Published online Jun 7, 2009. doi: 10.3748/wjg.15.2657
Revised: April 29, 2009
Accepted: May 6, 2009
Published online: June 7, 2009
AIM: To evaluate the number of bone marrow mononuclear cells (BMMC) that are migrated to the liver following transplantation of murine BMMC into mice with acute liver injury.
METHODS: BMMC were isolated from the bone marrow of mice in a lymphocyte separation medium and then labeled with PKH26. The labeled cells were subsequently infused into the caudal veins of BALB/c mice with hepatic injury induced by carbon tetrachloride and 2-acetylaminofluorene. Mice in experimental group were treated with stromal cell-derived factor-1 (SDF-1) which was injected intraperitoneally after transplantation of BMMC. Mice in control group were injected intraperitoneally with 0.1 mL of saline (0.9% NaCl) after transplantation of BMMC. After 2 wk, migration of the cells in experimental group was studied by fluorescence microscopy. The expression of proliferating cell nuclear antigen and albumin was quantified with manual methods in both groups. The serum transaminase levels at different time points were compared between the two groups.
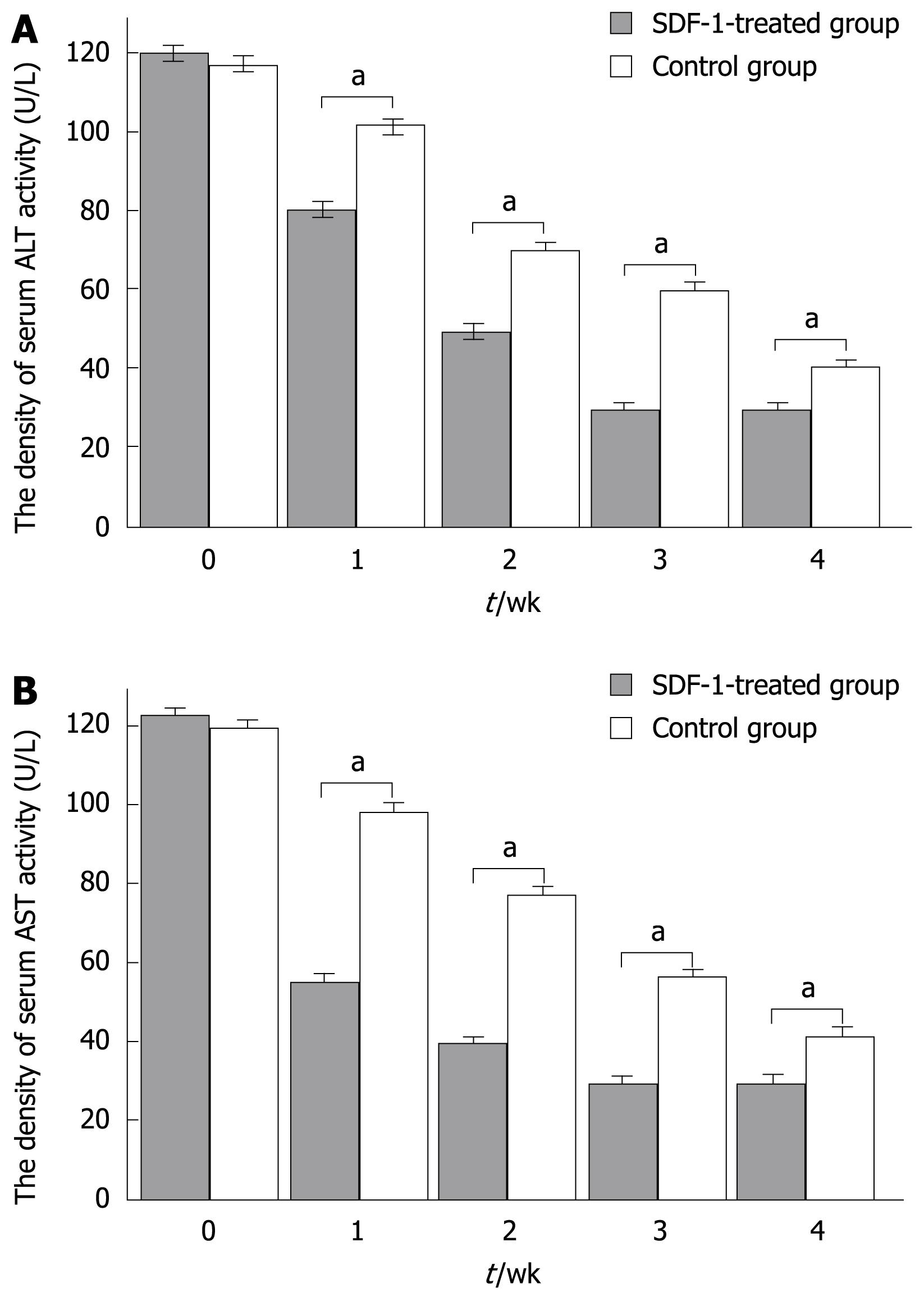
RESULTS: The labeled “cells” were found in the portal region and central veins of hepatic lobules. The PKH26-labeled cells appeared at an average frequency of 108 ± 8/high power field in the experiment group and 65 ± 8/high power field in the control group (P < 0.05). The total number of positive cells was 29 ± 7/high power field in the experimental group and 13 ± 2/high power field in the control group. The albumin expression level was also higher in the experimental group than in the control group (29 ± 7 vs 13 ± 2, P < 0.05). The total number of crossing points was 156 ± 5/high power field in the experimental group and 53 ± 5/high power field in the control group (P < 0.05). The serum alanine aminotransferase levels in experimental and control groups were measured at different time points (120 ± 40 vs 118.50 ± 1.75, P > 0.05; 80.60 ± 6.50 vs 101.08 ± 5.67, P < 0.05; 50.74 ± 5.38 vs 80.47 ± 4.62, P < 0.05; 30.54 ± 2.70 vs 60.72 ± 4.37, P < 0.05; 30.77 ± 5.36 vs 40.47 ± 6.50, P < 0.05). At the same time, the serum aspartate aminotransferase levels were measured in experimental and control groups at different time points (122.55 ± 1.46 vs 120.70 ± 4.22, P > 0.05; 54.26 ± 6.50 vs 98.70 ± 8.20, P < 0.05; 39.47 ± 5.39 vs 78.34 ± 4.50, P < 0.05; 28.94 ± 2.70 vs 56.44 ± 4.28, P < 0.05; 30.77 ± 5.45 vs 42.50 ± 6.28, P < 0.05).
