Published online Mar 7, 2008. doi: 10.3748/wjg.14.1463
Revised: December 6, 2007
Published online: March 7, 2008
Eosinophilic esophagitis (EE) and gastroesophageal reflux disease (GERD) have overlapping clinical, manometric, endoscopic and histopathologic features. The diagnosis of EE is nowadays based upon the presence of 15 or more eosinophils per high power field (eo/HPF) in esophageal biopsies. We report the cases of two young males suffering from dysphagia and recurrent food impaction with reflux esophagitis and more than 20 eo/HPF in upper-mid esophagus biopsies, both of which became asymptomatic on proton pump inhibitor (PPI) therapy. The first patient also achieved a histologic response, while EE remained in the other patient after effective PPI treatment, as shown by 24-h esophageal pH monitoring. Topical steroid therapy combined with PPI led to complete remission in this latter patient. GERD and EE may be undistinguishable, even by histology, so diagnosis of EE should only be established after a careful correlation of clinical, endoscopic and pathologic data obtained under vigorous acid suppression. These diagnostic difficulties are maximal when both diseases overlap. Limited data are available about this topic, and the interaction between EE and GERD is a matter of debate. In this setting, upper-mid esophagus step biopsies and esophageal pH monitoring of patients on PPI therapy are pivotal to evaluate the role of each disease. A PPI trial is mandatory in patients with a histopathologic diagnosis of EE; in those unresponsive to PPI treatment, EE should be suggested. However, a clinical response to PPI may not rule out quiescent EE, as shown in this report.
- Citation: Molina-Infante J, Ferrando-Lamana L, Mateos-Rodríguez JM, Pérez-Gallardo B, Prieto-Bermejo AB. Overlap of reflux and eosinophilic esophagitis in two patients requiring different therapies: A review of the literature. World J Gastroenterol 2008; 14(9): 1463-1466
- URL: https://www.wjgnet.com/1007-9327/full/v14/i9/1463.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.1463
Eosinophilic esophagitis (EE) is an emerging under-diagnosed disease in adults, associated with allergic or asthmatic disorders, which typically affect males in the second to fourth decades of life. The main symptoms are dysphagia, heartburn and chest pain, and it may be present in half of all adults with bolus food obstruction of the esophagus[12]. The classical endoscopic appearance of the esophagus is polymorphic and includes papular elevations, whitish exudates, corrugation, longitudinal furrows, undulated mucosa, reddening, multiple contraction rings and strictures, but may also be normal in 25% of cases[13]. Esophageal manometry may show non-specific motor disturbances, such as hypoperistaltic and hypercinetic disorders, or nontransmitted or simultaneous contraction waves[3].
Thus, EE and gastroesophageal reflux disease (GERD) can not be distinguished based on clinical, manometric or endoscopic features. Mild eosinophilic infiltration of the distal esophagus, usually less than 7-10 eosinophils per high power field (eo/HPF), is a hallmark of GERD, so on histological confirmation, which is required for the diagnosis of EE, dense eosinophil infiltrates (> 15-20 eo/HPF) must be observed along the length of the esophagus, in the absence of eosinophilic gastroenteritis, parasites or fungal infections, vasculitis or granulomatous diseases. The interaction between EE and GERD seems complex, and recently the importance of GERD in EE has been suggested due to its higher than expected prevalence, and the report of the resolution of EE with proton pump inhibitor (PPI) therapy[45].
A 21-year-old man (case 1) and a 35-year-old man (case 2) with no prior medical history both presented with acute-onset dysphagia and a complete inability to swallow saliva after food impaction in the mid and proximal esophagus, respectively. Both patients related a 2-year history of non-progressive intermittent dysphagia to solids and recurrent food impaction, which improved after drinking water. They had no previous asthma, allergic disorders or seasonal variations in their symptoms. Neither complained of heartburn, chest pain, or regurgitation. No peripheral eosinophilia or elevation in IgE level was detected in blood samples, while gastric and duodenal biopsies were normal.
