Published online Mar 28, 2009. doi: 10.3748/wjg.15.1518
Revised: February 27, 2009
Accepted: March 6, 2009
Published online: March 28, 2009
AIM: To observe the biotransformation process of a Chinese compound, aesculin, by human gut bacteria, and to identify its metabolites in rat urine.
METHODS: Representative human gut bacteria were collected from 20 healthy volunteers, and then utilized in vitro to biotransform aesculin under anaerobic conditions. At 0, 2, 4, 8, 12, 16, 24, 48 and 72 h post-incubation, 10 mL of culture medium was collected. Metabolites of aesculin were extracted 3 × from rat urine with methanol and analyzed by HPLC. For in vivo metabolite analysis, aesculetin (100 mg/kg) was administered to rats via stomach gavage, rat urine was collected from 6 to 48 h post-administration, and metabolite analysis was performed by LC/ESI-MS and MS/MS in the positive and negative modes.
RESULTS: Human gut bacteria could completely convert aesculin into aesculetin in vitro. The biotransformation process occurred from 8 to 24 h post-incubation, with its highest activity was seen from 8 to 12 h. The in vitro process was much slower than the in vivo process. In contrast to the in vitro model, six aesculetin metabolites were identified in rat urine, including 6-hydroxy-7-gluco-coumarin (M1), 6-hydroxy-7-sulf-coumarin (M2), 6, 7-di-gluco-coumarin (M3), 6-glc-7-gluco-coumarin (M4), 6-O-methyl-7-gluco-coumarin (M5) and 6-O-methyl-7-sulf-coumarin (M6). Of which, M2 and M6 were novel metabolites.
CONCLUSION: Aesculin can be transferred into aesculetin by human gut bacteria and is further modified by the host in vivo. The diverse metabolites of aesculin may explain its pleiotropic pharmaceutical effects.
- Citation: Ding WJ, Deng Y, Feng H, Liu WW, Hu R, Li X, Gu ZM, Dong XP. Biotransformation of aesculin by human gut bacteria and identification of its metabolites in rat urine. World J Gastroenterol 2009; 15(12): 1518-1523
- URL: https://www.wjgnet.com/1007-9327/full/v15/i12/1518.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1518
Aesculin, a 6, 7-dihydroxy derivative of coumarin with pleiotropic pharmacological and biochemical properties, has recently been analyzed for its biochemical activity[1]. Kaneko and colleagues[2] found that aesculetin and its 6-glycoside, aesculin, can inhibit oxidative DNA damage and formation of aberrant crypt foci and tumors. Furthermore, this compound shows an inhibitory effect on BOP-induced oxidative DNA damage and carcinogenesis in a hamster pancreatic tumor model[2], as well as chemo-preventive[3] and anti-tumor activity on cancer[4]. Aesculin and aesculetin have strong antioxidative and photo-protective activities, by scavenging 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radicals and superoxide anions from the xanthine/xanthine oxidase system and inhibiting oxidation of 5-(6-)-chloromethyl-2', 7'-dichlorodihydro-fluorescein diacetate[5]. Aesculetin increases apoptosis of 3T3-L1 adipocytes in a time- and dose-dependent manner[6], and inhibits cell growth and cell cycle progression by inducing G1 phase arrest in HL-60 leukaemia cells, which is a direct result of the inhibition of retinoblastoma protein phosphorylation[7]. Aesculetin also displays multiple immunomodulatory effects on murine lymphocytes and peritoneal macrophages in rat liver[8], including anti-inflammatory activity, inhibition of lipoxygenase and tyrosinase activity, scavenge of hydroxyl radicals and suppressing lipid peroxidation.
However, the biotransformation progress of aesculin and its derivatives has not been completely determined, let alone their complex structure-activity relationships. Aesculetin is one of the simplest coumarins with two hydroxyl groups at carbons 6 and 7 that serve as targets for O-methylation or O-glycosylatin. It has been reported that biotransformation of aesculetin with E. coli expressing O-methyltransferase (POMT-9) generates scopoletin, isoscopoletin, and scoparone[9]. The growth of E. coli is inhibited by aesculetin, but not by aesculin[10]. Hairy roots of medicinal morning glory (Pharbitis nil) show a potent glucosylation activity against aesculetin, especially at 7-hydroxyl group[11]. As one of the metabolites of caffeic acid oxidation, aesculetin observed in perfused rat liver may be responsible for its biological effects observed in vivo[12]. Aesculetin is a substrate of mushroom polyphenol oxidase and horseradish peroxidase enzyme resulting in the oxidization of aesculetin into its o-quinone[13]. Despite these accumulated data, we still do not fully understand the structural basis and dynamic pattern underlying the pleiotropic biological effects of aesculetin and there are few reports describing its derivatives or metabolites.
