Published online Jun 14, 2009. doi: 10.3748/wjg.15.2794
Revised: May 11, 2009
Accepted: May 18, 2009
Published online: June 14, 2009
AIM: To construct p27mt recombinant adenovirus, transfect the colorectal cell line Lovo and observe the effects of p27mt on Lovo cell apoptosis and cell cycle inhibition.
METHODS: We constructed recombinant adenovirus containing p27mt by homologous recombination in bacteria. The colorectal cancer cell line Lovo was infected with recombinant replication-defective adenovirus Ad-p27mt, and expression of p27mt was determined by Western blotting; the inhibitory effect of p27mt on Lovo cells was detected by cytometry. Cell cycle was determined by flow cytometry. DNA fragment analysis identified the occurrence of apoptosis.
RESULTS: The recombinant adenovirus which already contained p27mt target gene was successfully constructed. When multiplicity of infection was ≥ 50, the infection efficiency was 100%. After transfection of Lovo cells with Ad-p27mt the cells had high p27 expression which was identified by immunoblotting assay. PI staining and flow cytometry showed that 77.96% of colorectal cancer cells were inhibited in phase G0/G1, while in the Ad-LacZ group and blank control group, 27.57% and 25.29% cells were inhibited in the same phase, respectively. DNA fragment analysis, flow cytometry and TUNEL assay demonstrated that p27mt is able to induce apoptosis in colorectal cancer cells.
CONCLUSION: p27mt has an obvious blocking effect on colorectal cancer cell cycle, and most cells were inhibited in phase G0/G1. Therefore, p27mt can induce apoptosis in colorectal cells.
-
Citation: Chen J, Ding WH, Xu SY, Wang JN, Huang YZ, Deng CS. Effect of
p27mt gene on apoptosis of the colorectal cancer cell line Lovo. World J Gastroenterol 2009; 15(22): 2794-2799 - URL: https://www.wjgnet.com/1007-9327/full/v15/i22/2794.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2794
p27Kip1 (p27) is a cyclin dependent kinase inhibitor (CDKI), whose specific late G1 destruction allows progression of the cell across the G1/S boundary. The protein was ubiquitinated by S-phase kinase-interacting protein-2 (Skp2) following its specific phosphorylation, and was subsequently degraded by the 26S proteasome[1]. There was a direct relationship between the low level of p27 and rapid proliferation occurring in several benign states and in many malignancies. It has been reported that p27 levels were markedly reduced in several malignancies, such as those of the skin[2], liver[3], bladder[4], thyroid[5], breast[6], prostate[7] and endometrium[8]. In some of the tumors studied, a strong correlation was found between the low level of p27, the aggressiveness of the disease and poor prognosis of the patients[6]. Interestingly, p27 in all these tumors was of the wild-type species (p27wt), and its regulation has been attributed to phosphorylation of Thr-187 and subsequent ubiquitination[9]. Overexpression of p27 via adenoviral gene transfer could suppress cancer cell growth regardless of p27 mutation[10]. Montagnoli et al[11] showed that the ubiquitination of p27 did not occur in p27mt with Thr-187 to Ala [p27 (T187A)]. Sheaff et al[12] showed that the transfection of p27 (T187A) plasmid caused a G1 block, which was both resistant to and not modulated by cyclin E/Cdk2. On the basis of these observations of p27 regulation and the nature of the p27 tumor suppressor gene, we constructed Adenovirus expressing p27mt (Thr-187/Pro-188 to Met-187/Ile-188) to infect the colorectal cancer cell line Lovo, and then investigated its expression and functional significance in the cell proliferation and apoptosis of Lovo cells, by which we aimed to discuss novel methods of gene therapy in colorectal cancer.
The restriction endonucleases such as AgeI, NheI, KpnI, PacI and PmeI were purchased from New England Biolabs Co. HindIII, EcoRI, λDNA HindIII marker, 200 bp DNA ladder, dNTP, Tag enzyme and T4 DNA ligase were purchased from Huamei Biological Co. (China). The Western blotting kit was purchased from KPL Co. (USA). The rat anti-human p27kip1 multi-antibody was purchased from Santa Cruz Co. (USA). The horseradish peroxidase (HRP) labeled sheep anti-rat IgG monoclonal antibody was purchase from Zhongshan Co. (China). The p27mt primer was designed and synthesized by Beijing Saibaisheng Biological Co. (China). The fetal bovine serum (FBS) was purchased from Hangzhou Sijiqing Biological Engineering Materials Co. (China). The liposome (polyfect) was purchased from Qiagen Co. (USA). Trypsin, DMEM culture medium, Hepes and Cscl were purchased from Sigma Co. (USA).
