Published online Feb 28, 2008. doi: 10.3748/wjg.14.1274
Revised: December 6, 2007
Published online: February 28, 2008
AIM: To evaluate the therapeutic efficiency of replicative adenovirus CNHK300 targeted in telomerase-positive hepatocellular carcinoma.
METHODS: CNHK300, ONYX-015 (55 kDa protein deleted adenovirus) and wtAd5 (wild type adenovirus 5) were compared, and virus proliferation assay, cell viability assay, Western blot and fluorescence microscopy were used to evaluate the proliferation and cytolysis selectivity of CNHK300.
RESULTS: The replicative multiples in Hep3B and HepGII after 48 h of CNHK300 proliferation were 40 625 and 65 326 fold, respectively, similar to that of wtAd5.. However, CNHK300 exhibited attenuated replicative ability in normal fibroblast cell line BJ. CNHK300 could lyse hepatocellular carcinoma cells at a low multiplicity of infection (MOI), but could not affect growth of normal cells even at a high MOI.
CONCLUSION: CNHK300 is a cancer-selective replication-competent adenovirus which can cause oncolysis of liver cancer cells as well as wtAd5 (wild type adenovirus 5), but had severely attenuated replicative and cytolytic ability in normal cells. This novel strategy of cancer treatment offers a promising treatment platform.
- Citation: Li YM, Song ST, Jiang ZF, Zhang Q, Su CQ, Liao GQ, Qu YM, Xie GQ, Li MY, Ge FJ, Qian QJ. Telomerase-specific oncolytic virotherapy for human hepatocellular carcinoma. World J Gastroenterol 2008; 14(8): 1274-1279
- URL: https://www.wjgnet.com/1007-9327/full/v14/i8/1274.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.1274
Replication-defective adenoviruses have been successfully established to deliver genes for cancer gene therapy. But, progress in clinical practice has been hampered by lack of cell specific infectivity and poor viral distribution within the tumor mass[12]. To confer specificity of infection and increase viral spread in the tumor mass, replication-competent adenovirus is being developed as an promising cancer therapeutic agent. These replication-competent adenoviruses can cause oncolysis of tumor cells as part of the virus life cycle, the new virus progeny released from the dead cells infects the neighboring cells and continues to replicate and spread until the tumor is eradicated. Furthermore, tumor antigens released from the dead cells can enhance antitumor immunity[3]. Until now, more than 10 kinds of replication-competent oncolytic viruses have been evaluated in different phases of clinical trials. This novel strategy of cancer treatment offers a promising treatment platform[4–6].
The key of replication-competent oncolytic adenovirus modification is to constrict viral replication to tumor cells. One approach is to regulate expression of viral genes essential for replication with tumor-specific promoters that are highly active in tumor cells, but inactive in normal cells. For example, human breast cancer has been targeted with the adenovirus Ad. DF3-E1 in which the E1A gene is under the control of the DF3/MUC1 gene promoter/enhancer[7]. Several other tumor-specific promoters are also being evaluated to restrict viral replication to their cognate tumors, such as prostate-specific antigen enhancer element for prostate carcinoma and γ-fetoprotein for hepatocellular carcinoma[8–10]. Although these results demonstrated that these reconstructed adenovirus had high selectivity, their major drawback was that they were only available for treatment of a narrow range of tumors, because only a limited number of tumors could express the targeted tumor markers. Telomerase is the broadest spectrum molecular marker of malignancies, which is highly activated in immortalized cell lines and most of the malignant tumors, but is inactive in normal comatic cells. Human telomerase reverse transcriptase (hTERT) is the major determinant of human telomerase activity[11]. HTERT promoter is highly active in tumor cells but inactive in normal cells, and utilizing hTERT promoter to drive antitumoral genes in cancer gene therapy can target to and selectively kill the cancer cells with positive telomerase activity[12–14].
