expression levels of Bax and NF-κB P65 proteins, as well as apoptosis index of spleen and thymus were observed, respectively.
Envision two-step method was used to detect the expression levels of Bax and NF-κB P65 proteins in intestinal mucosa. The staining intensity was evaluated according to the extent of cell coloration: “-” represents negative staining; “+” represents mild staining with positively stained cells showing a yellow pigment; “++” represents moderate staining with positively stained cells showing a brown pigment; “+++” represents intense staining with positively stained cells showing a dark brown pigment; (-) indicates no positive cells; (+) indicates less than 25% of positive cells; (++) indicates 26%-50% of positive cells; and (+++) indicates over 50% of positive cells, each of which was scored as 0, 1, 2 and 3 points, respectively.
DNA nicking in tissue sections was observed with in situ end-labeling (TUNEL) staining. In brief, sections were baked at 60°C for 30 min, deparaffinaged, and washed with Milli-Q for 5 min. Tissue was processed with protease K (10 &mgr;g/&mgr;L) at room temperature for 15 min and washed with phosphate-buffered saline (PBS) for 5 min. A 3% H2O2 solution was used to block endogenous peroxydase for 5 min, washed twice with PBS, 5 min each time. Thirty microliters of reaction solution at freezing condition (TdT enzyme : labeling safe buffer = 1:10) was added, incubated at 37°C for 90 min, and washed twice with PBS, 5 min each time. Fifty microliters of anti-FITC HRP conjugate was added, incubated at 37°C for 30 min, and washed twice with PBS, 5 min each time. DAB was colored, washed with Milli-Q, counterstained with hematoxylin, and washed with water after differentiation till it became blue. The DAB was routinely dehydrated and mounted onto neutral gum. Apoptotic index was calculated following the equation: Apoptotic index = apoptotic cell count/total cell count × 100%.
Data were input into the Excel sheet and read into SPSS 15.0 for further analysis. Normal data were expressed as mean ± SD while abnormal data were expressed as median (interquartile range). Analysis of variance and pairwise comparison were used for normal data, whereas abnormal data were subjected to non-parametric tests, of which Kruskal-Wallis H test was used for pairwise comparison and Mann-Whitney U test was used for multiple comparisons. Yates’χ2 test was used for inter-group comparisons of mortality rates.
All rats in the sham-operation group were alive after operation. Two rats in the model control group and one rat in the treatment group died on day 7 after operation. Four rats in the model control group and three rats in the treatment group died on day 14 after operation. Four rats in the model control group and four rats in the treatment group died on day 21 after operation. Seven rats in the model control group and six rats in the treatment group died on day 28 after operation. The total mortality rate of rats in the model control and treatment groups on day 28 was significantly higher than that of rats in the sham-operation group (P < 0.001). No significant difference was found in mortality rate between the model control and treatment groups.
The serum TNF-α level was significantly lower in sham-operation group than in model control and treatment groups at different time points after operation (P < 0.01), and was significantly lower in treatment group than in model control group on days 7 and 21 after operation (P < 0.05, Table 1).
In the sham-operation group, the morphology of spleen was normal with no gross pathological changes under light microscope.