The pORF9-p27mt plasmid was purchased from Invivogen (USA). The pAdeasy-1 plasmid, pBluescript IIsk (+), Ad293 cell, E. coli BJ5183 and XL10-gold were purchased from Stratagene (USA). The LacZ recombinant adenovirus (Ad-LacZ with titer 7.15 × 1015/L)[13] and DH5α were gratefully provided by Doctor Wang Jianing, Clinical Research Institute, Yunyang Medical College. The Lovo cell line was purchased from Type Culture Collection Center, Wuhan University.
This equipment included a high speed freezing centrifuge (Universal 32R, Germany), the ultraspeed freezing centrifuge (Tokyo Cp80max, Japan), inverted phase contrast microscope (Nikon TE2000-u, Japan), CO2 culture box (CB150#00-17611, wtb-binder), PCR machine (Biometra. Germany), ultraviolet spectrophotometer (Auriud CE2401, UK), Coulter Epics XL flow cytometer (Beckman Co., USA), high speed table-top centrifuge and a water bath shaking table (China).
After pORF9-p27mt was digested by AgeI and NheI enzymes, the 619 bp fragment was recycled and subcloned into pBluescript IISK (+) which was digested by XmaI and XbaI enzymes, thus obtaining pBluescript-p27mt. Then pBluescript-p27mt was digested by NotI and KpnI enzymes, and the 699 bp fragment was recycled and inserted into the shuttle plasmid vector pShuttle-CMV which was digested by the same enzymes, thus the transfer plasmid pShuttle-CMV-p27mt was obtained. The competent E. coli was transformed by the adenoviral framework plasmid pAdeasy-1. According to the ampicillin-resistant gene, the BJ5183 containing pAdeasy-1 was picked out and prepared into the ultra-competence BJ5183 containing pAdeasy-1. Then, the ultra-competence BJ5183 was transformed by transfer plasmid pShuttle-CMV-p27mt which was digested by PmeI enzyme and dephosphorylated by alkaline phosphatase. A little DNA from the transformed clone bacterial plasmid was taken out and the suspect DNA of the recombinant adenovirus plasmid was chosen according to the size of the plasmid in agarose electrophoresis. If the chosen DNA was identified as the correct DNA by digestion of PacI enzyme, then the recombinant adenovirus plasmid pAdeasy-1-p27mt could be prepared. Recombinant adenovirus plasmid DNA was excised by PacI, then transfected by Ad293 via liposome polyfect mediation, where the change in cells at different times after transfection was determined. When it appeared that 90% had cell lesions, scratch 293 cells from the culture bottle were vortexed three times at -80°C to +37°C to lyse the cells, then centrifuged and the supernatant containing the virus was collected, the 293 cells were reinfected with the above virus and proliferation of the virus occurred at a large scale. Purification of recombinant adenovirus was similar to the method of Cortin et al[14]. After purification the adenovirus underwent dialysis, test virus titers were detected using an ultraviolet spectrophotometer. Fifty microliter of purified adenovirus liquid, 100 g/L SDS 20 &mgr;L, PBS 430 &mgr;L, were assayed at absorbance values of A260 and A280, then the granule numbers and purity of adenovirus were determined. If A260 = 1015pfu/L and A260/A280 > 1.3 this indicated that the purity was relatively high when virus titers (pfu/L) = A260× dilution × 1015. PCR identification of recombinant adenovirus Ad-p27mt was carried out. Recombinant adenovirus genome DNA from the high titer virus storage liquid served as a template. Using primer toward the reporter gene p27mt, the PCR reaction parameters were: pre-denaturation at 95°C for 5 min, denaturation at 94°C for 20 s, annealing at 56°C for 30 s, elongate at 72°C for 30 s, 30 cycles, elongate at 72°C for 10 min. Primer 1: 5’CCTAGAGGGCAAGTACGAGTG3’; Primer 2: 5’GAAGAATCGTCGGTTGCAGGTCGCT3’.