Therefore, in the present work, we analyzed the biotransformation process of aesculin in response to human gut microflora in a rat model, in order to identify its novel metabolites, which may enrich our understanding of the complicated structure-activity relationships between aesculin and its metabolites.
Twenty healthy volunteers (10 males and 10 females) at the age of 20-24 years, were recruited from students of Chengdu University of Traditional Chinese Medicine. Their faeces were collected and intestinal flora was identified as normal with an automatic system of bacteria identification (BD, USA). Then, fecal samples were pooled. The bacterial concentration was adjusted to 2 × 107 CFU/mL in culture medium, and 1 mL of aliquots was stored at -80°C until use.
Culture medium for anaerobic gut bacteria, GAM, was prepared according to the following formula: 3 g of soybean phytone, 10 g of peptone, 13.5 g digested blood serum powder, 5 g yeast extract, 2.2 g extractum carnis, 1.2 g beef liver extract, 3 g glucose, 5 g soluble starch, 2.5 g KH2PO4, 3 g NaCl, 0.3 g L-cysteine, 0.3 g sodiumthioglycollate, were added into 800 mL of dH2O. The pH was adjusted to 7.4 with 1 mol/L NaOH and the final volume was adjusted to 1000 mL with dH2O. The medium was sterilized by autoclaving at 121°C for 20 min and stored at 4°C for further use.
Aesculin (6-hydroxy, 7-glycoside coumarin), with an estimated purity of 98%, was purchased from Aldrich Chemical Co.(Milwaukee, WI). For the preparation of standard aesculin, 250 mg of aesculin was added to 25 mL of saline (0.9% NaCl solution) and dispensed into the solution by sonication. The mixture was then boiled for 10 min to dissolve aesculin. This stock solution of aesculin (10 mg/mL) was stored in the dark at -20°C until use.
Aesculetin (6, 7-dihydroxycoumarin) was also purchased from Aldrich Chemical Co. (Milwaukee, WI). The stock solution of aesculetin (10 mg/mL) was prepared as above and stored under the same condition for aesculin.
Twenty mL of aseculin stock solution was added to 179 mL of GAM in a 500 mL glass flask, followed by addition of 1 mL of human gut bacteria. The final concentration of aesculin was 1 mg/mL. The mixture was incubated at 37°C in DY-II anaerobic incubator containing 80% N2, 10% CO2, and 10% H2 (Yiwu Co. Ltd, Zhejiang Province, China). Ten mL of the solution was removed at 0, 2, 4, 8, 12, 16, 24, 48 and 72 h post-incubation, respectively, and 10 mL of methanol was then added to at each time point. The solutions were mixed well with hands for 5 min and the upper phase was transferred into a fresh flask. This extraction process was repeated two times and all the upper phase fractions were pooled for HPLC analysis.
The same protocol was employed to incubate aesculetin with human gut bacteria.
Preparation of control stock solution: Methanol was used as a solvent to prepare the aesculin and aesculetin stock solutions. The final concentration of aesculin and aesculetin was 10.08 mg/mL and 6.120 mg/mL, respectively. The solutions were kept in the dark at 4°C for up to one week.
HPLC was performed on a Waters Separation Module 2695 at a detection wavelength of 340 nm, at a screen wavelength of 210-400 nm, at a flow rate of 1.0 mL/min, at a sample size of 10 &mgr;L, and at a column temperature of 25°C, with mobile phase A set at 5% acetic acid : methanol (78:22), mobile phase B at 5% acetic acid: methanol gradient elution (0-20 min, 80:20; 20-35 min, linearity changed from 80:20 to 40:60), and mobile phase C at 5% acetic acid: methanol gradient elution (0-20 min, 90:10; 20-35 min, linearity changed from 90:10 to 50:50), respectively.