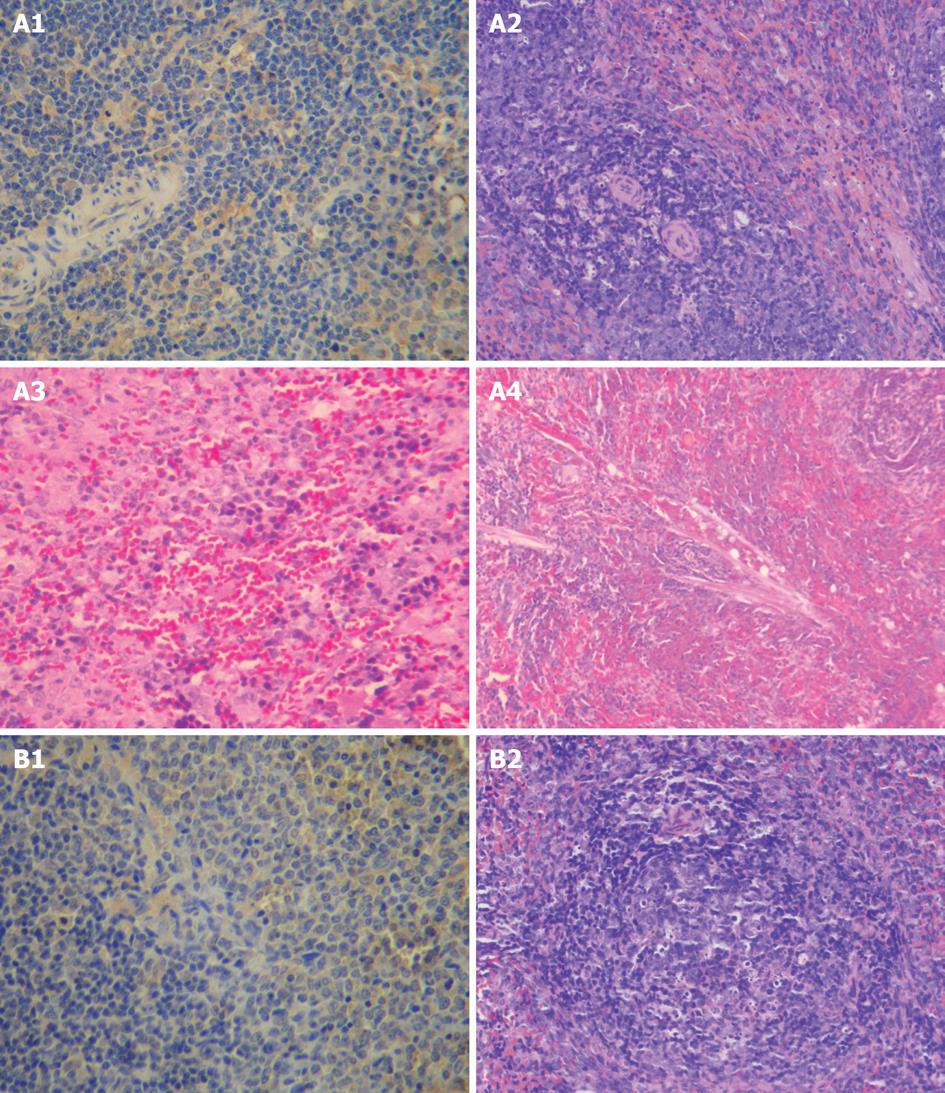
In the model control group, the size of spleen increased by 1.2-1.5 folds and the texture of spleen became fragile with no change in color on day 7 after operation. The spleen became enlarged with a thickness of above 0.6 cm and a deeper color and its texture became fragile on day 14 after operation. The spleen was 4 cm × 1 cm × 1 cm in size and its texture became fragile with a purple black color on days 21 and 28 after operation. Under light microscope, the spleen of all rats was grossly normal on day 7 after operation. Fusion, enlargement or spotty necrosis of follicular germinal center in the white pulp of spleen, hyperplasia of fibrous tissue in sinus, and arteriolar sclerosis in spleen of most rats were observed on day 14 after operation. The spleen of few rats was grossly normal. Fusion, enlargement or spotty necrosis of follicular germinal centers in the white pulp of spleen, hyperplasia of fibrous tissue in sinus, and arteriolar sclerosis in spleen of few rats were seen on days 21 and 28 after operation. The spleen of some rats was grossly normal (Figure 1A).
In the treatment group, no significant difference was found in pathological changes at all time points after operation compared to the model control group. Under light microscope, no significant difference in pathological changes was noted at each time point after operation. The spleen of most rats was grossly normal. Arteriolar sclerosis in spleen of few rats was seen (Figure 1B).
The pathological scoring standards for spleen are listed in Table 2. The pathological scores were significantly lower for sham-operation group than for model control group on day 14 after operation (P < 0.05), and were significantly lower for treatment group than for model control group on days 7 and 14 after operation (P < 0.05, Table 3).