CONCLUSION: SDF-1 can promote the migration of BMMC to the liver of mice with acute liver failure.
- Citation: Jin SZ, Meng XW, Han MZ, Sun X, Sun LY, Liu BR. Stromal cell derived factor-1 enhances bone marrow mononuclear cell migration in mice with acute liver failure. World J Gastroenterol 2009; 15(21): 2657-2664
- URL: https://www.wjgnet.com/1007-9327/full/v15/i21/2657.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2657
Stem cells have the potential ability of multi-directional differentiation and self-renewal. Under proper induction circumstances, they can differentiate into different functioning cells. Differentiation between groups is ongoing. Recent studies using experimental animal models and samples from clinical mobilization protocols demonstrated that chemokines such as stromal derived factor-1 (SDF-1) and IL-8 are involved in the mobilization process[12]. The central role of SDF-1 in induction of mobilization has been reviewed[3]. SDF-1, a kind of micro-molecular proteins, possesses a variety of biologic activities. It has been identified that SDF-1 can promote bone marrow stem cell directional differentiation both in heart tissue[4] and in nerve tissue[5]. Since mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential l[6], we hypothesize that SDF-1 can also mobilize migration of bone marrow mononuclear cells (BMMC) in mice with acute liver failure.
The aim of this study was to determine whether autologous BMMC can be mobilized by SDF-1 in BALB/c mice with experimental acute liver failure. To test this, BMMC were isolated from the bone marrow of mice in a lymphocyte separation medium and then labeled with the fluorochrome dye PKH26.The labeled cells were subsequently infused into the caudal veins of mice with hepatic injury induced by carbon tetrachloride and 2-acetylaminofluorene. Mice in experimental group were injected intraperitoneally with SDF-1 (5 &mgr;g/kg) and mice in control group were injected intraperitoneally with 0.1 mL of saline (0.9% NaCl) after transplantation of BMMC. After 2 wk, the migration of BMMC was studied by fluorescence microscopy. The number of migrated BMMC was calculated, and the expression of proliferating cell nuclear antigen (PCNA) and albumin in both groups was quantified with manual methods. In the following 4 wk, serum aminotransferase activity was detected to monitor the changes in liver function at different time points.
Male BALB/c mice, weighing 20-22 g, at the age 8-10 wk, were purchased from the Animal Center of Jilin University. All mice were housed in rooms at a constant temperature and humidity in a 12 h light/dark cycle with free access to normal rodent chow and water. Experiments were conducted according to the guidelines established by Jilin University. The procedures were approved by the Supervisor Committee of Jilin University Animal Council.
Recombinant murine SDF-1 α (CXCL12, Catalog #: 250-20A) was obtained from PeproTech EC Company (USA). Proliferating cell nuclear antigen, lymphocyte isolation medium (1.077 g/cm3) and red fluorochrome PKH26GL were purchased from Simga Company (Saint louis, Missouri USA). 2-acetylaminofluorene was purchased from Invitrogen Company (California, USA).
The animals were divided into donor and recipient groups. The recipient group was further divided into an experiment group and a control group (n = 30).
Femoral bones were aseptically removed from male BALB/c mice under anesthesia and bone marrow in the medullary cavity was bathed by heparin (50 U/mL) dissolved in normal saline. Bone marrow cells were suspended in a sterilized lymphocyte isolation medium. After dilution with 2 mL phosphate-buffered saline (PBS, 0.01 mol/L, pH = 7.4) at 1:1, the cells were slowly added at a relative matching density of 1.077 g/cm3 lymphocytes followed by centrifugation at 2000 r/min for 20 min. Cell groups were identified, washed with PBS, and centrifuged at 1200 r/min for 10 min. The top of centrifuge tube was shaken lightly to detach the cell groups. A DMEM/F12 medium was added (15% FBS, 100 000 U/L penicillin, pH = 7.4) to prepare cell suspension at a density of over 5 × 108 cells/L. Finally, BMMC were labeled with PKH26 according to its manufacture, s instructions. The density of labeled cell suspension was adjusted to 3 × 107 cells/mL. BMMC with a viability over 95%, measured by trypan blue exclusion, were used. One million of BMMC were isolated from the donor group, labeled with PKH26 and injected into mice of the experimental group via the tail vein. One hour later, mice in the experimental group were injected intraperitoneally with SDF-1 (5 &mgr;g/kg). Mice in the control group were injected intraperitoneally with 0.1 mL of saline (0.9% NaCl). The injection of SDF-1 or saline was repeated once a day in the following weeks. Two weeks later, the mice were euthanized by cervical spine dislocation with their livers removed immediately and frozen in liquid nitrogen. The number of migrated BMMC in hepatic tissue stained with hematoxylin-eosin and immunohistochemistry was calculated under a fluorescence microscope. The density of serum aminotransferase activity was detected with an automatic biochemistry analyzer.