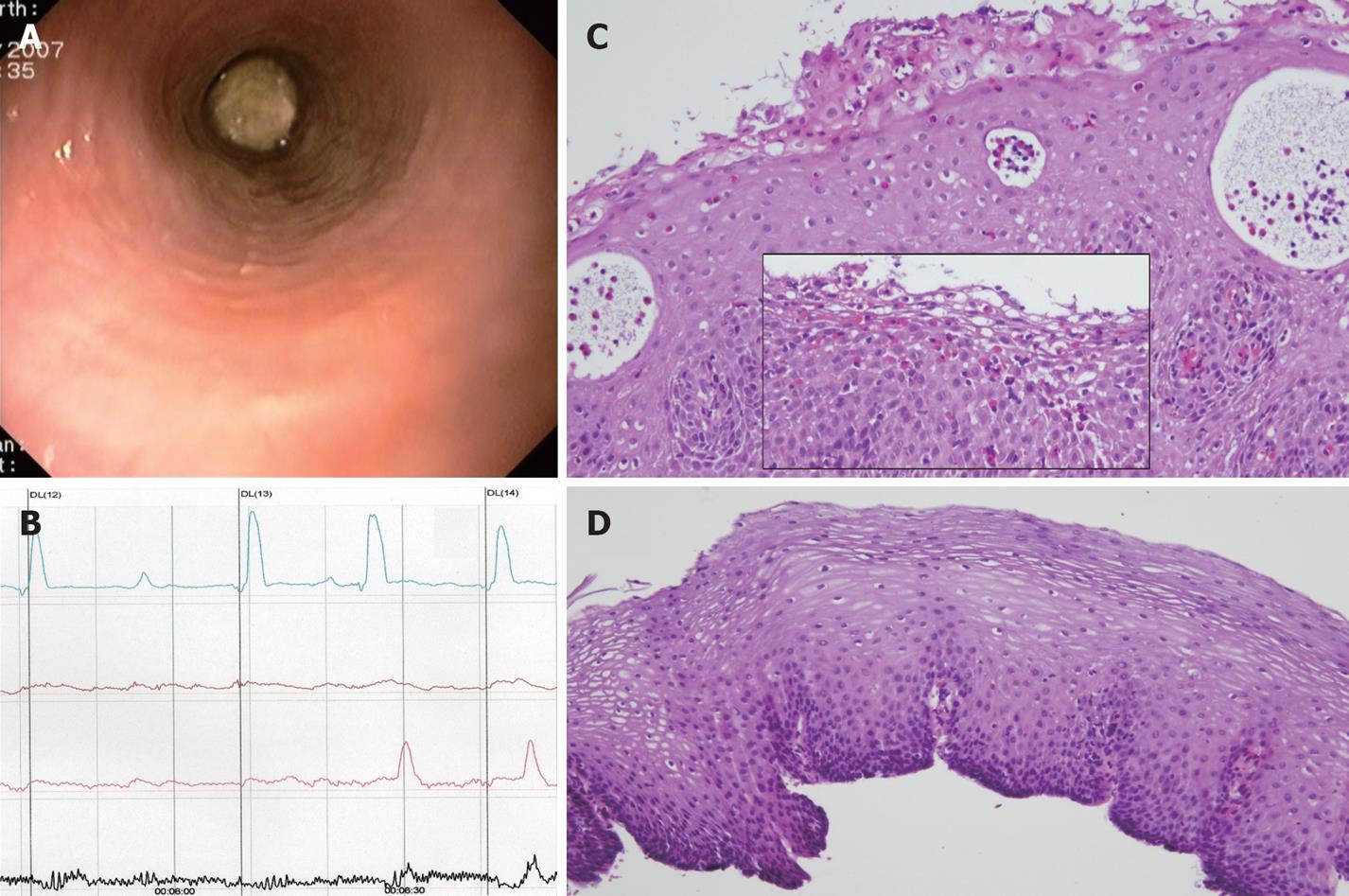
A meat bolus impacted at 32 cm from the incisors was removed in the emergency room with a polypectomy snare by endoscopy, which revealed no esophageal mucosa abnormalities (Figure 1A), but no biopsies were taken. A previous upper endoscopy due to meat impaction and a barium swallow revealed the esophagus to be normal. Esophageal manometry demonstrated severe dysmotility in the distal esophagus, with two thirds of all esophageal contractions waves measured being simultaneous and interrupted (Figure 1B) and a normal lower esophageal sphincter pressure. The patient refused 24 h esophageal pH monitoring because of severe intolerance. On this basis, a third esophagoscopy was performed to rule out EE. Upper endoscopy showed three mucosal breaks in the distal esophagus, more than 5 mm long, but not continuous between the tops of adjacent folds, compatible with grade B (Los Angeles classification) reflux esophagitis, while the rest of the mucosa was normal. Upper-mid esophagus step biopsies showed prominent eosinophil micro-abscesses (Figure 1C) and a high density eosinophilic infiltrate (31 eo/HPF, magnification × 400), with a predominantly superficial distribution (Figure 1C, box), mid basal zone hyperplasia and intercellular edema, which also contributed to the suspected diagnosis of EE. A 2-mo course on PPIs (omeprazole 40 mg/d) without steroids led to clinical, manometric and pathologic remission (Figure 1D).
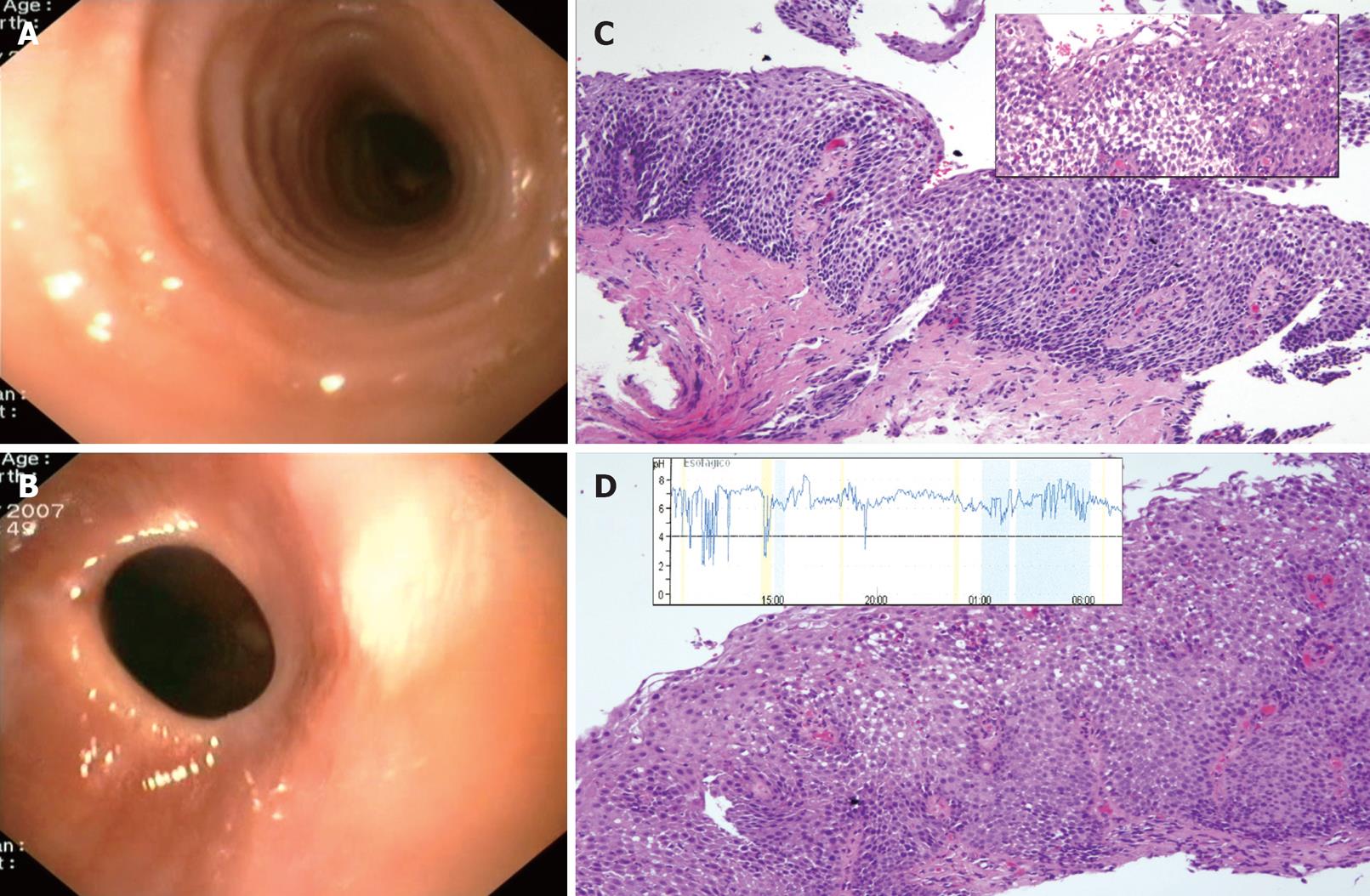