Lovo cells were incubated in 100 mL/L FCS and RPMI-1640 culture medium at 37°C in a 50 mL/L CO2 culture box until the cells spread to 70%-80% of the area and were used in the following experiment.
Lovo cells taken from the 15 cm culture flask were infected with Ad-LacZ according to an Multiplicity of infection (MOI) of 20, 40, 50 and 100 and then incubated for another 48 h. The cells were then fixed by 5 mL/L glutaral for 15 min and washed three times with PBS, X-gal staining solution (20:1) was added. The cells were then incubated at 37°C in the 50 mL/L CO2 culture box for 4-24 h. The blue-stained cells, i.e. the positive cells in which the LacZ gene was expressed, were observed under the microscope and the percentage of positive cells was calculated.
Lovo cells taken from the 75 cm culture flask were infected with Ad-p27mt (MOI 50) and Ad-LacZ (MOI 50), respectively. After incubation in the same conditions for 48 h, the cells were digested by 0.5 g/L trypsin, collected and washed twice with PBS. After lysis by 500 &mgr;L 1 × SDS-PAGE cell lysis solution and boiling for 5 min, the cells were centrifuged, the supernatant was collected and then detected by Western blotting.
Lovo cells cultured in the 75 cm culture flask were infected with Ad-p27mt (MOI 50). After incubation for 48 h, the cells were digested by 0.5 g/L trypsin, collected and then washed twice with PBS. The cell concentration was adjusted to 109/L with PBS. One hundred microliter of cell suspension was taken out and mixed with 200 &mgr;L DNA-PREP™ LPR and placed at room temperature in the dark for 3 min. The cell suspension was then mixed with 1000 &mgr;L DNA-PREP stain (PI staining). The cell cycle phase and apoptosis were detected by a Coulter Epics XL flow cytometer 15 min later. Ad-LacZ (MOI 50) group and normal controls (Lovo cells cultivated without adenovirus) were used as control groups.
The cells in the three groups (Ad-p27mt and Ad-LacZ for 48 h and the normal control), were collected and centrifuged at 1000 r/min for 5 min. The supernatant was discarded, and 500 &mgr;L of cell lysis solution [1% Np40, 20 mmol/L EDTA, 50 mmol/L Tris-HCl (pH7.5)] and 10 &mgr;L protease K were added to the cell sediment. Following incubation in a water bath (56°C) for 1-2 h and extraction with phenol and chloroform, DNA was precipitated by dehydrated alcohol. After washing once with 700 mL/L alcohol, 200 &mgr;L TE was added to lyse the DNA. Then RNase (final concentration 50 &mgr;g/mL) was added and placed at 37°C for one night. The DNA was electrophoresed in 10 g/L agarose gel and the results were observed under an ultraviolet lamp.
1 × 104 cell suspension was inoculated into a 60 mm dish with a cover glass (washed and high-pressure sterilized). Each of the Ad-p27mt group and the normal control were inoculated into 6 glasses and incubated for 24 h. The glasses were then taken out and washed twice with 1 × PBS and fixed with methanol and freezing acetic acid (3:1) for 30 min. The next procedure was carried out according to the kit instructions. One thousand cells were counted on each glass and the average number of apoptotic cells was determined. Then the apoptotic index (AI), i.e. the number of apoptotic cells in every 100 cancer cells, was calculated.
One way-ANOVA was used in processing the measured data, which were expressed as mean ± SD. χ2 test was adopted in the calculation of enumeration data.
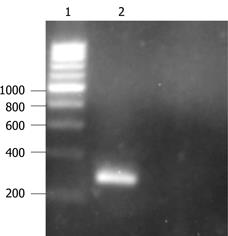
The pathologically changed 293 cells and the culture fluid were collected, frozen and thawed three times and centrifuged. Five millilitre of the supernatant was taken out and added to 1 mg protease K, 2 mL 1% SDS, 10 mmol/L EDTA and 20 mmol/L Tris-HCl for 2 h. After centrifugation, the supernatant was extracted with phenol and chloroform. After precipitation with dehydrated alcohol, the viral DNA was collected. Then forward and reverse primers were added and the PCR reaction was carried out. The 275 bp target gene was amplified, which showed that the recombinant adenovirus already contained the p27mt target gene (Figure 1).