| Score standards | Observation indexes |
| 0 | Normal |
| 1 | Necrosis of follicle center |
| 2 | Blood sinus dilation or arteriolar sclerosis |
| 3 | Necrosis of follicle center, blood sinus dilation and arteriolar sclerosis |
| Index | Time (d) | Sham-operation group | Model control group | Treatment group |
| Pathological severity score | 7 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0)c |
| 14 | 0.0 (1.0)c | 0.0 (2.0) | 0.0 (0.0)c | |
| 21 | 0.0 (0.0) | 1.0 (2.0) | 0.0 (0.0) | |
| 28 | 0.0 (0.0) | 0.0 (1.0) | 0.0 (0.0) | |
| Product staining intensity and positive rate of Bax | 7 | 0.0 (0.0)c | 1.0 (2.0) | 0.5 (1.0)a |
| 14 | 0.0 (1.0) | 1.0 (2.0) | 0.0 (0.0)c | |
| 21 | 0.0 (0.0)c | 1.0 (2.0) | 0.0 (2.0) | |
| 28 | 0.0 (0.0)c | 2.0 (1.5) | 0.0 (1.0)c | |
| Apoptosis index | 7 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| 14 | 0.0 (0.0) | 0.0 (0.01) | 0.0 (0.0)c | |
| 21 | 0.0 (0.0)c | 0.0 (0.01) | 0.0 (0.0) | |
| 28 | 0.0 (0.0)c | 0.01 (0.02) | 0.0 (0.0) | |
| Product staining intensity and positive rate of NF-κB | 7 | 0.0 (0.0) | 0.0 (1.0) | 0.0 (0.0) |
| 14 | 0.0 (1.0) | 0.0 (2.0) | 0.0 (0.0) | |
| 21 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | |
| 28 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
No significant difference was found in product staining intensity and in positive rate of NF-κB protein in spleens of all groups (Table 3).
The product staining intensity and positive rate of Bax protein were significantly lower in sham-operation and treatment groups than in model control group on day 28 after operation (P < 0.05, Table 3).
The apoptosis index was significantly lower in sham-operation group than in model control group on days 7, 14 and 28 after operation (P < 0.05), and was significantly lower in treatment group than in model control group on day 14 after operation (P < 0.05, Table 3).
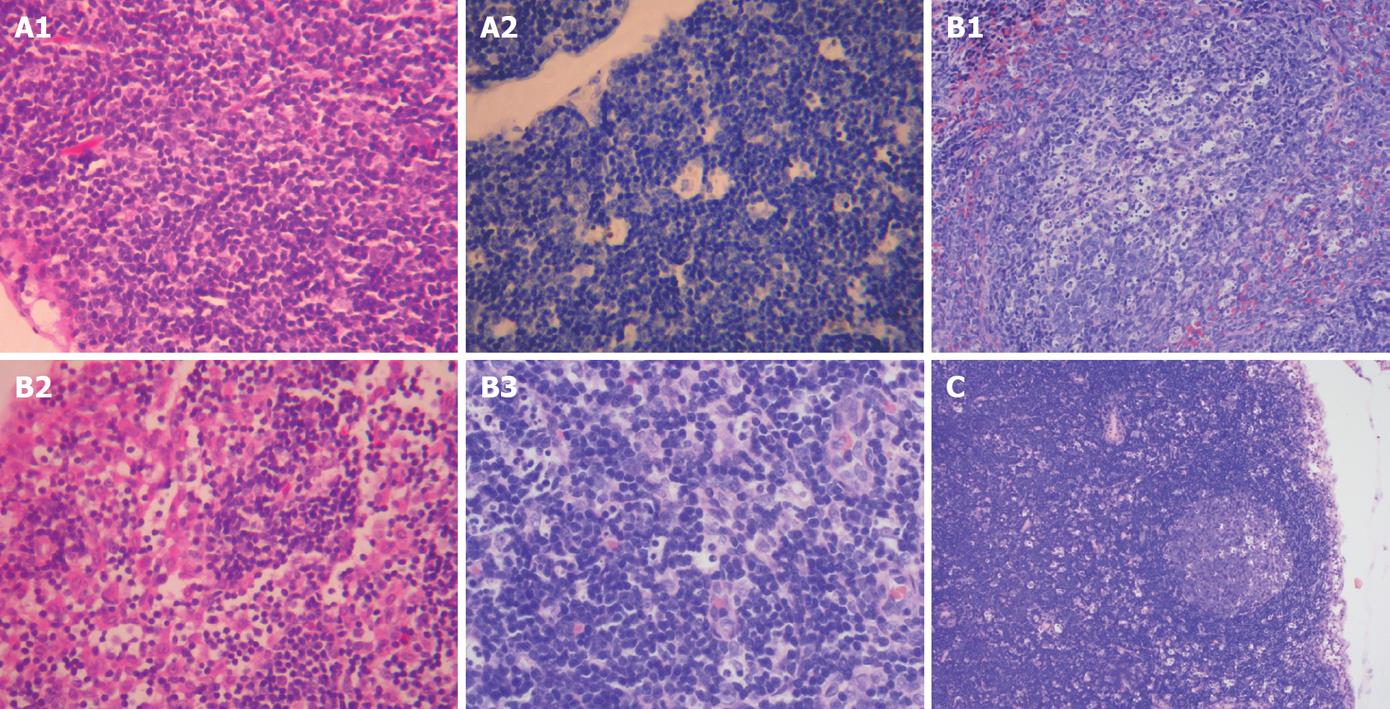
In sham-operation group, the gross morphology of lymph nodes was normal. Under light microscope, no significant difference was observed in pathological changes at different time points after operation. The morphology and structure of lymph nodes were grossly normal. Enlargement of follicular germinal centers and hyperplasia of sinus cells were seen in few rats (Figure 2A).
