INTRODUCTION
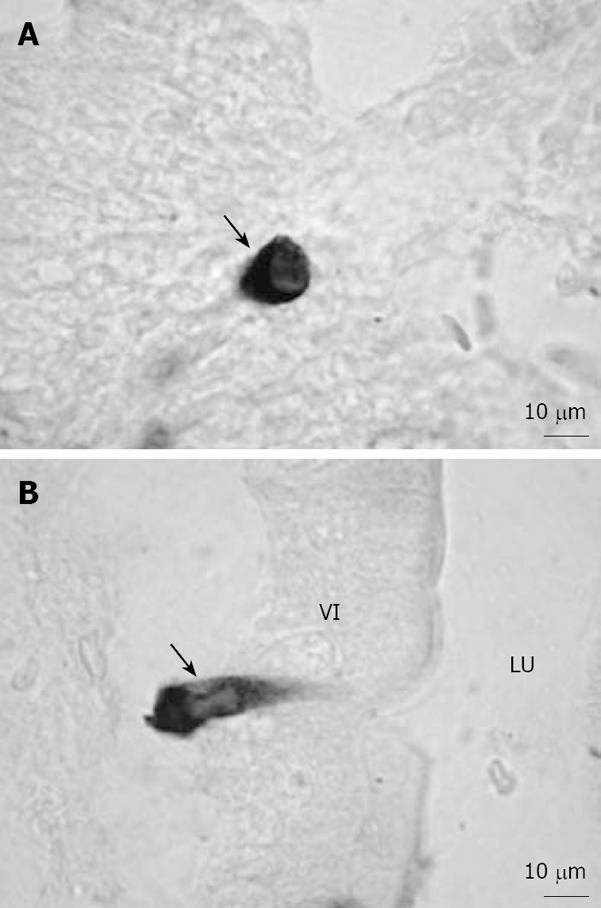
Figure 1 Representative microphotographs of ghrelin-ip cells in the rat gastrointestinal tract[16].
A: Closed-type ghrelin cells (arrow) were found in the crypt of the duodenum; B: Opened-type ghrelin cells (arrow) in contact with the lumen were found in the villi of the duodenum. Bar = 10 μm. VI: Villi; LU: Lumen.
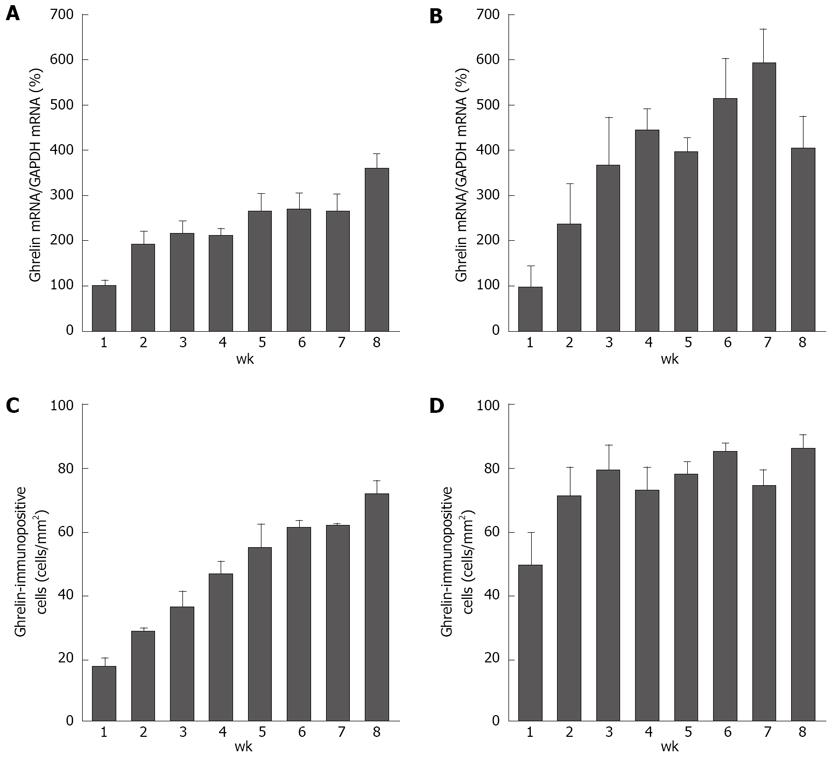
Figure 2 Changes in ghrelin mRNA levels and the densities of ghrelin-ip cells during the postneonatal period in the rat stomach[20].
The data in (A) and (B) are shown as the % of 1-wk-old (1 wk) rats (100%) and each bar represents the mean ± SE (n = 3). A: Ghrelin mRNA levels in the male stomach; B: Ghrelin mRNA levels in the female stomach; C: The densities of ghrelin-ip cells (cells/mm2) in the male stomach; D: The densities of ghrelin-ip cells (cells/mm2) in the female stomach.