In this study, we report a strategy for the development of a novel replication-competent oncolytic adenovirus. In this virus, the E1A gene was placed under the control of the human hTERT promoter to allow us to target a wide range of telomerase-positive tumors with little influence on the growth of normal cells.
CNHK300 is a tumor-specific replication competent adenovirus variant that was reconstructed at our lab. hTERT gene promoter was inserted at the upstream of the E1A encoding region of the viral genome. Because different cell lines may show various levels of susceptibility to adenovirus infection and virus production, non-selectively replicating wild type 5 adenovirus (wtAd5) was compared with the tested conditionally replicating adenovirus CNHK300. CNHK300-GFP virus carried green fluorescent protein (GFP) as reporter gene which inserted the upstream of hTERT promoter of CNHK300. ONYX-015 is an E1B 55 kDa deleted adenovirus kindly presented by Professor Berk AJ (University of California, USA)[15–17]. All adenoviruses were grown on the human embryonic kidney cell line HEK293. Virus titers were determined by plaque assay. Ratios of virus particle and plaque were between 20:1 and 100:1 normally. CNHK300 and ONYX-015 virus preparation were free of the wtAd5 and endotoxin contamination.
The human hepatocellular carcinoma cell lines HepGII (wild-type p53), Hep3B (mutated p53) and human normal fibroblast cell line BJ were purchased from the American Type Culture Collection (Manassas, VA). Transformed human embryonal kidney HEK293 cell line was obtained from Microbix Biosystem Inc. (Toronto, Ontario, Canada). HEK293, HepGII and Hep3B were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (Hyclone). BJ was maintained in Minimum Eagle Medium (MEM) with 10% fetal bovine serum (FBS), 2 mmol/L L-glutamine. All the media were supplemented with 100 U/mL penicillin and 100 &mgr;g/mL streptomycin and maintained at 37°C in 5% CO2.
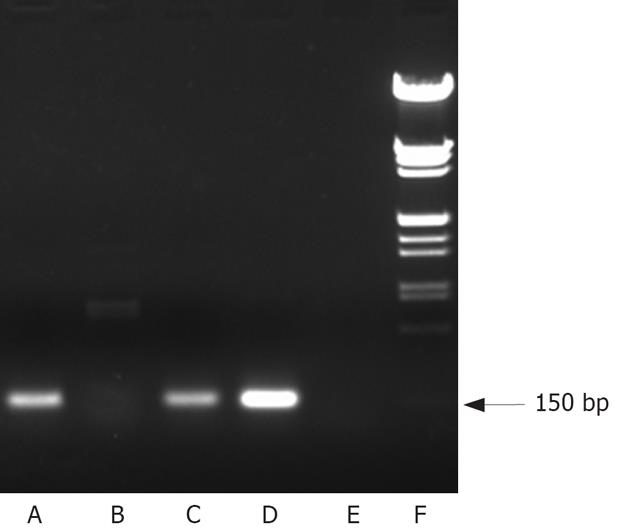
RT-PCR amplification method was used to detect the hTERT mRNA. Total RNA was extracted from cell lines by the Trizol Reagent (Invitrogen Corp., San Diego, CA). First strand cDNAs synthesis and PCR reaction were performed using the SuperScript one-step RT-PCR with Platinum Taq Kit (Invitrogen Corp.) according to the manufacturer’s protocol. The reaction mixture was heated at 50°C for 30 min, and then 30 cycles of PCR were performed, including denaturation at 94°C for 30 s, annealing at 60°C for 30 s and extension at 72°C for 60 s, and then 72°C for 2 min. For PCR reaction, sense and antisense oligonucleotide primers (5’-CGGAAGAGT GTCTGGAGCAA-3’; 5’-GGATGAAGCGGAGTC TGGA-3’) were designed. The PCR products (150 bp in length) were electrophoresed on 10% agarose gel.