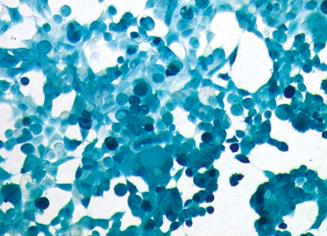
After Lovo cells were infected with Ad-LacZ, the adenovirus-mediated gene transfer rate was evaluated by X-gal staining. The results showed that the infection efficiency could reach 100% when MOI was larger than 50, which indicated that recombinant adenovirus could effectively transfer the gene to Lovo cells in vitro (Figure 2).
After Lovo cells were infected with Ad-p27mt (MOI 50) for 24 h, these cells were collected and lysed using 1 × SDS × PAGE cell lysis solution. After boiling at 100°C for 5 min, the cells were centrifuged. The supernatant was collected and the protein was detected by the TMB system Western blotting kit (KPL, USA). After staining with TMB stain, a high expression of 27 KD protein was observed in the Ad-p27mt group while only slight expression (endogenous expression) was observed in the Ad-LacZ group and the normal control group. This showed that the p27mt recombinant adenovirus constructed in the present study could express p27mt gene in Lovo cells and the protein could also be expressed at a high level in Lovo cells (Figure 3).
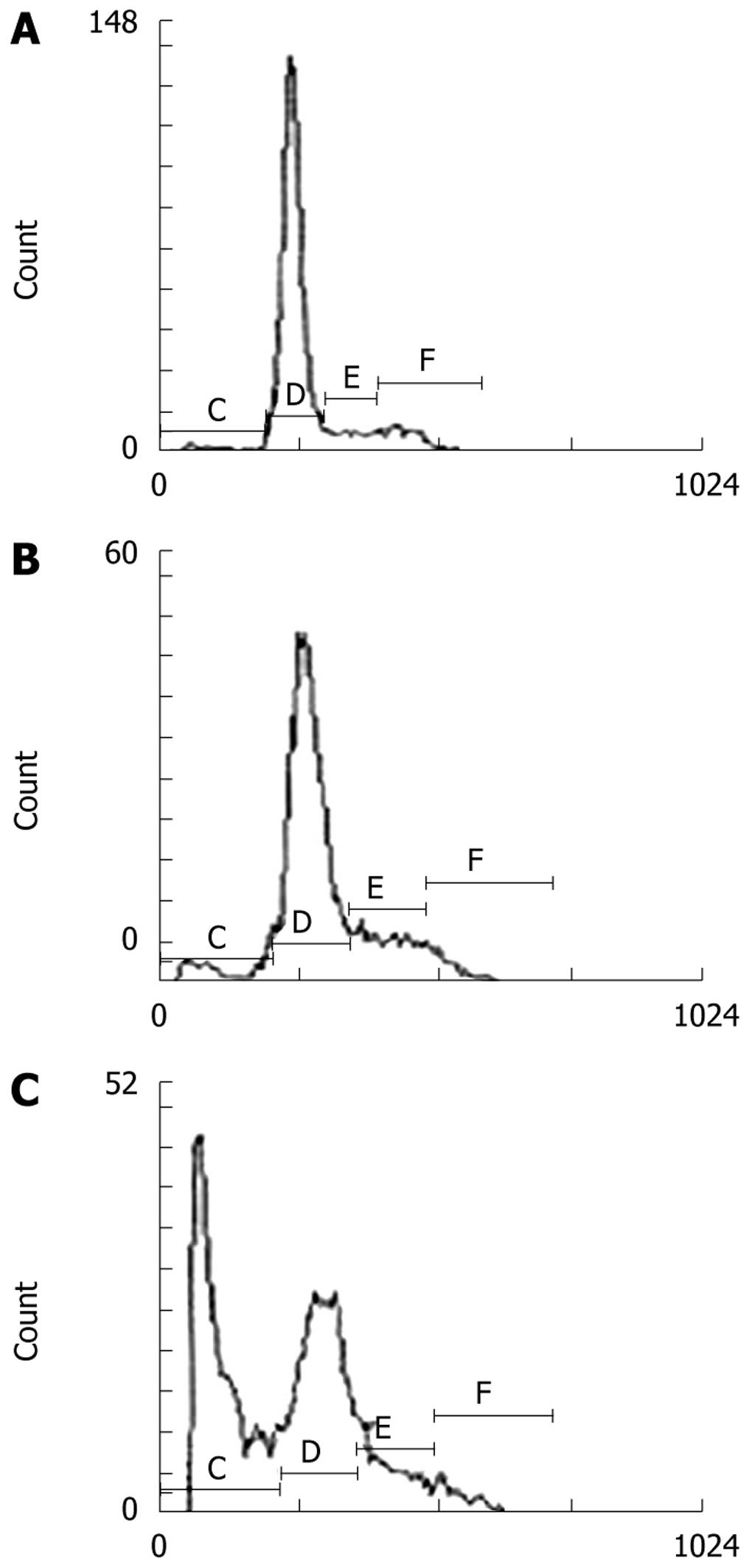
After Lovo cells were treated with Ad-p27mt, Ad-LacZ and without virus for 24 h, apoptosis was observed by flow cytometry and was repeated six times. The average value of hypodiploid cells was: 41.0%, 4.67% and 1.96%, respectively. After statistical analysis, there was a significant difference among the three groups, (P < 0.01) (Figure 4).