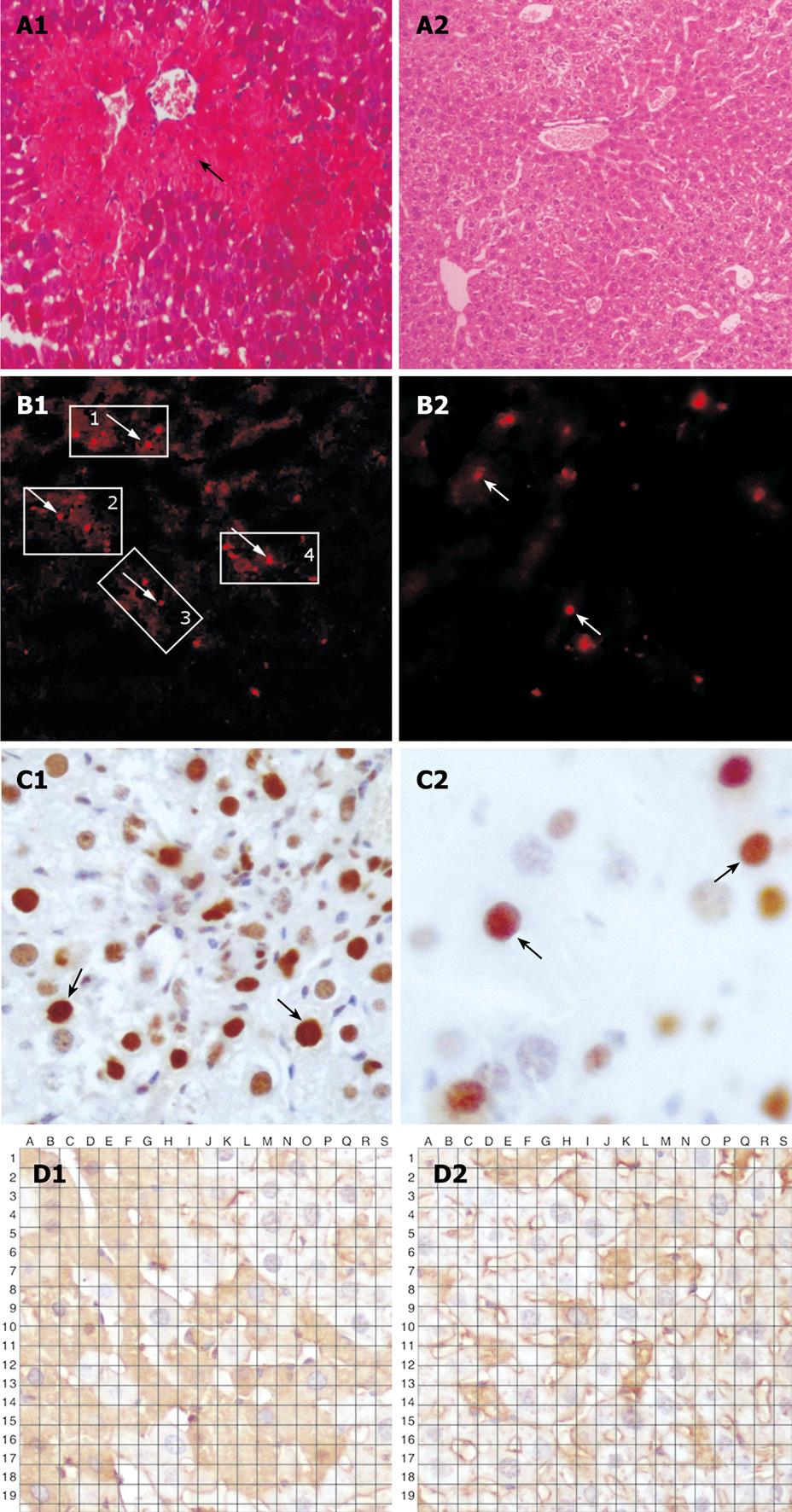
Carbon tetrachloride (CCl4) is widely used to generate an experimental model mimicking acute liver injury caused by toxic substances. Mice in the recipient group were treated with 2-acetaminofluorene dissolved in liquid macrogol (20 mg/kg) by lavage, once a day for 7 d. On day 8, the animals were injected intraperitoneally with 20% CCl4 (0.5 mL/kg, dissolved in vegetable oil). On day 9, one million of BMMC were transplanted into mice via the tail vein. From day 10, mice in the recipient group were given 2-acetaminofluorene dissolved in liquid macrogol (20 mg/kg), once a day for 7 d. On day 21, mice in the experimental group were euthanized by cervical spine dislocation. Liver tissue was resected immediately and frozen in liquid nitrogen. Liver injury was confirmed by fluorescence microscopy and hematoxylin-eosin staining. Extensive vacuolar degeneration and edema of hepatocytes were found in liver tissue (Figure 1A).