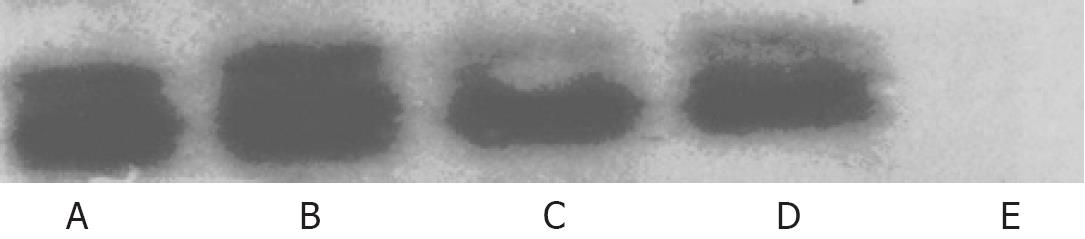
HepGII, Hep3B and BJ cells were plated at a density of 5 × 105 in 6-well plates (Falcon) and 24 h later infected with CNHK300, ONYX-015 or wtAd5 at a MOI corresponding to IC50. After a 2 h incubation at 37°C in 5% CO2, culture medium were removed, the cells were then washed twice with serum-free medium and incubated at 37°C in 5% CO2. Forty-eight hours later, culture medium were removed, total cell lysates were prepared using 150 &mgr;L/well of Mammalian Protein Extraction Reagent (M-PER, PIERCE, Rockford, IL). Twenty &mgr;L of aliquots were subjected to detection on 10% SDS-PAGE and E1A protein was detected with M73 antibody against adenovirus E1A (Santa Cruz Biotechnology, Santa Cruz, CA). The signals were visualized with LumiGLO chemiluminescent reagent and peroxide (Cell Signaling Technology, Bevery, MA).
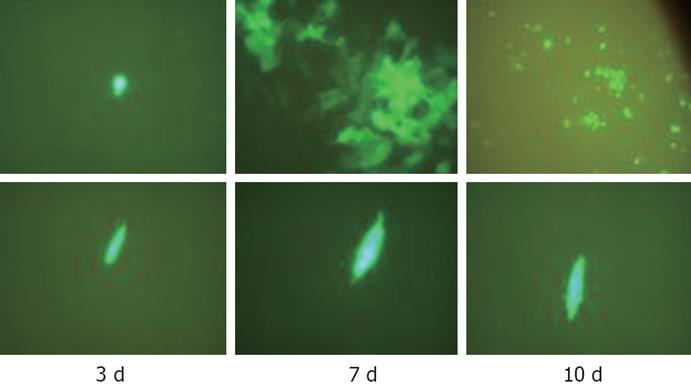
Hep3B and BJ cells were plated at a density of 2 × 105 in 6-well plates (Falcon). When the cells reached 80% confluence 2-4 d later, the cells were infected with CNHK300-GFP at a MOI of 0.01, 1, 10 plaque forming unit (pfu)/cell, respectively. The cells were observed and photographed at 3, 7 and 10 d after infection.
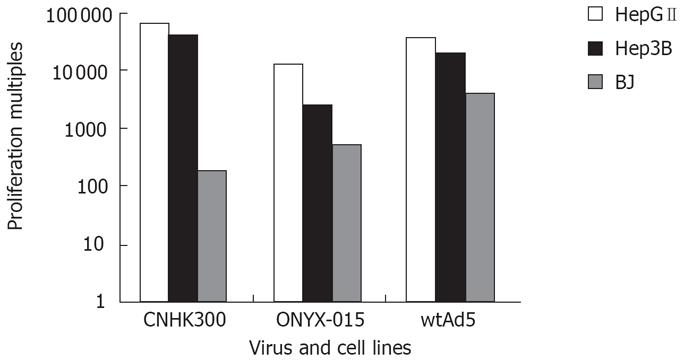
HepGII, Hep3B and BJ cells were plated at a density of 5 × 105 in 6-well plates (Falcon) and 24 h later infected with CNHK300, ONYX-015 or wtAd5 at MOI of 5 pfu/cell. After a 2 h incubation at 37°C in 5% CO2, culture medium were removed, the cells were then washed twice with serum-free medium and incubated at 37°C in 5% CO2 for varying periods of time (0 h, 12 h, 24 h, 48 h, 96 h), the cultures at each time point were harvested and lysed by three cycles of freeze and thaw. The supernatant of each time point was tested for virus production on 293 cells for 10 d with plaque assay. The relative ability of the viruses to amplify in these cells after infection was calculated by comparing the ratio of viral yields to initial virus input.