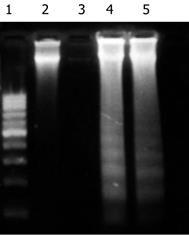
The results of DNA electrophoresis showed that the gene bands were intact in the Ad-LacZ and normal control group, while there were obvious 180-200 bp diploid “trapezia” bands in the Ad-p27mt infected group, which was in concordance with the characteristic changes of apoptosis (Figure 5).
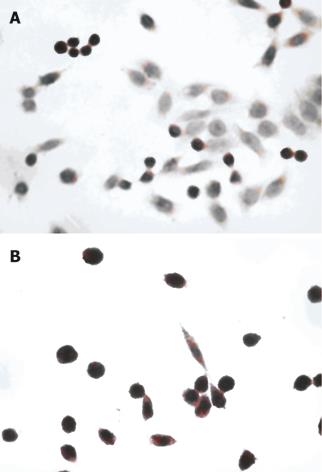
The nuclei of apoptotic cells were dark stained, the cytoplasm was concentrated and the cells had shrunk. The AI of the Ad-p27mt and the control group were (82.6% ± 3.2%) and (5.0% ± 3.5%), respectively and showed a significant difference (P < 0.05). This demonstrated that Ad-p27mt could obviously induce apoptosis in colorectal cancer cells (Figure 6).
The cell cycle of Lovo cells after the various treatments are shown in Table 1. It can be seen that in the Ad-LacZ and blank control groups, the number of cells in the G0/G1 phase decreased gradually and the percentage of cells in the S phase increased, which indicated that the transition time of the cell cycle was shortened and cell proliferation was active. However, the percentage of cells in the G0/G1 phase decreased and the percentage of cells in the S phase increased and the cell cycle was arrested in the G0/G1 phase in the Ad-p27mt group, which was significantly different from the blank and Ad-LacZ groups (P < 0.01).
Currently, functional reconstruction of anti-oncogenes is a reasonable strategy for tumor gene therapy. p27 protein degradation is mainly caused by phosphorylation of the 187th threonine of p27 which is mediated by ubiquitin[15–17]. Park et al[1819] replaced the 187th threonine and the 188th praline of the wild-type p27 with methionine and isoleucine, respectively, by which the target phosphorylation site of CDK would be protected from phosphorylation, and thus the mutant p27 was prepared. These authors constructed a replication-deficient recombinant adenovirus which carried p27mt and p27wt, respectively and proved that the inhibition and apoptotic effects in cancer cells was more obvious in the mutant p27 (p27mt) than in the wild-type p27 (p27wt).
This study constructed a replication-deficient recombinant adenovirus which successfully carried p27mt (Ad-p27mt). Ad-p27mt was then transfected into Lovo cells. When the MOI ≥ 50, the infection efficiency was 100%. Using Western blotting, high expression of p27 was observed, and using FACS, the rate of apoptosis was up to 41.0% in the Ad-p27mt group which was significantly different compared to the control group (P < 0.01). DNA analysis showed a 180-200 bp DNA ladder, and using the TUNEL technique, the apoptotic index was sharply upregulated to 82.6% which was significantly different compared to the control group (P < 0.05). These results showed that p27mt is an important gene and is related to the incidence of colorectal carcinoma. The downregulation of p27 may be the main cause of cell differentiation dysfunction and apoptosis dysfunction. Mutant p27 promoted the expression of p27 in Lovo cells, which led to apoptosis in tumor cells. This may be a potential new therapy for colorectal carcinoma.