The number of fluorescence PKH26-labeled cells in liver sections stained with immunohostochemistry (FITC) was counted. Albumin and PCNA in liver tissue sections stained with FITC were also calculated. The density of serum aminotransferase activity was detected with an automatic biochemistry analyzer which can test the liver function at different time points following the transplantation of BMMC.
Livers were perfused with 4% paraformaldehyde in 0.01 mol/L phosphate-buffered saline (pH 7.4, PBS) following anesthesia with sodium pentobarbital 100 mg/kg (ip), fixed overnight and cryoprotected in 30% sucrose at 4°C. Liver tissue was cut into 6-&mgr;m thick sections.
Immunofluorescence was carried out by incubating the sections in PBS containing 5% donkey serum and primary antibody-rabbit anti-mouse serum albumin(1:1000, ab19196, abcam, MA, USA), followed by a 2-h reaction with Alexa Fluor® 488-conjugated donkey anti-rabbit antibody (1:200, A21206, Invitrogen, Carlsbad, CA). Liver tissue sections were then mounted in an anti-fading medium.
Whole blood samples were collected after bulbus oculi in mice with acute hepatic failure were exposed to light ether anesthesia. Serum was separated by centrifugation and stored at -30°C. Alanine aminotransferase activity was detected with an automatic biochemistry analyzer (Sinnowa D336, SINNOWA Medical Science & Technology Co., Ltd, Nanjing, China). The density of serum alanine aminotransferase (ALT) activity in experimental and control groups was compared. Serum ALT levels in experimental and control groups were measured in the following weeks.
Liver tissue from each mouse was cut into five sections. Each frozen section was examined under a microscope at a magnification × 200 under 10 microscopic fields. The total number of fluorescence-labeled cells in each section was calculated. At the same time, the serum aminotransferase activity was determined with an automatic biochemistry analyzer. Results were expressed as mean ± SD. Statistically significant differences between groups were compared with the t test. Paired t test was used to compare the PKH26 fluorescence intensity values and albumin expression. P < 0.05 was considered statistically significant. All data were processed using statistical software SPSS 10.0.
Extensive vacuolar degeneration and edema of hepatocytes were observed in mice of the experimental group, implying that an animal acute liver failure model can be successfully established.
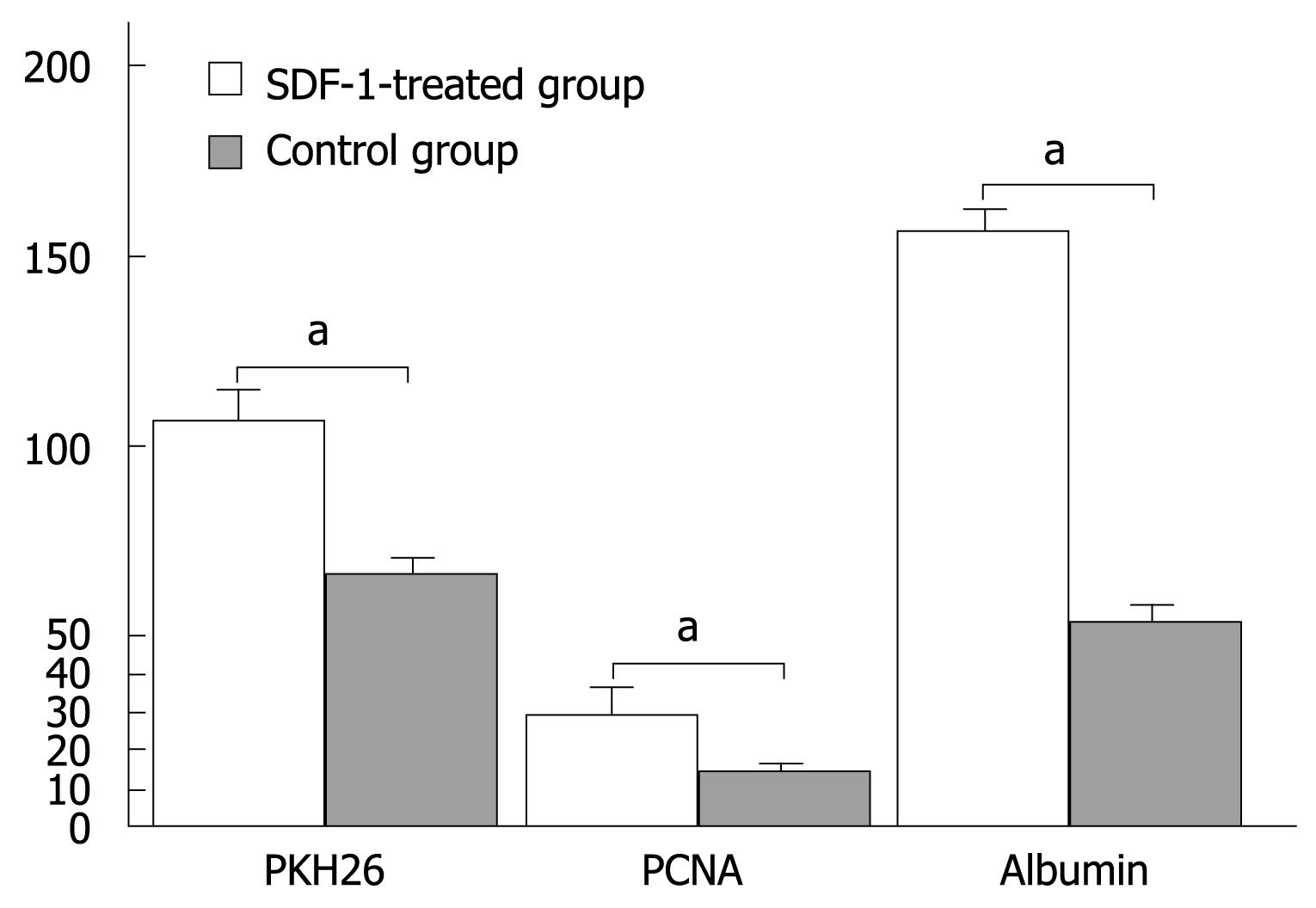
PKH26-labeled cells were detected in liver tissue sections following injection of SDF-1 via the tail vein. The total number of positive PKH26-labeled cells was 108 ± 8/high power field in the experimental group and 65 ± 8/high power field in the control group, respectively (P < 0.05, Figure 1B and Figure 2).