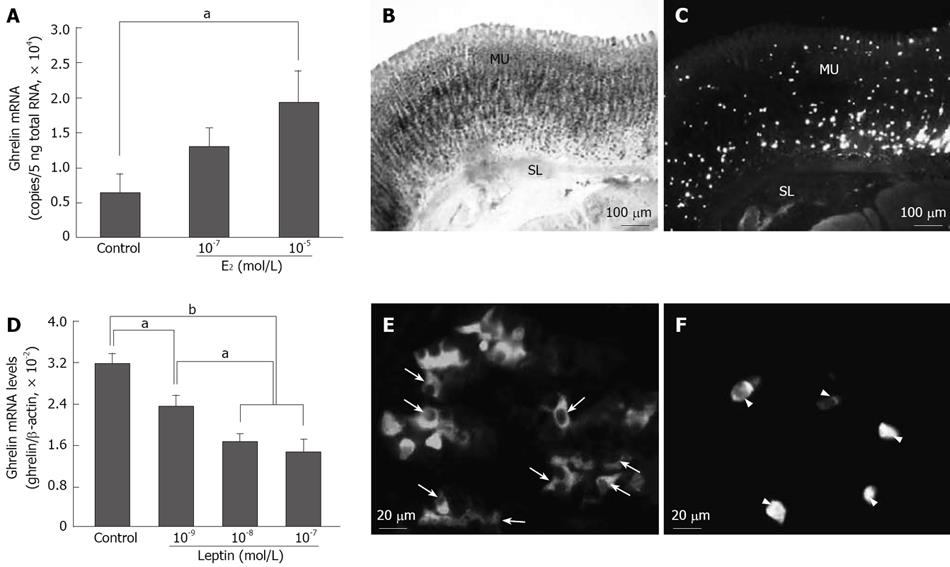
Figure 3 In vitro effects of estrogen and leptin on ghrelin mRNA expression and distributions of aromatase mRNA-expressing, leptin-ip and ghrelin-ip cells in the gastric mucosa[23,27].
A: Changes in ghrelin mRNA expression after estrogen treatment; B: Aromatase mRNA-expressing cells were found in the glandular body of the fundic gland; C: Ghrelin-ip cells were found sporadically throughout the gastric mucosa; D: Changes in ghrelin mRNA expression after leptin treatment. Ghrelin mRNA level is expressed relative to β-actin mRNA level; E: Leptin-ip cells (arrow) were found in the lower half of the fundic gland; F: Ghrelin-ip cells (arrowhead) were found in the gastric mucosa. (A, D) Data are presented as mean ± SE. n = 3-4/group. aP < 0.05; bP < 0.01. Bar = 100 μm (B, C) and 20 μm (E, F) respectively. MU: Mucosa; SL: Smooth muscle layer.
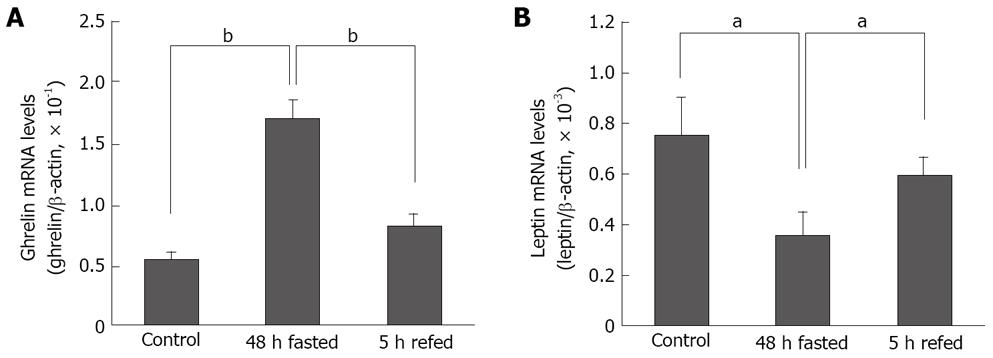
Figure 4 mRNA expression levels of gastric ghrelin and leptin under different feeding conditions[27].
(A) Effects of 48 h of fasting and 5 h of refeeding on gastric ghrelin expression; (B) Effects of 48 h of fasting and 5 h of refeeding on gastric leptin expression. Ghrelin and leptin mRNA levels are expressed relative to β-actin mRNA levels. Data are presented as mean ± SE. n = 3-4/group. aP < 0.05; bP < 0.01.