MTT assay was performed to determine cell viability at various viral MOIs. HepGII, Hep3B and BJ cells were plated at a density of 1 × 104 in 96-well plates (Falcon) and 24 h later, the cells were infected with CNHK300 at increasing MOIs. After 7 d of incubation, cell viability was measured by the MTT assay using the non-radioactive cell proliferation kit (Roche Molecular Biochemicals) according to the kit protocol and the spectrophotometrical absorbance of the samples using a microtiter plate (ELISA) reader at 570 nm (the reference wavelength was 650 nm). The percentage of cell survival was calculated using the formula: Cell survival (%) = (A value of infected cells/A value of uninfected control cell) × 100%. Eight replicate samples were taken at each MOIs, and each experiment was performed at least three times.
RT-PCR was performed to detect the hTERT mRNA expression in HepGII, Hep3B cells and normal cells. As shown in Figure 1, telomerase hTERT mRNA expression was demonstrated in both cancer cells tested. In contrast, BJ fibroblast cells did not express hTERT mRNA.
The Western blotting revealed that CNHK300 could express E1A in the HepGII and Hep3B cells but not in the BJ human normal fibroblast cells. As controls, BJ cells were also infected with wtAd5, and the results indicated that wtAd5 could effectively express E1A in BJ cells. These findings demonstrated that CNHK300 selectively expressed E1A in telomerase positive hepatocellular cancer cells, whereas wtAd5 did not show any selectivity in E1A expression (Figure 2).
As shown in Figure 3, CNHK300-GFP could infect both BJ and Hep3B cells and expressed GFP within 3 d. But after 7 d of infection, CNHK300-GFP virus could proliferate effectively and express GFP protein. When the CNHK300-GFP was released from the infected cancer cells, it could infect the adjacent cells and enter into new life cycles. Therefore, the cancer cells showed obvious proliferation and cytopathologic effect (CPE) at the 7th day. Because many cancer cells died from virus infection after 10 d of infection, the GFP protein was degraded and extincted gradually. Whereas in normal cell lines, CNHK300-GFP proliferation was attenuated, and BJ cells did not show CPE after days of infection (Figure 3).
To determine whether the CNHK300 viruses we reconstructed replicate preferentially in tumor cells and replicatively attenuated in normal cells, we performed virus yield assay in HepGII and Hep3B tumor cell lines and BJ normal cell lines, and because different cell lines may show different susceptibility to adenovirus infection and replication ability to correct the difference in infectivity and virus production between various tumor cell lines and normal cell lines, wtAd5 served as a control. As shown in Figure 4, CNHK300 replicated by 40 625 and 65 326 folds on tumor cell HepGII and Hep3B, respectively, similar to those of wtAd5. However, the CNHK300 replication (180-folds) was attenuated significantly as compared with wtAd5 (4000-folds) in BJ normal cell lines.