Twelve adult SD rats (6 males and 6 females), weighing 200 ± 5.6 g, were raised under specific pathogen-free conditions at the University Laboratory Animal Service Center of Chengdu University of Traditional Chinese Medicine, China. Each rat was raised in a solitary metabolic cage, with free access to normal food in a 12 h light and dark cycle. After adaptive nurture for one week, the aesculetin solution was administrated at a dosage of 100 mg per kilogram. Rat urine produced from 6 to 48 h after administration of aesculetin solution was collected for analysis. Five millilitre of urine sample from each rat was mixed for HPLC analysis.
Aesculin metabolites were identified in rat urine by XenoBiotic Laboratories, Inc., Plainsboro, NJ, USA. Control solutions of aesculetin and aesculin, and rat urine sample were analyzed by LC/ESI-MS and MS/MS in the positive and negative modes. The actual instrument conditions were modified to optimize chromatography and instrument sensitivity.
HPLC: HPLC System:Waters Separation Module 2695. Column: Ace 3, C18, 3 &mgr;m, 150 mm × 4.6 mm; guard column: Ace 3, C18, 10 × 3.2 mm; column temperature: 25°C; autosampler temperature: 4°C; mobile phase A: 0.4% HCOOH in H2O; mobile phase B: CAN.
MS: Mass spectrometer: Finnigan LCQ™ mass spectrometer. Data system: ThermoQuest Xcalibur Version1.3; ionization mode: positive or negative electrospray ion modes [(+)/(-) ESI]; ion spray (IS): 4.5 kV; capillary temperature: 240°C; sheath gas flow: N2, ~80 units; auxiliary gas flow: N2, ~20 units; collision gas: helium.
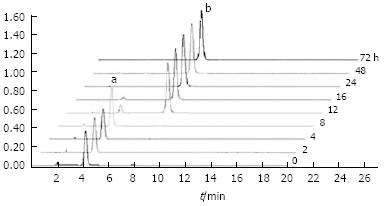
HPLC analysis revealed that our representative human gut bacteria degraded the glycoside of aesculin, thus completely converting it into aesculetin (Figure 1). The biotransformation process occurred between 8 and 24 h post-culture. After 24 h, aesculin was almost transferred into aesculetin. No other metabolite was observed, indicating that human gut bacteria cannot further modify aesculetin. No conversion of aesculin to aesculetin was observed in the absence of human gut bacteria 72 h after incubation (data not shown), indicating that human gut bacteria are a prerequisite for the biotransformation of aesculin.
The biotansformation efficiency of aesculin was quantitatively determined according to the following equation: Transformation ratio= (aesculetin concentration/178)/(aesculetin concentration/178 + aesculin concentration/340), where 178 represents the molecular weight of aesculetin and 340 represents the molecular weight of aesculin. Almost 80% of aesculin biotransformation occurred from 8 to 12 h post-incubation (Table 1).
| Incubation time (h) | 0 | 2 | 4 | 8 | 12 | 16 | 24 | 48 | 72 |
| Aesculin Conc. (mg/mL) | 0.886 | 0.848 | 0.832 | 0.873 | 0.166 | 0.043 | ND | ND | ND |
| Aesculetin Conc. (mg/mL) | ND | ND | ND | ND | 0.332 | 0.418 | 0.434 | 0.466 | 0.462 |
| Transformation ratio (%) | 0 | 0 | 0 | 0 | 79.20 | 94.90 | 100 | 100 | 100 |
The mixture of human gut bacteria did modify aesculetin under the same culture conditions (data not shown), indicating that human gut bacteria can only biotransform aesculin but not further modify its molecules.