The expression level of PCNA was higher in the experimental group than in the control group. The total number of positive cells was 29 ± 7/high power field in the experimental group and 13 ± 2/high power field in the control group, respectively (P < 0.05, Figure 1C and Figure 2).
The albumin expression level was also higher in the experimental group than in the control group. The percentage area of histologic field was calculated as previously described[7] and compared with albumin cells. The manual count set-up consisting of albumin image printouts and the transparent grid overlay used for point count are shown in Figure 1D. The total number of bone marrow stem cells at crossing points was 156 ± 5/high power field in the experimental group and 53 ± 5/high power field in the control group, respectively (P < 0.05, Figure 1D and Figure 2).
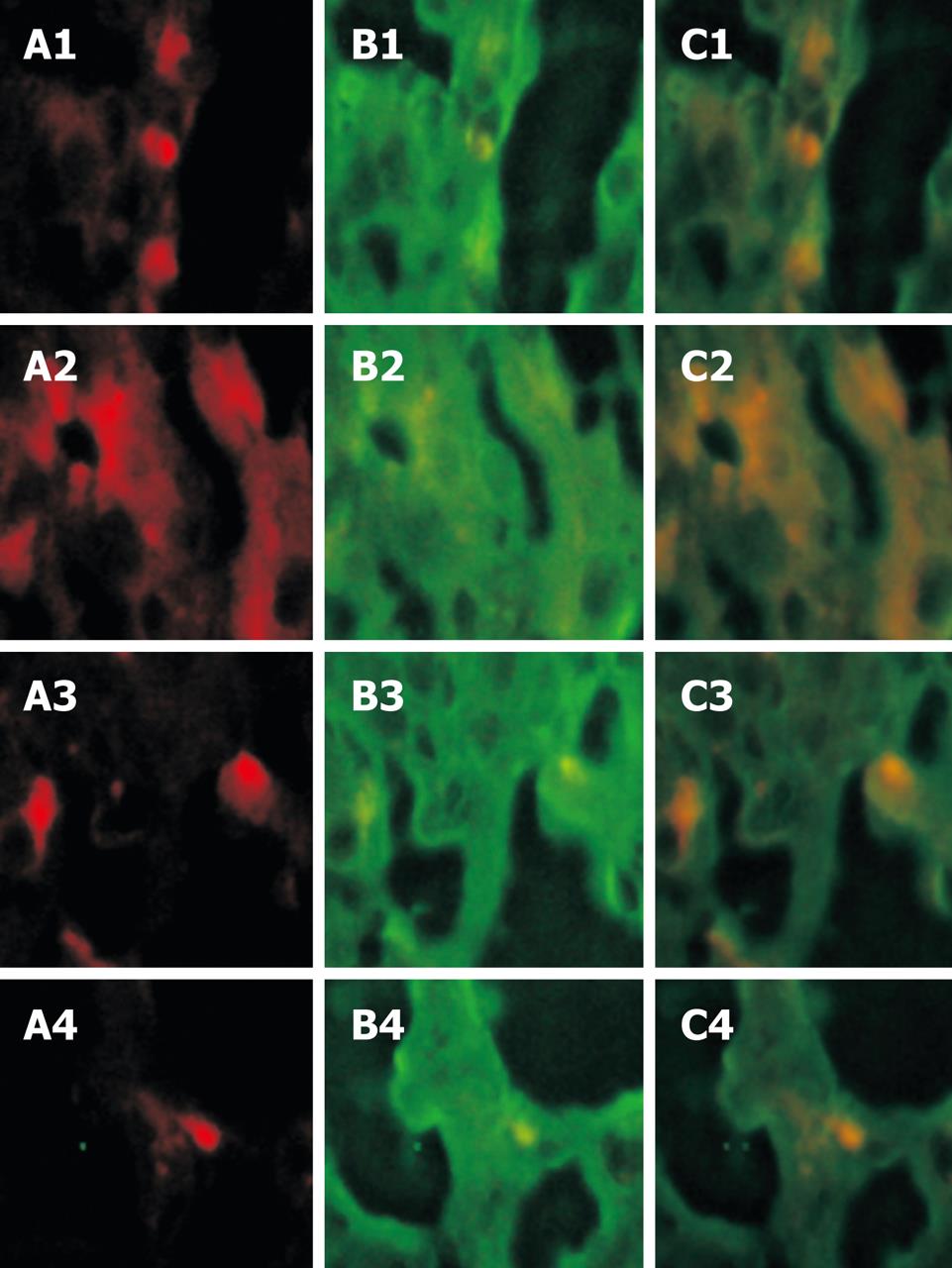
Green fluorescence was observed in FITC-labeled albumin antibodies at the 494 nm excitation light both in the experimental group and in the control group. Albumin in hepatocytes was detected with indirect labeling antibodies and expressed widely with green fluorescence. After the red and green fluorescence were focused, yellow fluorescence emerged at a suitable position, confirming that the yellow cells come from PKH26-labeled positive BMMC in vitro and show albumin (Figure 3).