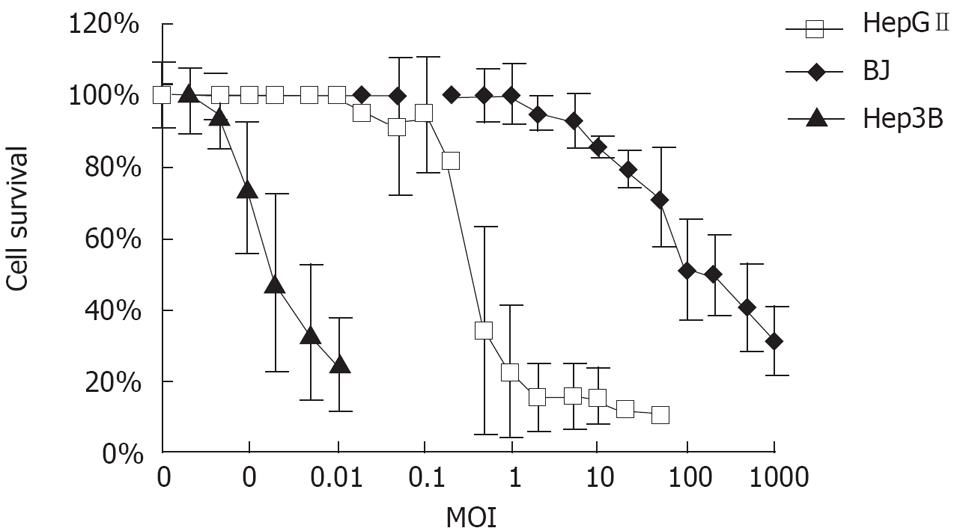
MTT assay was performed to characterize the specificity of CNHK300 on tumor cells with no or less toxicity than normal cells. As shown in Figure 5, CNHK300 could cause significant cytolysis in HepGII and Hep3B tumor cell lines with a MOI of 0.5 pfu/cell and 0.0002 pfu/cell, respectively. However, cells infected with CNHK300 showed an over 50% cell viability rate at the same time points with MOI of 100 pfu/cell, suggesting that more than 200-500 000 folds of CNHK300 were needed to kill half of normal fibroblast cells compared with HepGII and Hep3B tumor cells.
Many independent studies indicated that telomerase was active in over 85% malignant cancers, but not in normal tissues except hematopoietic stem cells and germ cells in the ovary and testis. Telomerase activation has been regarded as the critical characteristic of malignant tumors[18]. hTERT is the major determinant of human telomerase activity, and hTERT promoter has been successfully used to drive antitumoral genes in cancer gene therapy to target to and selectively kill the cancer cells with positive telomerase activity[19–21]. In our study, we constructed a replicative adenovirus CNHK300, in which, the E1A gene was placed under the control of the human hTERT promoter to allow us to target a wide range of telomerase-positive tumors[22]. A key requirement for clinical application of tumor-specific oncolytic virus is their attenuation in non-target tissues. So, in vitro viral replication assay and in vitro cell viability MTT assay were used to test the replicative specificity and cytotoxicity of CNHK300 in cancer cell lines and attenuated cytolysis in normal cells. Our experiments achieved similar results with some foreign studies[23–25]. As shown in our results, CNHK300 showed strong differences in replication and cytotoxicity in different hepatocellular cell lines. Though we tested higher titers of CNHK300 in HepGII cells than in Hep3B cells after 48 h of virus exposure, CNHK300 did not show stonger cytotoxicity on HepGII cells. There might be some other cell-killing mechanisms besides oncolytic effect due to virus replication.
Hematopoietic stem cells and germ cells in the ovary and testis are all telomerase-positive cells. Therefore, CNHK300 may replicate in above normal cells if it infects these cells. Fortunately, CNHK300 can not get into these cells because they lack adenovirus-specific CAR receptor on their cell surface[26].