Emergency endoscopy showed a mixed vegetable bolus impacted at 23 cm from the dental margin, which was removed with a polypectomy snare and a Roth retrieval net. A normal caliber upper-mid esophagus with undulated mucosa was detected (Figure 2A), as well as a peptic fibrotic stricture at the cardias (Figure 2B), which was dilated using gentle pressure of the endoscope, with a medium size sliding hiatal hernia below. Esophageal step biopsies from the upper-mid esophagus showed severe lamina propia fibrosis (Figure 2C) and a dense intraepithelial eosinophilic infiltration (37 eo/HPF, magnification × 400) predominantly in the luminal surface of epithelium, with intercellular edema, basal cell hyperplasia and papillae elongation (Figure 2C, box). After a 2 mo course of PPIs (omeprazole 40 mg per day) the patient became asymptomatic, but endoscopic and all pathologic features remained at follow-up endoscopy (Figure 2D), despite PPI therapy, including 32 eo/HPF with degranulating superficial eosinophils. 24-h esophageal pH monitoring of the patient on PPIs ruled out persistent acid reflux (total time pH < 4.0, 4%, DeMeester score 3) (Figure 2D, box). Due to these findings, a 3 mo course of fluticasone propionate, at a dosage of 500 &mgr;g per 12 h, on top of PPIs, was started. The patient finally achieved histopathologic remission with this combined therapy.
Until recently, diagnosis of EE has been based upon the presence of more than 15-20 eo/HPF in esophageal biopsies. Due to the patchy distribution of EE, multiple-step biopsies of the mid and proximal esophagus are advisable to maximize diagnosis yield, since GERD may also induce esophageal eosinophilia, normally less than 7-10 eo/HPF, although this has been reported to be typically