The concentration of cyclin/CDK is the key factor for cells passing the G1-S threshold. Cell cycle analysis showed that the cleavage of tumor cells was stopped at the G1 stage by p27mt suppressing the activity of the cyclin/CDK kinase. Hurteau et al[20] reported that the accumulation of p27 played a role in the cell cycle arrest mechanism at the initiation of cell differentiation. A related report[21] in China showed that the p27 gene suppressed DNA replication and protein synthesis and reduced cell mitosis division and inhibited cell generation.
In our study, apoptosis of colorectal carcinoma cells was successfully induced by the application of the mutant gene p27. The apoptosis rate was significantly higher than that of wild-type p27 reported in our previous study[21], which serves as a very useful experimental support for tumor suppression function reconstruction in the gene therapy of colorectal carcinoma. The efficacy of this method in vivo and the mechanism of apoptosis should be determined in future studies.
Along with the improvement in living standards and a change in diet, there has been a gradual increase in the incidence of colorectal cancer in China. However, no effective therapeutic modalities are available for this condition. Gene therapy for the restoration of p27 expression is a promising therapy. A mutant type p27 gene, with a mutation of Thr-187/Pro-188 to Met-187/Ile-188, can inhibit degradation of p27 protein by the ubiquitin-mediated pathway. Inhibition of mutant p27 (p27mt) in tumor cells was stronger than that in wild-type p27 (p27wt), which was demonstrated by cells arresting in the G0/G1 phase. In addition, the apoptosis promoting activity of p27mt was also stronger. However, few studies on the p27 gene in colorectal cancer have been reported.
p27, as a cyclin-dependent kinase inhibitor, tumor suppressor gene, and promoter of apoptosis, has been widely investigated in malignancies such as skin, liver, bladder, thyroid, breast, prostate and endometrium cancer. However, the apoptotic bioactivity of p27mt has not been studied in colorectal cancer.
The study indicates that Ad-p27mt has a strong apoptosis inducing bioactivity as well as a cell cycle inhibitory effect in colorectal cancer in vitro.
This is a nice article. This in vitro effect of p27mt should be examined in vivo to determine the safety and efficacy.
| 1. | Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501-1512. [Cited in This Article: ] |
| 2. | Tchernev G, Orfanos CE. Downregulation of cell cycle modulators p21, p27, p53, Rb and proapoptotic Bcl-2-related proteins Bax and Bak in cutaneous melanoma is associated with worse patient prognosis: preliminary findings. J Cutan Pathol. 2007;34:247-256. [Cited in This Article: ] |
| 3. | Matsuda Y, Ichida T. p16 and p27 are functionally correlated during the progress of hepatocarcinogenesis. Med Mol Morphol. 2006;39:169-175. [Cited in This Article: ] |
| 4. | Shariat SF, Ashfaq R, Sagalowsky AI, Lotan Y. Predictive value of cell cycle biomarkers in nonmuscle invasive bladder transitional cell carcinoma. J Urol. 2007;177:481-487; discussion 487. [Cited in This Article: ] |
| 5. | Saltman B, Singh B, Hedvat CV, Wreesmann VB, Ghossein R. Patterns of expression of cell cycle/apoptosis genes along the spectrum of thyroid carcinoma progression. Surgery. 2006;140:899-905; discussion 905-906. [Cited in This Article: ] |
| 6. | Tan P, Cady B, Wanner M, Worland P, Cukor B, Magi-Galluzzi C, Lavin P, Draetta G, Pagano M, Loda M. The cell cycle inhibitor p27 is an independent prognostic marker in small (T1a,b) invasive breast carcinomas. Cancer Res. 1997;57:1259-1263. [Cited in This Article: ] |