It is well known that albumin is associated with the maturity of hepatocytes[8]. The obvious albumin expression in the experimental group implied that BMMC could differentiate into hepatocytes.
A significant difference in serum aminotransferase activity was observed between the two groups at different time points (120 ± 40 vs 118.50 ± 1.75, P > 0.05; 80.60 ± 6.50 vs 101.08 ± 5.67, P < 0.05; 50.74 ± 5.38 vs 80.47 ± 4.62, P < 0.05; 30.54 ± 2.70 vs 60.72 ± 4.37, P < 0.05; 30.77 ± 5.36 vs 40.47 ± 6.50, P < 0.05). At the same time, the serum AST levels in the experimental and control groups were measured at different time points (122.55 ± 1.46 vs 120.70 ± 4.22, P > 0.05; 54.26 ± 6.50 vs 98.70 ± 8.20, P < 0.05; 39.47 ± 5.39 vs 78.34 ± 4.50, P < 0.05; 28.94 ± 2.70 vs 56.44 ± 4.28, P < 0.05; 30.77 ± 5.45 vs 42.50 ± 6.28, P < 0.05) (Figure 4A and B).
In regards to the genetic identity of inbred animals, use of BALB/c mice in the study allowed us to avoid immunologic rejection, thus the transplantation procedure represents an auto graft. Use of PKH26 fluorescent labeling permitted us to observe the homing of BMMC in the recipient group and to quantify the phenomenon by counting the fluorescencence-labeled cells as previously described[9]. By expressing albumin, the experimental data suggest that these cells can differentiate into hepatic cells. The expression of proliferating cell nuclear antigen suggested that the cells subsequently underwent cell division. PCNA is a nuclear antigen related with the cell life cycle, and is synthesized in cell nuclei. The PCNA is expressed in the G1 and S phases, and functions essentially as replicative DNA polymerases in eukaryotic cells[10]. The quantity of PCNA is low in resting cells but is substantially increased in multiplying and transformed cells. In this study, the expression levels of albumin and proliferating cell nuclear antigen were higher in the experimental than in the control group. Albumin was widely expressed in liver tissue. After red fluorescence was cofocused with green fluorescence, yellow fluorescence emerged at a suitable position, confirming that the yellow cells coming from PKH26-labeled positive BMMC in vitro can show albumin. Serum aminotransferase activity was detected. The density of serum ALT and AST was changed obviously after transplantation of BMMC, confirming that the liver function can be ameliorated by transplanted BMMC and the effect is more significant in the experimental group than in the control group, indicating that SDF-1 can promote BMMC homing to injured livers of mice.
BMMC mainly consist of HSC, BMSC and endodermis progenitor cells. Density gradient centrifugation was performed to remove adipocytes, erythrocytes and apocyte. BMMC were collected to enrich rudimentary bone marrow stem cells. SDF gene can code two proteins, namely SDF-1α and SDF-1β. The SDF-1α gene is expressed in bone marrow stromal cells. The homology of human SDF-1 and mouse SDF-1 can reach 99%[11]. In the study, the SDF-1 receptor gene (LESTER, CXCR4) carrying 7 membrane spanning domains, was successfully cloned when the SDF gene, a kind of G protein linkage receptors, was cloned. CXCR4 is widely expressed in leucocytes, CD34+ HSC and CD34+ progenitor cells[12]. Initially, CXCR4 is regarded as a unique SDF-1 receptor[13] and the specific combination of SDF-1 and CXCR4, is named as SDF-1/CXCR4 biology axis. Another kind of SDF-1 receptors (CXCR7), known as an orphan receptor (RDC), has been recently found and is[14], mainly expressed in tumor cell line, activated endotheliocytes and fetal liver cells. CXCR7 is detected but not expressed in normal cells[15].
Aiuti et al[16] showed that SDF-1 is a chemotactic factor of CD34+ HSC, and CD34+ cells including endothelium progenitor cells, which can migrate and home along the concentration gradient of SDF-1. It has been shown that SDF-1 can play an important role in promoting migration of cells including endothelium progenitor cells from bone marrow to target tissue[17]. Bhakta et al[18] reported that marrow stromal cells can also express CXCR4 and SDF-1 is a chemotactic factor for the homing of marrow stromal cells in vivo and in vitro, suggesting that stem cells expressing CXCR4 can migrate and home along the concentration gradient. Moreover, the concentration gradient between inner and outer bone marrow can decrease the inner concentration gradient[1920] or increase the outer concentration gradient[21] of bone marrow, thus promoting mobilization of bone marrow stem cells.