confined to the distal esophagus[13]. The diagnosis criteria of EE (> 15-20 eo/HPF) are based on the unlikelihood to observe an eosinophil count above this cut-off value in individuals with GERD and other secondary causes of eosinophilia. This cut-off value is arbitrary, and strict consensus on histopathologic diagnostic criteria of EE is lacking as a recent systematic review has shown a wide variety of eosinophil count cut-off points, methods for counting eosinophils and esophageal biopsy protocols in the literature on EE[67]. In this setting, eosinophil counts may lose diagnostic power, so the prominence of eosinophils towards the luminal surface, eosinophil micro-abscesses (defined as a cluster of 4 or more eosinophils), degranulating eosinophils and lamina propia fibrosis have been reported as secondary pathologic markers of EE[78]. Moreover, and to complicate the scenario, there has been a recent report of three cases fulfilling all clinical, endoscopic and histopathologic diagnosis criteria of EE, who achieved complete response to PPI therapy[5]. To avoid these diagnostic difficulties, consensus recommendations have recently been made by the First International Gastrointestinal Eosinophil Research Symposium (FIGERS) subcommittees[9], which define EE with three statements: (1) Symptoms including, but not restricted to food impaction and dysphagia in adults, and feeding intolerance and GERD symptoms in children; (2) 15 or more eosinophils/HPF; (3) exclusion of other disorders associated with similar clinical, histological or endoscopic features, especially GERD, with PPI treatment or esophageal pH monitoring.