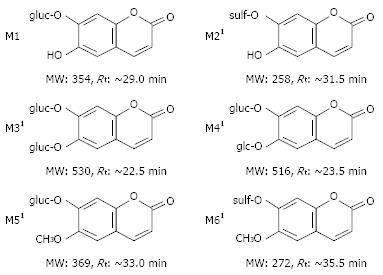
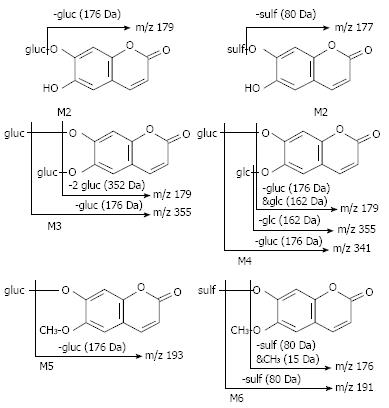
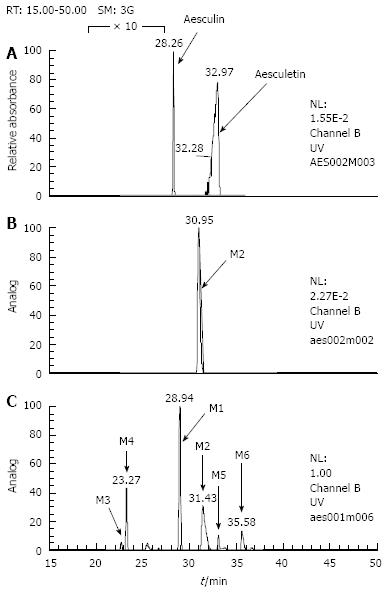
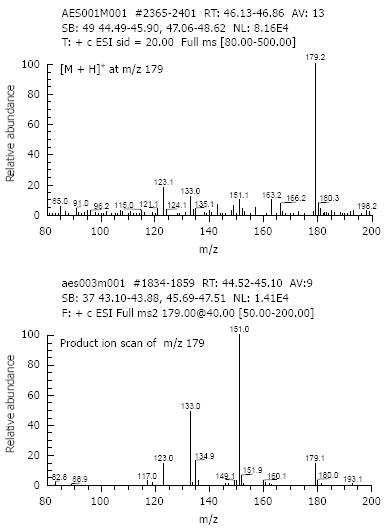
Six metabolites of aesculin were detected in rat urine (Figure 2). The primary characteristics of each metabolite were obtained by CAD-MS/MS analysis (Figure 3). One or two hydrogens in these metabolites [6-hydroxy-7-gluco-coumarin (M1), 6-hydroxy-7-sulf-coumarin (M2), 6, 7-di-gluco-coumarin (M3), 6-glc-7-gluco-coumarin (M4), 6-O-methyl-7-gluco-coumarin (M5) and 6-O-methyl-7-sulf-coumarin (M6)] of the hydroxyl groups of aesculetin were modified by glucosylation, glycoside, sulfation, and/or methylation. In the present study, M2 and M6 were found to be novel aesculin metabolites compared with the reported data[12].
Compared with the reference standards for aesculetin and aesculin, six aesculetin metabolites in rat urine were clearly observed on HPLC UV-chromatogram (Figure 4). The spectral data of aesculetin using LC/(+)ESI-MS and MS/MS are shown in Figure 5. By comparing the data, we could identify the substitution position of aesculetin metabolites in rat urine in vivo.
Unlike gut microflora, mammalian physiology could further modify aesculin and the pharmaceutical effects of aesculin might be a result of these metabolic modifications.
Natural coumarins, widely distributed in plants, fungi and bacteria, have pleiotropic biological effects. Coumarins, made of fused benzene and a-pyrone rings, have phenolic hydroxyl groups in their structures that can be easily replaced and/or modified, forming various metabolites or derivatives. Aesculin, one of the simplest coumarins known to have multiple pharmacological and biochemical activities, is an established inhibitor of lipoxygenase and cyclo-oxygenase and shows scavenging effects on ROS[13]. It has been reported that aesculetin has chemo-preventive and anti-tumor activities in vivo against cancer[514] and also induces apoptosis in several types of human cancer cells by diverse pathways[1671516]. Hence, it is a great challenge to determine the complex structure-activity relationships between aesculin/aesculetin and their metabolites. The aesculin metabolites in rat urine were identified both in vitro and in vivo.
The results of this study show that human gut bacteria could completely biotransform aesculin into aesculetin. About 80 % of aesculin was converted into aesculetin in less than 12 h post-incubation. The reason why the in vitro biotranformation speed of aesculin was so slow remains unclear. The universal time lag of our bacterial culture conditions might be a major reason, since human gut bacteria must first synthesize enough enzymes in order to adapt to any new environment. Since the starting concentration (105 CFU/mL) of human gut bacteria was markedly lower than that of human gut flora (about 1012 CFU/gram), some additional time is necessary for the exponent growth of seeded bacteria in order to reach the maximum bacterial concentration in the culture medium. In addition, we only employed anaerobic gut bacteria for the biotransformation of aesculin, which should be somewhat slower than in vivo conditions, since both anaerobic and aerobic bacteria may be involved in the in vivo aesculin biotransformation process.