In the present study, the number of PKH26-labeled cells was obviously higher in the experimental group than in the control group and the expression level of albumin and PCNA was markedly higher in the experimental group than in the control group, demonstrating that SDF-1 can promote bone marrow stem cell migration into the liver and SDF-1 mobilized BMMC can be used to promote liver regeneration after liver injury. Moreover, use of SDF-1 can avoid immune suppression so that liver injury can be repaired. BMMC can be easily harvested and applied in clinical practice.
Unfortunately, the mechanism by which SDF-1 becomes chemotactic to bone marrow stem cells is unclear. It has been shown that SDF-1 can ignite multiple signal pathways in cells and is regulated by different regulatory factors[22–24]. When SDF-1 binds to CXCR4, certain second messengers, such as NO and IP3, lead to a series of related kinase phosphorylation[23] and the production of actin, and rapid or transient polymerization of biological effects by changing the hereditary information of stem cells[22], all of which may be due to the stem cell migration induced by SDF-1.Protein kinase B, ecto-signal regulatory protein-2 and JAK2 also participate in the signal conduction pathways[24].
In addition, SDF-1 can act as a chemoattractant to promote migration of stem cells[2526] and strengthen their locomotory capacity[27]. When stem cells are migrated to the target tissue, SDF-1 facilitates their adhesion to fibrinogen, fibronectin, interstitium and endotheliocytes[28]. Then the cells, adhered to blood vessel endothelium, permeate vessel walls to ingress target tissue. With the help of SDF-1, more secreted MMP-9, NO and VEGF promote the mobilization of stem cells[232930]. It has been reported that SDF-1 may participate in tumor development[3132].
In conclusion, with the rapid progress in the field of stem cells, various kinds of stem cells are widely used to treat different organ diseases. SDF-1 can play an important role in bone marrow stem cells expressing CXCR4, thus promoting migration of BMMC into liver tissue. However, the precise mechanism by which SDF-1 mobilizes stem cells is unclear. Further study is needed to determine the dose, administration route and safety of SDF-1.
Stem cells have the potential ability of multi-directional differentiation and self-renewal. Under proper induction circumstances, stem cells can differentiate into different functioning cells. Differentiation between the groups is ongoing. The new treatment strategy for acute and chronic hepatitis is of potential importance.
Stromal cell derived factor-1 (SDF-1), a kind of micro-molecular proteins, possesses a variety of biologic activities. It has been shown that SDF-1 can promote bone marrow stem cell directional differentiation into heart and nerve tissues. The results of this study demonstrated that SDF-1 could promote the homing of bone marrow mononuclear cells (BMMC) to the liver of mice with acute liver failure.
Recent reports have highlighted that the mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype, but a heterogeneous multilineage differentiation potential. We hypothesize that SDF-1 can also mobilize the homing of bone marrow mononuclear cells in mice with acute liver failure. This is the first study to report that SDF-1 can promote the homing of BMMC to the liver in mice with acute liver failure.
This study showed how SDF-1 promotes the homing of BMMC to the liver, thus providing a future strategy for therapeutic intervention in the treatment of acute liver failure.
CXCL12 is a recombinant murine SDF-1-α. SDF-1-α and β are small cytokines belonging to members of the intercrine family, can activate leukocytes, and are often induced by proinflammatory stimuli such as lipopolysaccharide, TNF, or IL-1.The intercrines are characterized by the presence of four conserved cysteines which form two disulfide bonds, and can be classified into two subfamilies. In the CXC subfamily including β and α chemokines, cysteine residues are adjacent to each other and separated by an intervening amino acid, respectively. SDF-1 proteins belong to the latter group.
In this manuscript, SDF-1 was found to facilitate migration of infused bone marrow cells to liver. The total number of PKH26-labled bone marrow cells in the liver was higher in the experimental group than in the control group. Moreover, the number of albumin-producing cells and proliferating cells was higher in the experimental group than in the control group. Although the data are preliminary, they are important and encouraging.