| 7. | Tsihlias J, Kapusta LR, DeBoer G, Morava-Protzner I, Zbieranowski I, Bhattacharya N, Catzavelos GC, Klotz LH, Slingerland JM. Loss of cyclin-dependent kinase inhibitor p27Kip1 is a novel prognostic factor in localized human prostate adenocarcinoma. Cancer Res. 1998;58:542-548. [Cited in This Article: ] |
| 8. | Lahav-Baratz S, Ben-Izhak O, Sabo E, Ben-Eliezer S, Lavie O, Ishai D, Ciechanover A, Dirnfeld M. Decreased level of the cell cycle regulator p27 and increased level of its ubiquitin ligase Skp2 in endometrial carcinoma but not in normal secretory or in hyperstimulated endometrium. Mol Hum Reprod. 2004;10:567-572. [Cited in This Article: ] |
| 9. | Wang Z, Yu BW, Rahman KM, Ahmad F, Sarkar FH. Induction of growth arrest and apoptosis in human breast cancer cells by 3,3-diindolylmethane is associated with induction and nuclear localization of p27kip. Mol Cancer Ther. 2008;7:341-349. [Cited in This Article: ] |
| 10. | Craig C, Wersto R, Kim M, Ohri E, Li Z, Katayose D, Lee SJ, Trepel J, Cowan K, Seth P. A recombinant adenovirus expressing p27Kip1 induces cell cycle arrest and loss of cyclin-Cdk activity in human breast cancer cells. Oncogene. 1997;14:2283-2289. [Cited in This Article: ] |
| 11. | Montagnoli A, Fiore F, Eytan E, Carrano AC, Draetta GF, Hershko A, Pagano M. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev. 1999;13:1181-1189. [Cited in This Article: ] |
| 12. | Sheaff RJ, Groudine M, Gordon M, Roberts JM, Clurman BE. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464-1478. [Cited in This Article: ] |
| 13. | Wang JN, Huang YZ, Kong X, Guo LY. The construction of recombinant adenoviral plasmid by homologous recombination in bacteria and the preparation of recombinant adenovirus expressing β-galactosidase. Yunyang Yixueyuan Xuebao. 2004;23:1-5. [Cited in This Article: ] |
| 14. | Cortin V, Thibault J, Jacob D, Garnier A. High-titer adenovirus vector production in 293S cell perfusion culture. Biotechnol Prog. 2004;20:858-863. [Cited in This Article: ] |
| 15. | Kwon TK, Park JW. Low levels of cyclin D and nonfunctional Rb protein affect cdk6 association with cyclin-dependent kinase inhibitor p27 (Kip1). Biochem Biophys Res Commun. 2001;284:106-111. [Cited in This Article: ] |
| 16. | Tsvetkov LM, Yeh KH, Lee SJ, Sun H, Zhang H. p27 (Kip1) ubiquitination and degradation is regulated by the SCF (Skp2) complex through phosphorylated Thr187 in p27. Curr Biol. 1999;9:661-664. [Cited in This Article: ] |
| 17. | Bloom J, Pagano M. Deregulated degradation of the cdk inhibitor p27 and malignant transformation. Semin Cancer Biol. 2003;13:41-47. [Cited in This Article: ] |
| 18. | Park KH, Seol JY, Kim TY, Yoo CG, Kim YW, Han SK, Shim YS, Lee CT. An adenovirus expressing mutant p27 showed more potent antitumor effects than adenovirus-p27 wild type. Cancer Res. 2001;61:6163-6169. [Cited in This Article: ] |
| 19. | Park KH, Lee J, Yoo CG, Kim YW, Han SK, Shim YS, Kim SK, Wang KC, Cho BK, Lee CT. Application of p27 gene therapy for human malignant glioma potentiated by using mutant p27. J Neurosurg. 2004;101:505-510. [Cited in This Article: ] |
| 20. | Hurteau JA, Brutkiewicz SA, Wang Q, Allison BM, Goebl MG, Harrington MA. Overexpression of a stabilized mutant form of the cyclin-dependent kinase inhibitor p27 (Kip1) inhibits cell growth. Gynecol Oncol. 2002;86:19-23. [Cited in This Article: ] |
| 21. | Zhang WG, Yu JP, Wu QM, Tong Q, Li SB, Wang XH, Xie GJ. Inhibitory effect of ubiquitin-proteasome pathway on proliferation of esophageal carcinoma cells. World J Gastroenterol. 2004;10:2779-2784. [Cited in This Article: ] |