The report by Ngo[5] and case 1 of this manuscript highlight that, in contrast to previously reported data[13], it is possible that GERD may cause identical high-density esophageal eosinophilia and histopathologic changes suggestive of EE in both the proximal and distal esophagus. According to initial endoscopic and pathologic data, case 1 would have been unequivocally diagnosed of EE. However, concomitant esophageal reflux lesions at the third endoscopy and complete remission on PPI treatment suggest the main diagnosis was GERD, which may have been triggering EE, or simply GERD mimicking EE histologically. Conversely, case 2 also had histological features of EE and esophageal acid reflux lesions, and became asymptomatic on PPI therapy, which made GERD the most probable diagnosis. However, persistent histopathologic features on effective PPI therapy and resolution on topical steroids showed the simultaneous existence EE and GERD.
These cases emphasize the importance of thoughtful consideration of clinical and histopathologic responses to acid blockade on the whole, and not only eosinophils count, to establish an accurate diagnosis of EE[1011]. The relationship between EE and GERD in adults is a controversial topic nowadays. Some authors defend their unrelated co-existence while others try to search for a plausible casual association. Contradictory data reported on the frequency of acid reflux observed by 24 h esophageal pH monitoring described in adults with EE (38%[4] against 11%[8]) does not clarify the situation. It has been suggested motor esophageal changes related to EE may contribute to GERD by decreasing lower esophageal sphincter pressure and delaying esophageal clearance, which would expose longer esophageal mucosa to the acid noxa[1011]; this theory may explain the symptomatic response in case 2 under PPI therapy, although endoscopic and pathologic features remained. On the other hand, the previously mentioned report by Ngo[5] and case 1 of the present manuscript, with documented complete responses of EE to PPI therapy, support the hypothesis that, at least in a small subset of patients with EE, GERD may cause EE, probably by means of an acid-mediated increase in epithelial permeability, allowing immune cell recruitment or access to allergenic peptides[1011]; in this setting, PPI could represent an effective therapy for both diseases. In patients with confirmed EE, alimentary allergic study is warranted since an elimination diet, mainly based on skin-prick tests, has demonstrated its effectiveness in terms of clinical and pathologic remission[12]. In case 2, there were no food allergens detected in a subcutaneous allergic test.
The real interplay between these two diseases is still to be elucidated. Nevertheless, when EE and GERD overlap, the clinical, endoscopic and histologic findings are non-specific in distinguishing both entities. Despite the growing recognition of EE in recent years, the more alert we are to EE, maybe the more we are likely to miss GERD. Indeed, the estimated prevalence of probable EE is 1% in the general population[13], while GERD is a much more frequent condition which may affect 10%-20% of adults in Western countries[14]; on this basis, the likelihood to find unrelated GERD in patients with EE is high, especially if we consider that they both commonly affect young males. Then, irrespective of diagnosis criteria of EE and the validation of the relationship between GERD and EE, it is mandatory an initial trial of PPI therapy[9–11] for patients with histopathologic diagnosis of EE. In those unresponsive to acid suppression, EE should be suggested. However, a clinical response to PPI may not either preclude underlying EE, as demonstrated in case 2.