In model control group, lymph nodes became yellow in 50% of the rats on day 7 after operation, but no changes were found in the texture of lymph nodes at all time points after operation. Under light microscope, no significant difference in pathological changes was observed at all time points after operation. Enlargement of follicular germinal centers and hyperplasia of sinus cells were seen in most rats and few rats showed no obvious pathological changes in lymph nodes with spotty necrosis in the mantle zone and germinal centers on days 7, 14, 21 and 28 after operation (Figure 2B).
In the treatment group, no significant difference in pathological changes was observed at all time points after operation compared to the model control group. Under light microscope, no obvious difference was found in lymph node pathological changes at all time points after operation. In most rats, enlargement of lymph nodes in germinal centers and hyperplasia of cells in lymph sinus were observed with spotty necrosis of lymph nodes in the mantle zone and germinal centers of (Figure 2C).
The pathological scoring standards for lymph nodes have been described elsewhere[17]. The pathological score was significantly lower for sham-operation group than for model control group on days 7, 14 and 21 after operation (P < 0.05). The pathological score was significantly lower for sham-operation group than for treatment group on day 7 after operation (P < 0.05) and was significantly lower for treatment group than for model control group on days 21 and 28 after operation (P < 0.05, Table 4).
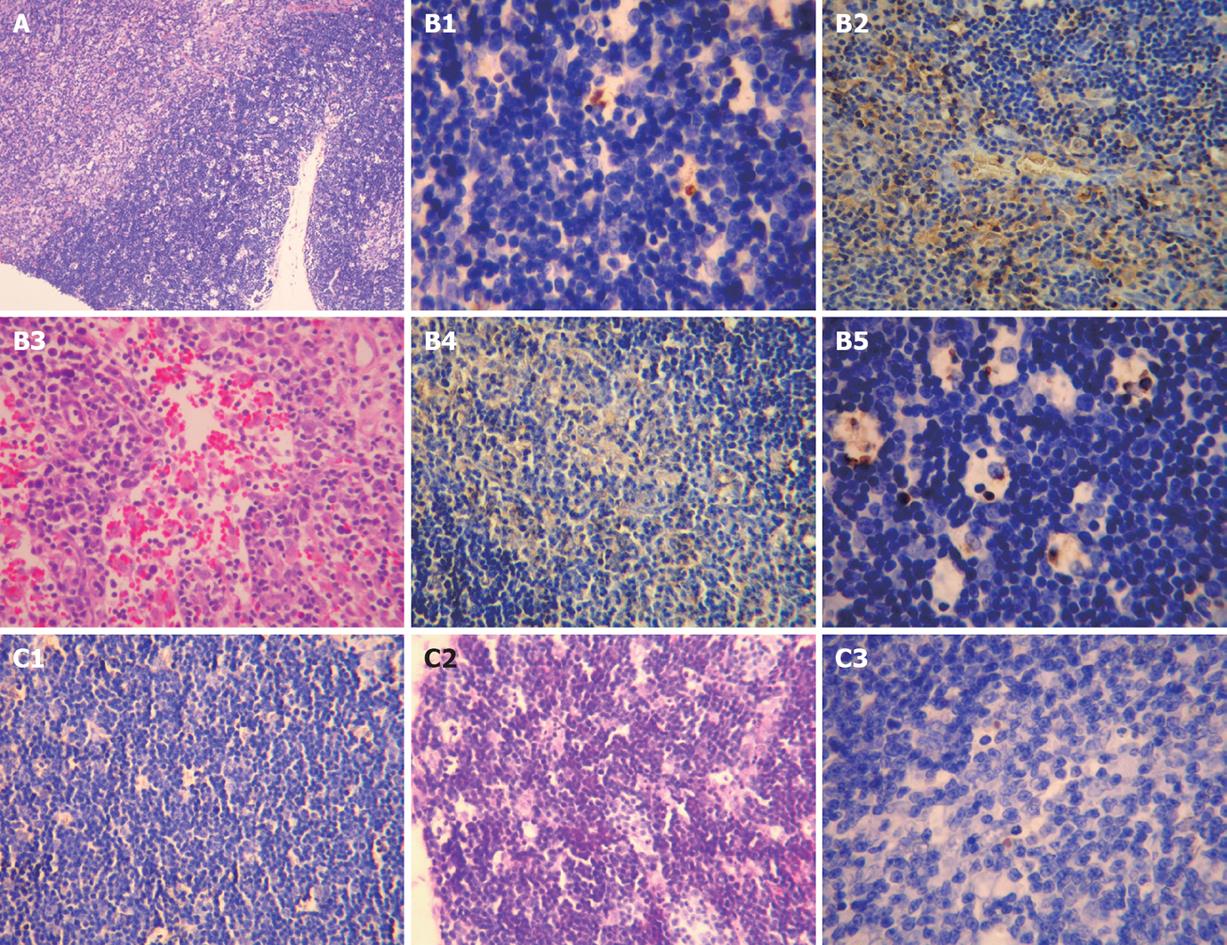
In sham-operation group, no significant difference was found in thymus pathological changes at all time points after operation compared to model control group, and the thymus tissue of all rats was grossly normal (Figure 3A).
In model control group, the thymus of rats was mildly shrunken on day 7 after operation, moderately shrunken and jaundiced on day 14 after operation, and severely shrunken and jaundiced on days 21 and 28 after operation. Under light microscope, no significant difference was noted in thymus pathological changes at all time points after operation. The thymus tissue of most rats was grossly normal. An obscure boundary between thymus cortex and medulla was occasionally seen. The thymus tissue was grossly normal on day 14 after operation. The thymus pathological changes were similar on days 14, 21 and 28 after operation (Figure 3B).