Adenovirus is widely used in generating replication-selective viruses. In recent years, two major strategies have come into force. In the first one, viral genes that become dispensable in tumor cells, such as the genes responsible for activating the cell cycle through p53 or Rb binding, have been completely or partially deleted[15–17]. E1b 55 kDa deleted adenovirus Onyx-015 was proposed to replicate selectively in p53-deficient cells. Yet, several studies indicated that Onyx-015 was independent of the p53 status[15–17]. In the second strategy, transcription of viral genes has been controlled by replacing the native viral promoters with tumor-specific promoters[8–1022–25]. In contrast, great progress has been made in the study of tumor-specific promoter regulated replication-selective adenovirus, Paul Hallenbeck (Genetic Therapy-Novartis, Gaithersburg, MD) and Daniel R. Henderson (Calydon, Sunnyvale, CA) have initiated the efforts in this direction using the α-fetoprotein (AFP) and prostate specific antigen (PSA) promoters to drive the adenovirus E1A gene. It has been proved that modified adenovirus using this strategy has some advantages compared with Onyx-015[27–30].
Viruses can infect, replicate in and kill human cells through diverse mechanisms. Clinicians have treated hundreds of cancer patients with a wide variety of wild-type viruses over the last century, but the approach was abandoned due to toxicity. However, with the advance of recombinant DNA technology, it became possible to genetically engineer viruses to enhance their safety and antitumoral potency. Conditionally replicating adenoviruses (CRAds) represent a promising new platform for the treatment of malignant tumors. Recently, the promoter of human telomerase reverse transcriptase (hTERT) was used to restrict adenoviral replication to telomerase-positive cancer cells through controlling the adenoviral E1a gene. Compared with other promoters, the hTERT promoter is highly active in more than 85% of different human cancers, but inactive in most normal somatic cells, therefore. this mechanism can be applied to a wide range of cancers. In this study, the authors tested the antitumor activity of the hTERT promoter regulated tumor-selective RCAd CNHK300, and confirmed its highly efficient antitumor activity in vitro in HCC cell lines.
In recent years, two major strategies have come into force. In the first one, viral genes that become dispensable in tumor cells, such as the genes responsible for activating the cell cycle through p53 or Rb binding, have been completely or partially deleted. In the second strategy, transcription of viral genes has been controlled by replacing the native viral promoters with tumor-specific promoters. McCormick's group at Onyx proposed that an E1b 55 kDa deleted adenovirus Onyx-015 would replicate selectively in p53-deficient cells. Recently, the progress made in the study of tumor-specific promoter regulated replication-selective adenovirus has proved that modified adenovirus using this strategy has some advantages compared with Onyx-015.
Tumor specificity is a major challenge in viral therapy. For adenovirus, one strategy is the development of promoter-driven replicative adenovirus to achieve selective-replication adenovirus. The promoters of AFP, MUC-1 and PSA have been studied to control the adenovirus E1A expression targeting hepatocellular, breast and prostate carcinomas, respectively. In CNHK300, the E1A gene was placed under the control of the human hTERT promoter to allow us to target a wide range of telomerase-positive tumors with little influence on the growth of normal cells.
The results demonstrated CNHK300 could replicate in liver cancer cells efficiently as well as wtAd5 and kill heptocellular carcinoma, but had severely attenuated proliferation and cytolysis in normal cells. It may has a strong potential for targeting therapy of a wide range of cancers including hepatocellular carcinoma.
This is a paper worthy of publishing. The design of the study in all aspects is methodologically sound. hTERT promoter-regulated replicative adenovirus CNHK300 can specifically kill a wide range of cancer cells including HCC. This new cancer therapy strategy may be proven to be very promising in clinical practice.