The results of aesculin transformation suggest a simple but vivid example of host-gut bacteria cooperation in the dynamic utilization and subsequent modification of xenobiotics. Human gut bacteria degrade the glycoside of aesculin to facilitate its absorbtion in intestine, then modification of aesculetin into various derivatives with the potential of having multiple biological activities, occurs in the host. It was reported that the distal human intestine represents an anaerobic bioreactor programmed with a large population of bacteria, providing us with genetic and metabolic attributes, including the ability to harvest otherwise inaccessible nutrients and to metabolize diverse xenobiotics[17–19]. Microbiome is still a largely under explored regulator of drug metabolism and bioavailability[17–19]. Our data present here provide additional evidence for the metabolism of aesculin by human gut microbiota.
In this study, two novel sulfated aesculetin metabolites were identified in rat urine, which might represent very interesting modifications of coumarins. As aesculetin has two hydroxyl groups at carbons 6 and 7, it can serve as targets for O-methylation or O-glycosylatin. It has been reported that O-methylated products of aesculetin are scopoletin (6-O-methyl aesculetin), isoscopoletin (7-O-methyl aesculetin), and scoparone (6, 7-O-dimethyl aesculetin), which possess antimicrobial, immunosuppressive, and hypolipidermic activities[79]. The hydroxyl groups of aesculetin, especially the 7-hydroxyl group, can also be glucosylated in plants such as hairy roots of medicinal morning glory[11]. In addition, mushroom polyphenol oxidase (PPO) and horseradish peroxidase (POD) can oxidize aesculetin and generate its o-quinone[13]. However, as far as we are know, no sulfated derivative of aesculetin has been identified. Indeed, sulfation is one of the most important modifications in many natural compounds. As their sulfate groups accumulate negative charges, sulfated cumarins can interact with certain molecular domains that usually have positive charges, resulting in compounds with anti-viral, anti-tumor and anti-oxidative effects[12]. Therefore, these novel derivatives identified in rat urine offer new evidence for the biological activities ascribed to aesculetin.
Aesculin and its metabolites have pleiotropic pharmacological and biochemical properties, such as antioxidative, photo-protective and multiple immunomodulatory effects. However, their biotransformation has not been extensively studied, let alone their complex structure-activity relationships.
To uncover the dynamic progress and identify the pharmacological metabolites of natural products is one of the hotspots in development of traditional Chinese herbal drugs.
The biotransformation of aesculin was investigated in this study. Human gut bacteria could completely convert aesculin into aesculetin in vitro. Six aesculetin metabolites were identified in rat urine, 2 of which were first found. These novel sulfated aesculetin metabolites represent one of the most important modifications of cumarins. As their sulfate groups accumulate negative charges, sulfated cumarins can interact with certain molecular domains that usually have positive charges, resulting in compounds with anti-viral, anti-tumor and anti-oxidative effects. Therefore, these novel derivatives offer new evidence for the biological activities ascribed to aesculin.
The results of this study can direct the development of anti-viral, anti-tumor and/or anti-oxidative natural herbal drugs, and display a useful design for retrieval of the complex biotranformation process of ceterin natural compounds.
LC/ESI-MS stands for Liquid chromatography-electrospray ion trap mass spectrometry; CFU indicates that colony forming units.
The authors studied the biotranformation of aesculin by gut bacteria in rats and showed that aesculin had a wide range of biological activities that may have important pharmaceutical applications. Hence, knowledge about its metabolism is essential for understanding its therapeutic effects. The findings are interesting. Aesculin was converted to aesculetin in an in vitro bacterial culture system and 6 aesculetin metabolites were identified in rat urine, 2 of which were first observed.