In treatment group, no significant difference in thymus pathological changes was observed on days 7 and 14 after operation compared with model control group. The thymus became mildly jaundiced with no obvious shrinkage on days 21 and 28 after operation. Under light microscope, the thymus tissue of most rats was grossly normal. An obscure boundary between thymus cortex and medulla was seen in few rats (Figure 3C).
The pathological scoring standards for thymus have been described elsewhere [18]. No significant difference was observed in pathological scores for different groups (Table 5).
| Index | Time (d) | Sham-operation group | Model control group | Treatment group |
| Product staining intensity and positive rate of Bax | 7 | 0.0 (0.0)c | 1.0 (2.0) | 0.5 (1.0)a |
| 14 | 0.0 (1.0) | 1.0 (2.0) | 0.0 (0.0)c | |
| 21 | 0.0 (0.0)c | 1.0 (2.0) | 0.0 (2.0) | |
| 28 | 0.0 (0.0)c | 2.0 (1.5) | 0.0 (1.0)c | |
| Apoptosis index | 7 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| 14 | 0.0 (0.0) | 0.0 (0.01) | 0.0 (0.0)c | |
| 21 | 0.0 (0.0)c | 0.0 (0.01) | 0.0 (0.0)c | |
| 28 | 0.0 (0.0)c | 0.01 (0.02) | 0.0 (0.0) | |
| Product of the staining intensity and positive rate of NF-κB | 7 | 0.0 (0.0) | 0.0 (2.0) | 0.0 (0.0) |
| 14 | 0.0 (4.0) | 2.0 (4.0) | 0.0 (2.0) | |
| 21 | 0.0 (2.0) | 0.0 (2.0) | 0.0 (0.0) | |
| 28 | 0.0 (1.0) | 0.0 (3.0) | 0.0 (2.0) |
No significant difference was found in product staining intensity and positive rate of NF-κB protein in thymus of different groups (Table 5).
The product staining intensity and positive rate of Bax protein were significantly lower in sham-operation group than in model control group on days 7, 21 and 28 after operation (P < 0.05), in sham-operation group than in treatment group on day 7 after operation (P < 0.05), and in treatment group than in model control group on days 14 and 28 after operation (P < 0.05).
The apoptosis index for thymus was significantly lower in sham-operation group than in model control group on days 21 and 28 after operation (P < 0.05), and in treatment group than in model control group on days 14 and 21 after operation (P < 0.05).