Ghrelin, a 28-amino-acid peptide with an essential n-octanoyl modification on the third amino acid, was purified as an endogenous ligand for the growth hormone (GH) secretagogue receptor from rat stomach in 1999[1]. In initial studies, ghrelin was shown to stimulate GH release from the pituitary both in vivo and in vitro[1,2], and ghrelin was later found to be also deeply involved in the regulation of feeding behavior and energy homeostasis[3-7].
Ghrelin is predominantly produced in the stomach[1]. Date et al[8] further revealed that X/A-like cells in the stomach were responsible for ghrelin production. They also found that in the gastrointestinal tract, the greatest amount of ghrelin was in the gastric mucosa, and smaller amounts were in the small and large intestines[8]. However, the detailed distribution and morphological characteristics of ghrelin cells in the whole gastrointestinal tract have not been elucidated.
On the other hand, it has been reported that both the expression and secretion of ghrelin are mainly influenced by changes in energy balance, i.e. increased by fasting and decreased after refeeding[3,9-11]. In addition, many other factors such as leptin, insulin, somatostatin and vagal activity have also been shown to be involved in the regulation of stomach ghrelin[9,12-14]. However, the factor that directly regulates ghrelin expression or production remains unclear, and little is known about the regulation of gastric ghrelin expression in various physiological states.
Therefore, in this review, we summarize the data obtained by our group regarding the distribution and morphological characteristics of gastrointestinal ghrelin cells, postnatal changes in stomach ghrelin, and the regulation of stomach ghrelin in some physiological states.
DISTRIBUTION OF GHRELIN CELLS IN THE RAT GASTROINTESTINAL TRACT
It was found that plasma ghrelin-like immunoreactivity levels were reduced by 65% in totally gastrectomized patients, suggesting that the stomach is the major source of circulating ghrelin[15]. To investigate the morphological characteristics and distribution of ghrelin cells, we used anti-acylated rat ghrelin antiserum to detect ghrelin-immunopositive (ip) cells in the rat gastrointestinal tract in a previous study[16]. We found that ghrelin cells were present throughout the whole gastrointestinal mucosa from the stomach to the colon, and that they could be classified into two types, i.e. closed-type cells (Figure 1A) and lumen-contacted opened-type cells (Figure 1B). Interestingly, in ghrelin cells, des-acylated ghrelin was found to be mainly localized to the perinucleus, while acylated ghrelin was found to be distributed in the periphery of the cytoplasm. These findings suggest that des-acylated ghrelin, which is from the Golgi complex, undergoes acylation in secretory granules in the periphery of the cytoplasm.
Further morphometric analysis revealed that the largest number of ghrelin cells was in the stomach and the next-largest number was in the duodenum, and very small numbers of ghrelin cells were observed in the ileum, cecum and colon[16]. These findings are in agreement with the results of another study regarding gastrointestinal ghrelin content[8]. On the other hand, in the stomach, very few opened-type ghrelin cells were observed; but it was found that the percentages of opened-type ghrelin cells in all ghrelin cells in various regions of the gastrointestine gradually increased in the direction from the stomach to the lower intestine, being particularly high in the ileum, cecum and colon[16]. It is generally accepted that opened-type endocrine cells in the gastrointestinal tract are mainly regulated by luminal signals, whereas closed-type cells in the gastrointestinal tract receive modulation from hormones, neuronal stimulation or mechanical distension[17]. Therefore, the distinct distributions of opened- and closed-type ghrelin cells in the gastrointestine suggest that the ghrelin cells may be modulated by different stimulators and may play different physiological roles in various regions of the gastrointestinal tract.
POSTNATAL CHANGES IN STOMACH GHRELIN
In a recent study, the mRNA expression level of gastric ghrelin was shown to be elevated progressively during the second and third postnatal weeks[18]. Gualillo et al[19] also reported a gradual increase in the expression level of the gastric ghrelin gene after birth in the rat (up to 90 days). In agreement with these results, we found that gastric ghrelin expression was detectable just after birth in both male and female rats, and that expression levels of gastric ghrelin gradually increased during postnatal development (up to 8 wk of age) (Figure 2A and B).
Accordingly, ghrelin-ip and ghrelin mRNA-expressing cells were also observed just after birth[20]. At 1 wk of age, these ghrelin cells were mainly localized in the glandular base of the fundic gland, and then the distribution of ghrelin cells gradually extended from the glandular base to the glandular neck with increasing age in both sexes[20]. An interesting finding in that study was that two kinds of stained ghrelin cells, weakly stained and strongly stained cells, were found in female rats at the early stage of development (1 and 3 wk of age), whereas staining in most of the ghrelin cells was strong in male rats and 7-wk-old female rats[20]. With increasing age, weakly stained cells were replaced by strongly stained cells, resulting in almost no change in the density of ghrelin cells in female rats throughout the whole period of postnatal development (Figure 2D), in contrast to the increase in ghrelin mRNA expression level. On the other hand, in male rats, ghrelin cell density showed an age-dependent increase after birth (Figure 2C), and the increase was in concert with the increase in ghrelin expression level. The sexual dimorphism of ghrelin cell density suggests that ghrelin cells in female rats differentiate at an earlier stage of development than they do in male rats, and that sex steroids such as estrogen may be involved in the regulation of stomach ghrelin.