| 1. | Kaneko T, Tahara S, Takabayashi F. Inhibitory effect of natural coumarin compounds, esculetin and esculin, on oxidative DNA damage and formation of aberrant crypt foci and tumors induced by 1,2-dimethylhydrazine in rat colons. Biol Pharm Bull. 2007;30:2052-2057. [Cited in This Article: ] |
| 2. | Kaneko T, Tahara S, Takabayashi F, Harada N. Suppression of 8-oxo-2'-deoxyguanosine formation and carcinogenesis induced by N-nitrosobis (2-oxopropyl)amine in hamsters by esculetin and esculin. Free Radic Res. 2004;38:839-846. [Cited in This Article: ] |
| 3. | Masamoto Y, Murata Y, Baba K, Shimoishi Y, Tada M, Takahata K. Inhibitory effects of esculetin on melanin biosynthesis. Biol Pharm Bull. 2004;27:422-425. [Cited in This Article: ] |
| 4. | Wang CJ, Hsieh YJ, Chu CY, Lin YL, Tseng TH. Inhibition of cell cycle progression in human leukemia HL-60 cells by esculetin. Cancer Lett. 2002;183:163-168. [Cited in This Article: ] |
| 5. | Lee BC, Lee SY, Lee HJ, Sim GS, Kim JH, Kim JH, Cho YH, Lee DH, Pyo HB, Choe TB. Anti-oxidative and photo-protective effects of coumarins isolated from Fraxinus chinensis. Arch Pharm Res. 2007;30:1293-1301. [Cited in This Article: ] |
| 6. | Yang JY, Della-Fera MA, Hartzell DL, Nelson-Dooley C, Hausman DB, Baile CA. Esculetin induces apoptosis and inhibits adipogenesis in 3T3-L1 cells. Obesity (Silver Spring). 2006;14:1691-1699. [Cited in This Article: ] |
| 7. | Lacy A, O'Kennedy R. Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr Pharm Des. 2004;10:3797-3811. [Cited in This Article: ] |
| 8. | Leung KN, Leung PY, Kong LP, Leung PK. Immunomo-dulatory effects of esculetin (6,7-dihydroxycoumarin) on murine lymphocytes and peritoneal macrophages. Cell Mol Immunol. 2005;2:181-188. [Cited in This Article: ] |
| 9. | Kim BG, Lee Y, Hur HG, Lim Y, Ahn JH. Production of three O-methhylated esculetins with Escherichia coli expressing O-methyltransferase from poplar. Biosci Biotechnol Biochem. 2006;70:1269-1272. [Cited in This Article: ] |
| 10. | Duncan SH, Flint HJ, Stewart CS. Inhibitory activity of gut bacteria against Escherichia coli O157 mediated by dietary plant metabolites. FEMS Microbiol Lett. 1998;164:283-288. [Cited in This Article: ] |
| 11. | Kanho H, Yaoya S, Itani T, Nakane T, Kawahara N, Takase Y, Masuda K, Kuroyanagi M. Glucosylation of phenolic compounds by Pharbitis nil hairy roots: I. Glucosylation of coumarin and flavone derivatives. Biosci Biotechnol Biochem. 2004;68:2032-2039. [Cited in This Article: ] |
| 12. | Gumbinger HG, Vahlensieck U, Winterhoff H. Metabolism of caffeic acid in the isolated perfused rat liver. Planta Med. 1993;59:491-493. [Cited in This Article: ] |
| 13. | Stern N, Nozawa K, Kisch E, Tuck ML, Golub M, Eggena P, Knoll E. Tonic inhibition of renin secretion by the 12 lipoxygenase pathway: augmentation by high salt intake. Endocrinology. 1996;137:1878-1884. [Cited in This Article: ] |
| 14. | Munoz-Munoz JL, Garcia-Molina F, Varon R, Rodriguez-Lopez JN, Garcia-Canovas F, Tudela J. Kinetic chara-cterization of the oxidation of esculetin by polyphenol oxidase and peroxidase. Biosci Biotechnol Biochem. 2007;71:390-396. [Cited in This Article: ] |
| 15. | Park C, Jin CY, Kim GY, Choi IW, Kwon TK, Choi BT, Lee SJ, Lee WH, Choi YH. Induction of apoptosis by esculetin in human leukemia U937 cells through activation of JNK and ERK. Toxicol Appl Pharmacol. 2008;227:219-228. [Cited in This Article: ] |
| 16. | Yang JY, Della-Fera MA, Baile CA. Esculetin induces mitochondria-mediated apoptosis in 3T3-L1 adipocytes. Apoptosis. 2006;11:1371-1378. [Cited in This Article: ] |
| 17. | Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027-1031. [Cited in This Article: ] |