It has been shown that the incidence rate of endotoxemia in OJ patients is as high as 39.3%[19], which is mainly due to insufficient intestinal bile salt that leads to excessive multiplication of intestinal bacteria and decreased inactivation of endotoxins. Since the function of reticuloendothelial system is inhibited, gut-derived endotoxins are not effectively eliminated. As a result, large amounts of endotoxin enter into the blood resulting in endotoxemia. Endotoxin is a main factor for immune function impairment during OJ since it can directly stimulate Kupffer cells to release inflammatory mediators, including oxygen free radicals, TNF-α, IL-6 and IL-8, and thereby aggravates the inflammatory response of body[19–24]. TNF-α is the most important factor for mediating toxic effects of endotoxin. Excessive release of TNF-α can induce multiple organ injuries. We speculate that immune function impairment results from the damage to immune organs. The results of this study show that the serum TNF-α level and pathological scores for spleen and lymph nodes were significantly lower in sham-operation and treatment groups than in model control group, suggesting that TNF-α is involved in OJ-induced damage to immune organs, and Astragalus injection can significantly lessen the toxic effects of TNF-α on and improve the pathological changes in immune organs. We think that Astragalus injection exerts, to a certain degree, its protective effects on immune organs by suppressing the production of TNF-α. Although no statistically significant difference was observed in pathological scores for thymus, the pathological changes in thymus of Astragalus treatment group showed varying degrees of improvement compared with model control group. In model control group, the thymus showed varying degrees of jaundice and atrophy at all time points after operation, and an obscure boundary between the thymus cortex and medulla in parts of thymus tissue under light microscope. In contrast, the thymus in treatment group became jaundiced with no atrophy on days 21 and 28 after operation, and the boundary between the thymus cortex and medulla was obscure in few parts of thymus tissue under light microscope, suggesting that Astragalus has protective effects on immune organs.
NF-κB p65, a protein that is extensively distributed in cytoplasm of many cells, can regulate gene transcription in nuclei. It is a member of Rel family of transcriptional regulatory proteins and is involved in gene expression regulation of many inflammatory factors. When the body is under stress, NF-κB p65 is activated and binds to specific κB gene sequences, and thereby promotes gene transcription and protein synthesis of pro-inflammatory molecules, causes strong expression of inflammatory cytokines such as TNF-α and IL-6mRNA, accelerates toxic effect on cells in multiple organs, eventually leading to multiple organ dysfunction. Based on the expression levels of NF-κB p65 protein in spleen and thymus, we speculate that Astragalus has no inhibitory effects on the expression of NF-κB p65 protein in spleen and thymus of OJ rats.
Apoptosis is a self-protective strategy employed by the body for removal of the destroyed cells through initiating programmed gene expression under certain pathophysiological conditions[25]. In contrast to cell necrosis, apoptosis is an active and spontaneous process and does not induce dramatic inflammatory reaction. However, apoptosis as a mode of cell loss can also induce functional impairment of immune organs. Bax, a soluble protein encoded by a recently discovered apoptosis-promoting gene, shares the same protein family as Bcl-2 and is able to promote cell apoptosis[2627]. In this study, the expression level of Bax protein was higher in spleen and thymus of model group than in those of sham-operation group. As a result, the apoptosis index was increased and pathological injury was aggravated, suggesting that Bax protein is involved in physiological or pathological cellular apoptosis of spleen and thymus. After treatment with Astragalus injection, the pathological changes in immune organs were improved and the expression level of Bax protein in spleen and thymus, apoptosis index and pathological scores for spleen and thymus were significantly lower in treatment group than in model control group, indicating that Astragalus injection can down-regulate the expression of Bax protein, suppress cell apoptosis and exert protective effects on immune organs.
In summary, Astragalus injection can improve pathological changes in immune organs, reduce serum TNF-α level, down-regulate expression of Bax protein in spleen and thymus, and suppress cell apoptosis, thereby exerting its protective effects on immune organs of OJ rats. Since Astragalus has diverse pharmacological actions, low cost and few side effects, it has a better application prospect and economic value.
Obstructive jaundice (OJ) is a kind of common clinical manifestation. The pathogenesis and treatment of OJ have been a hot topic in medical field for a long time. As systemic inflammatory response syndrome and multiple organ dysfunction syndrome were studied in recent years, immune function impairment concomitant with OJ has gradually attracted wide attention and is considered as a cause of death in OJ patients. Therefore, one of the important approaches to treatment of OJ is to restore the functions of immune organs.