| 1. | Russell WC. Update on adenovirus and its vectors. J Gen Virol. 2000;81:2573-2604. [Cited in This Article: ] |
| 2. | Vile RG, Russell SJ, Lemoine NR. Cancer gene therapy: hard lessons and new courses. Gene Ther. 2000;7:2-8. [Cited in This Article: ] |
| 3. | Ring CJ. Cytolytic viruses as potential anti-cancer agents. J Gen Virol. 2002;83:491-502. [Cited in This Article: ] |
| 4. | Kasuya H, Nishiyama Y, Nomoto S, Goshima F, Takeda S, Watanabe I, Nomura N, Shikano T, Fujii T, Kanazumi N. Suitability of a US3-inactivated HSV mutant (L1BR1) as an oncolytic virus for pancreatic cancer therapy. Cancer Gene Ther. 2007;14:533-542. [Cited in This Article: ] |
| 5. | Wollmann G, Robek MD, van den Pol AN. Variable deficiencies in the interferon response enhance susceptibility to vesicular stomatitis virus oncolytic actions in glioblastoma cells but not in normal human glial cells. J Virol. 2007;81:1479-1491. [Cited in This Article: ] |
| 6. | Lamfers ML, Fulci G, Gianni D, Tang Y, Kurozumi K, Kaur B, Moeniralm S, Saeki Y, Carette JE, Weissleder R. Cyclophosphamide increases transgene expression mediated by an oncolytic adenovirus in glioma-bearing mice monitored by bioluminescence imaging. Mol Ther. 2006;14:779-788. [Cited in This Article: ] |
| 7. | Kurihara T, Brough DE, Kovesdi I, Kufe DW. Selectivity of a replication-competent adenovirus for human breast carcinoma cells expressing the MUC1 antigen. J Clin Invest. 2000;106:763-771. [Cited in This Article: ] |
| 8. | Dilley J, Reddy S, Ko D, Nguyen N, Rojas G, Working P, Yu DC. Oncolytic adenovirus CG7870 in combination with radiation demonstrates synergistic enhancements of antitumor efficacy without loss of specificity. Cancer Gene Ther. 2005;12:715-722. [Cited in This Article: ] |
| 9. | Small EJ, Carducci MA, Burke JM, Rodriguez R, Fong L, van Ummersen L, Yu DC, Aimi J, Ando D, Working P. A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol Ther. 2006;14:107-117. [Cited in This Article: ] |
| 10. | Ren XW, Liang M, Meng X, Ye X, Ma H, Zhao Y, Guo J, Cai N, Chen HZ, Ye SL. A tumor-specific conditionally replicative adenovirus vector expressing TRAIL for gene therapy of hepatocellular carcinoma. Cancer Gene Ther. 2006;13:159-168. [Cited in This Article: ] |
| 11. | Poole JC, Andrews LG, Tollefsbol TO. Activity, function, and gene regulation of the catalytic subunit of telomerase (hTERT). Gene. 2001;269:1-12. [Cited in This Article: ] |
| 12. | Koga S, Hirohata S, Kondo Y, Komata T, Takakura M, Inoue M, Kyo S, Kondo S. A novel telomerase-specific gene therapy: gene transfer of caspase-8 utilizing the human telomerase catalytic subunit gene promoter. Hum Gene Ther. 2000;11:1397-1406. [Cited in This Article: ] |
| 13. | Bilsland AE, Anderson CJ, Fletcher-Monaghan AJ, McGregor F, Evans TR, Ganly I, Knox RJ, Plumb JA, Keith WN. Selective ablation of human cancer cells by telomerase-specific adenoviral suicide gene therapy vectors expressing bacterial nitroreductase. Oncogene. 2003;22:370-380. [Cited in This Article: ] |
| 14. | Takeuchi H, Kanzawa T, Kondo Y, Komata T, Hirohata S, Kyo S, Kondo S. Combination of caspase transfer using the human telomerase reverse transcriptase promoter and conventional therapies for malignant glioma cells. Int J Oncol. 2004;25:57-63. [Cited in This Article: ] |
| 15. | Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373-376. [Cited in This Article: ] |
| 16. | Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, Gore M, Ironside J, MacDougall RH, Heise C. a controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879-885. [Cited in This Article: ] |