In conclusion, we report two cases in which reflux esophagitis and EE overlap, which were similar in terms of symptoms, endoscopic reflux lesions, motor esophageal alterations, high-density eosinophilia in upper-mid esophagus biopsies and good clinical response to PPI, but with different endoscopic and histopathologic outcomes after PPI therapy. This fact may reflect that the interaction between these diseases may be more complex than originally thought and may depend more on individual patient characteristics. An initial trial of PPI therapy in patients with clinical, endoscopic and pathologic findings of EE is warranted. Lack of a response to PPI may reinforce a diagnosis of EE, but a clinical response to PPI may not rule out quiescent EE, as shown in this report. Esophageal pH measurements and histopathologic data on patients on PPI treatment are pivotal in cases with overlapping GERD and EE in order to evaluate the role of each disease. Due to the fact that EE is an uncommon disease, multicentric studies are needed to establish the real incidence and prevalence of EE and GERD in EE, and to clarify the effects of GERD, food allergy and EE on esophageal eosinophilia.
| 1. | Muller S, Puhl S, Vieth M, Stolte M. Analysis of symptoms and endoscopic findings in 117 patients with histological diagnoses of eosinophilic esophagitis. Endoscopy. 2007;39:339-344. [Cited in This Article: ] |
| 2. | Kerlin P, Jones D, Remedios M, Campbell C. Prevalence of eosinophilic esophagitis in adults with food bolus obstruction of the esophagus. J Clin Gastroenterol. 2007;41:356-361. [Cited in This Article: ] |
| 3. | Lucendo AJ, Pascual-Turrion JM, Navarro M, Comas C, Castillo P, Letran A, Caballero MT, Larrauri J. Endoscopic, bioptic, and manometric findings in eosinophilic esophagitis before and after steroid therapy: a case series. Endoscopy. 2007;39:765-771. [Cited in This Article: ] |
| 4. | Remedios M, Campbell C, Jones DM, Kerlin P. Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc. 2006;63:3-12. [Cited in This Article: ] |
| 5. | Ngo P, Furuta GT, Antonioli DA, Fox VL. Eosinophils in the esophagus--peptic or allergic eosinophilic esophagitis? Case series of three patients with esophageal eosinophilia. Am J Gastroenterol. 2006;101:1666-1670. [Cited in This Article: ] |
| 6. | Dellon ES, Aderoju A, Woosley JT, Sandler RS, Shaheen NJ. Variability in diagnostic criteria for eosinophilic esophagitis: a systematic review. Am J Gastroenterol. 2007;102:2300-2313. [Cited in This Article: ] |
| 7. | Parfitt JR, Gregor JC, Suskin NG, Jawa HA, Driman DK. Eosinophilic esophagitis in adults: distinguishing features from gastroesophageal reflux disease: a study of 41 patients. Mod Pathol. 2006;19:90-96. [Cited in This Article: ] |
| 8. | Pasha SF, DiBaise JK, Kim HJ, De Petris G, Crowell MD, Fleischer DE, Sharma VK. Patient characteristics, clinical, endoscopic, and histologic findings in adult eosinophilic esophagitis: a case series and systematic review of the medical literature. Dis Esophagus. 2007;20:311-319. [Cited in This Article: ] |
| 9. | Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, Bonis P, Hassall E, Straumann A, Rothenberg ME. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342-1363. [Cited in This Article: ] |
| 10. | Spechler SJ, Genta RM, Souza RF. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol. 2007;102:1301-1306. [Cited in This Article: ] |
| 11. | Antonioli DA, Furuta GT. Allergic eosinophilic esophagitis: a primer for pathologists. Semin Diagn Pathol. 2005;22:266-272. [Cited in This Article: ] |
| 12. | Spergel JM. Eosinophilic esophagitis in adults and children: evidence for a food allergy component in many patients. Curr Opin Allergy Clin Immunol. 2007;7:274-278. [Cited in This Article: ] |