Development and utilization of traditional Chinese medicine have good prospects in therapy for OJ since it has lower cost, more extensive pharmacological effects and fewer side effects. Since Astragalus injection contains polysaccharide, saponin, flavone and trace elements, it has a variety of pharmacological effects and plays an important role in increasing the immunity of body and protecting the liver and kidney. This study demonstrated that Radix Astragali could exert its protective effects on immune organs of OJ rats by relieving the pathological changes in immune organs, reducing tumor necrosis factor (TNF)-α level, and inhibiting Bax expression and apoptosis in spleen and thymus.
At present, no studies about the effects of Astragalus on immune organs during OJ are available. In the present study, we investigated the protective effect of Astragalus injection on immune organs of OJ rats and its mechanism, which may provide an experimental basis for its application in clinical practice.
Astragalus has diverse pharmacological actions, low cost and few side effects, and thus can be applied in clinical practice.
Nuclear factor (NF)-κB p65, a protein that is extensively distributed in cytoplasm of many cells, is able to regulate gene transcription in nuclei. TNF-α is a most important factor for mediating the toxic effects of endotoxins. Bax, a soluble protein encoded by a recently discovered apoptosis-promoting gene, shares the same protein family as Bcl-2 and is able to promote cell apoptosis.
The manuscript describes the protective effects of Radix astragali injection on immune organs of rats with OJ. Radix astragali injection could reverse elevated TNF-α level, and spleen, thymus and lymph node lesions. Bax immunoreactivity and apoptosis could be observed after obstructive jaundice. This manuscript is largely descriptive by providing novel insights into the mechanism underlying the beneficial effect of Radix astragali on obstructive jaundice.
| 1. | Ljungdahl M, Osterberg J, Ransjö U, Engstrand L, Haglund U. Inflammatory response in patients with malignant obstructive jaundice. Scand J Gastroenterol. 2007;42:94-102. [Cited in This Article: ] |
| 2. | Tsuyuguchi T, Takada T, Miyazaki M, Miyakawa S, Tsukada K, Nagino M, Kondo S, Furuse J, Saito H, Suyama M. Stenting and interventional radiology for obstructive jaundice in patients with unresectable biliary tract carcinomas. J Hepatobiliary Pancreat Surg. 2008;15:69-73. [Cited in This Article: ] |
| 3. | Comert M, Ustundag Y, Tekin IO, Gun BD, Barut F. Obstructive jaundice leads to accumulation of oxidized low density lipoprotein in human liver tissue. World J Gastroenterol. 2006;12:5094-5095. [Cited in This Article: ] |
| 4. | Sano T, Ajiki T, Takeyama Y, Kuroda Y. Internal biliary drainage improves decreased number of gut mucosal T lymphocytes and MAdCAM-1 expression in jaundiced rats. Surgery. 2004;136:693-699. [Cited in This Article: ] |
| 5. | Sakrak O, Akpinar M, Bedirli A, Akyurek N, Aritas Y. Short and long-term effects of bacterial translocation due to obstructive jaundice on liver damage. Hepatogastroenterology. 2003;50:1542-1546. [Cited in This Article: ] |
| 6. | Veligostkiĭ NN, Veligotsiĭ AN, Obuobi RB, Okleĭ DV, Maslov SP, Komarchuk VV. [The choice of surgical strategy for patients with obstructive jaundice and high risk of multi-organ insufficiency syndrome]. Klin Khir. 2001;10-13. [Cited in This Article: ] |
| 7. | Nehéz L, Andersson R. Compromise of immune function in obstructive jaundice. Eur J Surg. 2002;168:315-328. [Cited in This Article: ] |
| 8. | Padillo FJ, Cruz A, Segura-Jiménez I, Ruiz-Rabelo J, Vázquez-Ezquerra MR, Perea-Alvarez MD, Peña J, Briceño J, Muntané J. Anti-TNF-alpha treatment and bile duct drainage restore cellular immunity and prevent tissue injury in experimental obstructive jaundice. Int J Immunopathol Pharmacol. 2007;20:855-860. [Cited in This Article: ] |