| 17. | Rothmann T, Hengstermann A, Whitaker NJ, Scheffner M, zur Hausen H. Replication of ONYX-015, a potential anticancer adenovirus, is independent of p53 status in tumor cells. J Virol. 1998;72:9470-9478. [Cited in This Article: ] |
| 18. | Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787-791. [Cited in This Article: ] |
| 19. | Kim SJ, Lee HS, Shin JH, Kim CG, Jeong S, Park K, Choe H, Lee H. Preferentially enhanced gene expression from a synthetic human telomerase reverse transcriptase promoter in human cancer cells. Oncol Rep. 2006;16:975-979. [Cited in This Article: ] |
| 20. | Braunstein I, Cohen-Barak O, Shachaf C, Ravel Y, Yalon-Hacohen M, Mills GB, Tzukerman M, Skorecki KL. Human telomerase reverse transcriptase promoter regulation in normal and malignant human ovarian epithelial cells. Cancer Res. 2001;61:5529-5536. [Cited in This Article: ] |
| 21. | Murofushi Y, Nagano S, Kamizono J, Takahashi T, Fujiwara H, Komiya S, Matsuishi T, Kosai K. Cell cycle-specific changes in hTERT promoter activity in normal and cancerous cells in adenoviral gene therapy: a promising implication of telomerase-dependent targeted cancer gene therapy. Int J Oncol. 2006;29:681-688. [Cited in This Article: ] |
| 22. | Su CQ, Sham J, Xue HB, Wang XH, Chua D, Cui ZF, Peng LH, Li LF, Jiang LH, Wu MC. Potent antitumoral efficacy of a novel replicative adenovirus CNHK300 targeting telomerase-positive cancer cells. J Cancer Res Clin Oncol. 2004;130:591-603. [Cited in This Article: ] |
| 23. | Irving J, Wang Z, Powell S, O’Sullivan C, Mok M, Murphy B, Cardoza L, Lebkowski JS, Majumdar AS. Conditionally replicative adenovirus driven by the human telomerase promoter provides broad-spectrum antitumor activity without liver toxicity. Cancer Gene Ther. 2004;11:174-185. [Cited in This Article: ] |
| 24. | Huang TG, Savontaus MJ, Shinozaki K, Sauter BV, Woo SL. Telomerase-dependent oncolytic adenovirus for cancer treatment. Gene Ther. 2003;10:1241-1247. [Cited in This Article: ] |
| 25. | Wirth T, Zender L, Schulte B, Mundt B, Plentz R, Rudolph KL, Manns M, Kubicka S, Kuhnel F. A telomerase-dependent conditionally replicating adenovirus for selective treatment of cancer. Cancer Res. 2003;63:3181-3188. [Cited in This Article: ] |
| 26. | Kawabata K, Sakurai F, Koizumi N, Hayakawa T, Mizuguchi H. Adenovirus vector-mediated gene transfer into stem cells. Mol Pharm. 2006;3:95-103. [Cited in This Article: ] |
| 27. | Ryan PC, Jakubczak JL, Stewart DA, Hawkins LK, Cheng C, Clarke LM, Ganesh S, Hay C, Huang Y, Kaloss M. Antitumor efficacy and tumor-selective replication with a single intravenous injection of OAS403, an oncolytic adenovirus dependent on two prevalent alterations in human cancer. Cancer Gene Ther. 2004;11:555-569. [Cited in This Article: ] |
| 28. | Chen Y, DeWeese T, Dilley J, Zhang Y, Li Y, Ramesh N, Lee J, Pennathur-Das R, Radzyminski J, Wypych J. CV706, a prostate cancer-specific adenovirus variant, in combination with radiotherapy produces synergistic antitumor efficacy without increasing toxicity. Cancer Res. 2001;61:5453-5460. [Cited in This Article: ] |
| 29. | Banerjee NS, Rivera AA, Wang M, Chow LT, Broker TR, Curiel DT, Nettelbeck DM. Analyses of melanoma-targeted oncolytic adenoviruses with tyrosinase enhancer/promoter-driven E1A, E4, or both in submerged cells and organotypic cultures. Mol Cancer Ther. 2004;3:437-449. [Cited in This Article: ] |