| 1. | Masson S, Harrison DJ, Plevris JN, Newsome PN. Potential of hematopoietic stem cell therapy in hepatology: a critical review. Stem Cells. 2004;22:897-907. [Cited in This Article: ] |
| 2. | Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697-703. [Cited in This Article: ] |
| 3. | Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973-981. [Cited in This Article: ] |
| 4. | Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300-3305. [Cited in This Article: ] |
| 5. | Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA. 2004;101:18117-18122. [Cited in This Article: ] |
| 6. | in ‘t Anker PS, Noort WA, Scherjon SA, Kleijburg-van der Keur C, Kruisselbrink AB, van Bezooijen RL, Beekhuizen W, Willemze R, Kanhai HH, Fibbe WE. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003;88:845-852. [Cited in This Article: ] |
| 7. | Deb-Joardar N, Thuret G, Gavet Y, Acquart S, Garraud O, Egelhoffer H, Gain P. Reproducibility of endothelial assessment during corneal organ culture: comparison of a computer-assisted analyzer with manual methods. Invest Ophthalmol Vis Sci. 2007;48:2062-2067. [Cited in This Article: ] |
| 8. | Laszlo V, Dezso K, Baghy K, Papp V, Kovalszky I, Safrany G, Thorgeirsson SS, Nagy P, Paku S. Triiodothyronine accelerates differentiation of rat liver progenitor cells into hepatocytes. Histochem Cell Biol. 2008;130:1005-1014. [Cited in This Article: ] |
| 9. | Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291-1302. [Cited in This Article: ] |
| 10. | Maga G, Hubscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci. 2003;116:3051-3060. [Cited in This Article: ] |
| 11. | Shirozu M, Nakano T, Inazawa J, Tashiro K, Tada H, Shinohara T, Honjo T. Structure and chromosomal localization of the human stromal cell-derived factor 1 (SDF1) gene. Genomics. 1995;28:495-500. [Cited in This Article: ] |
| 12. | Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872-877. [Cited in This Article: ] |
| 13. | Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829-833. [Cited in This Article: ] |
| 14. | Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M, Bachelerie F. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760-35766. [Cited in This Article: ] |
| 15. | Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201-2213. [Cited in This Article: ] |
| 16. | Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111-120. [Cited in This Article: ] |
| 17. | Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322-1328. [Cited in This Article: ] |
| 18. | Bhakta S, Hong P, Koc O. The surface adhesion molecule CXCR4 stimulates mesenchymal stem cell migration to stromal cell-derived factor-1 in vitro but does not decrease apoptosis under serum deprivation. Cardiovasc Revasc Med. 2006;7:19-24. [Cited in This Article: ] |
| 19. | Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687-694. [Cited in This Article: ] |
| 20. | Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia. 2002;16:1992-2003. [Cited in This Article: ] |
| 21. | Hattori K, Heissig B, Tashiro K, Honjo T, Tateno M, Shieh JH, Hackett NR, Quitoriano MS, Crystal RG, Rafii S. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001;97:3354-3360. [Cited in This Article: ] |
| 22. | Wang JF, Park IW, Groopman JE. Stromal cell-derived factor-1alpha stimulates tyrosine phosphorylation of multiple focal adhesion proteins and induces migration of hematopoietic progenitor cells: roles of phosphoinositide-3 kinase and protein kinase C. Blood. 2000;95:2505-2513. [Cited in This Article: ] |
| 23. | Kijowski J, Baj-Krzyworzeka M, Majka M, Reca R, Marquez LA, Christofidou-Solomidou M, Janowska-Wieczorek A, Ratajczak MZ. The SDF-1-CXCR4 axis stimulates VEGF secretion and activates integrins but does not affect proliferation and survival in lymphohematopoietic cells. Stem Cells. 2001;19:453-466. [Cited in This Article: ] |
| 24. | Tilton B, Ho L, Oberlin E, Loetscher P, Baleux F, Clark-Lewis I, Thelen M. Signal transduction by CXC chemokine receptor 4.Stromal cell-derived factor 1 stimulates prolonged protein kinase B and extracellular signal-regulated kinase 2 activation in T lymphocytes. J Exp Med. 2000;192:313-324. [Cited in This Article: ] |
| 25. | Ratajczak MZ, Majka M, Kucia M, Drukala J, Pietrzkowski Z, Peiper S, Janowska-Wieczorek A. Expression of functional CXCR4 by muscle satellite cells and secretion of SDF-1 by muscle-derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/progenitor cells in muscles. Stem Cells. 2003;21:363-371. [Cited in This Article: ] |
| 26. | Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845-848. [Cited in This Article: ] |
| 27. | Reca R, Mastellos D, Majka M, Marquez L, Ratajczak J, Franchini S, Glodek A, Honczarenko M, Spruce LA, Janowska-Wieczorek A. Functional receptor for C3a anaphylatoxin is expressed by normal hematopoietic stem/progenitor cells, and C3a enhances their homing-related responses to SDF-1. Blood. 2003;101:3784-3793. [Cited in This Article: ] |
| 28. | Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, Slav MM, Nagler A, Lider O, Alon R. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289-3296. [Cited in This Article: ] |
| 29. | Majka M, Janowska-Wieczorek A, Ratajczak J, Kowalska MA, Vilaire G, Pan ZK, Honczarenko M, Marquez LA, Poncz M, Ratajczak MZ. Stromal-derived factor 1 and thrombopoietin regulate distinct aspects of human megakaryopoiesis. Blood. 2000;96:4142-4151. [Cited in This Article: ] |
| 30. | Janowska-Wieczorek A, Marquez LA, Dobrowsky A, Ratajczak MZ, Cabuhat ML. Differential MMP and TIMP production by human marrow and peripheral blood CD34(+) cells in response to chemokines. Exp Hematol. 2000;28:1274-1285. [Cited in This Article: ] |
| 31. | Peng SB, Peek V, Zhai Y, Paul DC, Lou Q, Xia X, Eessalu T, Kohn W, Tang S. Akt activation, but not extracellular signal-regulated kinase activation, is required for SDF-1alpha/CXCR4-mediated migration of epitheloid carcinoma cells. Mol Cancer Res. 2005;3:227-236. [Cited in This Article: ] |
| 32. | Sutton A, Friand V, Brule-Donneger S, Chaigneau T, Ziol M, Sainte-Catherine O, Poire A, Saffar L, Kraemer M, Vassy J. Stromal cell-derived factor-1/chemokine (C-X-C motif) ligand 12 stimulates human hepatoma cell growth, migration, and invasion. Mol Cancer Res. 2007;5:21-33. [Cited in This Article: ] |