| 9. | Zulfikaroglu B, Zulfikaroglu E, Ozmen MM, Ozalp N, Berkem R, Erdogan S, Besler HT, Koc M, Korkmaz A. The effect of immunonutrition on bacterial translocation, and intestinal villus atrophy in experimental obstructive jaundice. Clin Nutr. 2003;22:277-281. [Cited in This Article: ] |
| 10. | Jiang WG, Puntis MC. Immune dysfunction in patients with obstructive jaundice, mediators and implications for treatments. HPB Surg. 1997;10:129-142. [Cited in This Article: ] |
| 11. | Yuan W, Zhang Y, Ge Y, Yan M, Kuang R, Zheng X. Astragaloside IV inhibits proliferation and promotes apoptosis in rat vascular smooth muscle cells under high glucose concentration in vitro. Planta Med. 2008;74:1259-1264. [Cited in This Article: ] |
| 12. | Gao QT, Cheung JK, Choi RC, Cheung AW, Li J, Jiang ZY, Duan R, Zhao KJ, Ding AW, Dong TT. A Chinese herbal decoction prepared from Radix Astragali and Radix Angelicae Sinensis induces the expression of erythropoietin in cultured Hep3B cells. Planta Med. 2008;74:392-395. [Cited in This Article: ] |
| 13. | Mou S, Ni ZH, Zhang QY. [Expression of c-met in human kidney fibroblasts induced by high glucose in vitro and the regulation of Radix Astragali]. Zhongxiyi Jiehe Xuebao. 2008;6:482-487. [Cited in This Article: ] |
| 14. | Gui SY, Wei W, Wang H, Wu L, Sun WY, Chen WB, Wu CY. Effects and mechanisms of crude astragalosides fraction on liver fibrosis in rats. J Ethnopharmacol. 2006;103:154-159. [Cited in This Article: ] |
| 15. | Mou S, Ni ZH, Zhang QY. [Expression of c-met in human kidney fibroblasts induced by high glucose in vitro and the regulation of Radix Astragali]. Zhongxiyi Jiehe Xuebao. 2008;6:482-487. [Cited in This Article: ] |
| 16. | Wang Y, Hu ZQ, Cheng ZG, Zhang LZ, Wang YH. Effect of astragalus on cellular immune function of rats with obstructive jaundice. Gandan Waike Zazhi. 2000;8:64-66. [Cited in This Article: ] |
| 17. | Zhang XP, Xu HM, Jiang YY, Yu S, Cai Y, Lu B, Xie Q, Ju TF. Influence of dexamethasone on mesenteric lymph node of rats with severe acute pancreatitis. World J Gastroenterol. 2008;14:3511-3517. [Cited in This Article: ] |
| 18. | Xiping Z, Li C, Miao L, Hua T. Protecting effects of dexamethasone on thymus of rats with severe acute pancreatitis. Mediators Inflamm. 2007;2007:72361. [Cited in This Article: ] |
| 19. | Zhang J, Liu YH, Jiang XH, Xu KS. Relationship between endotoxemia and cellular immunity in obstructive jaundice. Huaren Xiaohua Zazhi. 1998;6:305-306. [Cited in This Article: ] |
| 20. | Ding XZ, Li H, Xiong ST, Zhang SX, Lu KZ, Shao JF, Shen GX, Yang J. Effects of cimetidine on IL-2 and T suppressor cell function in rats with obstructive jaundice. J Tongji Med Univ. 1994;14:94-97. [Cited in This Article: ] |
| 21. | Zhan SL. [Clinical study on the immune function of patients with obstructive jaundice]. Zhonghua Waike Zazhi. 1993;31:480-483. [Cited in This Article: ] |
| 22. | Greve JW, Gouma DJ, Soeters PB, Buurman WA. Suppression of cellular immunity in obstructive jaundice is caused by endotoxins: a study with germ-free rats. Gastroenterology. 1990;98:478-485. [Cited in This Article: ] |
| 23. | Li YG, Li QL. Effect of obstructive jaundice on the blood and immune system. Zhongguo Shiyong Waike Zazhi. 1996;16:17-20. [Cited in This Article: ] |
| 24. | Ji F, Chen JX, Shi WJ. Changes in the body's immune function in obstructive jaundice complicated with endotoxemia. Gandanyi Waike Zazhi. 1997;9:100-102. [Cited in This Article: ] |
| 25. | Thatte U, Dahanukar S. Apoptosis: clinical relevance and pharmacological manipulation. Drugs. 1997;54:511-532. [Cited in This Article: ] |
| 26. | Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281-1292. [Cited in This Article: ] |
| 27. | Maurer M, Tsai M, Metz M, Fish S, Korsmeyer SJ, Galli SJ. A role for Bax in the regulation of apoptosis in mouse mast cells. J Invest Dermatol. 2000;114:1205-1206. [Cited in This Article: ] |