REGULATION OF STOMACH GHRELIN BY GASTRIC ESTROGEN AND LEPTIN IN SOME PHYSIOLOGICAL STATES
Regulatory role of gastric estrogen
A role of estrogen in the regulation of stomach ghrelin has also been suggested by several studies. In a previous study, the levels of gastric ghrelin mRNA and plasma ghrelin and the number of ghrelin cells were found to be transiently increased by ovariectomy in female rats[21]. In addition, it was found that ghrelin cells express estrogen receptor α (ERα)[21]. On the other hand, Ueyama et al[22] recently demonstrated that aromatase, an estrogen synthetase, is expressed in parietal cells of the rat stomach and that gastric parietal cells are capable of producing and secreting a substantial amount of estrogen. These findings indicate that gastric estrogen plays a role in the regulation of stomach ghrelin.
Therefore, in a previous study, using isolated stomach cells, which are rich in ghrelin cells, we determined the direct effect of estrogen on stomach ghrelin expression and production[23]. In that study, we found that estrogen treatment significantly stimulated ghrelin mRNA expression (Figure 3A) and production in a dose-dependent manner and that treatment of minced stomach tissue with formestane, an aromatase inhibitor, decreased ghrelin expression level[23]. Given that a significant increase in estrogen concentration in the portal vein compared with that in the artery was observed in intact rats, but not in gastrectomized rats[22], the concentration of gastric estrogen must be higher than that of plasma estrogen. Moreover, neither the gastric ghrelin expression nor the plasma ghrelin concentration was altered by gonadectomy[23]. Furthermore, ghrelin cells and aromatase mRNA-expressing cells were found to be located close together in the gastric mucosa (Figure 3B and C), suggesting that ghrelin cells are exposed to gastric estrogen. All of these results strongly suggest that estrogen produced in the stomach directly stimulates ghrelin expression and production in the rat stomach.
Regulatory role of gastric leptin
Reciprocal circadian rhythms in circulating ghrelin and leptin levels and antagonistic hypothalamus-mediated control of appetite by ghrelin and leptin have been revealed by several studies[3,6,24]; however, results of studies on the modulation of ghrelin expression by leptin are inconsistent. One group showed that leptin administration to rats for five days stimulated gastric ghrelin mRNA expression[11], whereas another group reported the opposite results[9]. Due to these conflicting results, the role of leptin in regulation of ghrelin expression remains unclear. On the other hand, leptin was initially thought to be adipocyte-derived[25], and it has also been identified in various tissues, including the stomach[26]. The fact that the release of gastric leptin is rapidly stimulated by food intake or CCK treatment suggests that gastric leptin is involved in the short-term control of energy balance[26], although adipocyte leptin is known to be a long-term regulator of energy balance.
In contrast to the stimulatory effect of estrogen, in another study, we found that leptin inhibits both basal (Figure 3D) and estrogen-stimulated ghrelin expression in vitro[27]. The fact that both long and short forms of the leptin receptor have been identified in the human and rat gastric mucosa[28,29] strongly suggests that the gastric epithelium could be a direct target of gastric leptin. Consistent with results of these studies, mRNAs of both OB-Ra and OB-Rb were found to be expressed in the rat gastric fundus, and no inhibitory effect of leptin on ghrelin expression was observed in leptin receptor-defective Zucker fatty (fa/fa) rats, indicating that leptin inhibits ghrelin expression via the leptin receptor[27]. Furthermore, it was revealed that leptin cells were mainly located in the lower half of the gastric mucosa, where most of the ghrelin cells were tightly surrounded by leptin cells (Figure 3E and F), suggesting that gastric leptin has a paracrine role in regulation of ghrelin cells, and that ghrelin cells may be exposed to a higher concentration of gastric leptin than that of plasma leptin since leptin infusion at 0.1 nmol/L, which can mimic the plasma leptin concentration under basal conditions in rats, has been shown to be incapable of suppressing ghrelin release from the isolated rat stomach[30].
Relative changes in gastric ghrelin, estrogen and leptin levels in some physiological states
Direct regulation of stomach ghrelin by gastric estrogen and leptin raises the possibility that gastric estrogen and leptin contribute to the altered ghrelin level in some physiological states. It is generally accepted that the most important physiological state for the regulation of stomach ghrelin is fasting. Results of numerous studies have shown that levels of both ghrelin expression and secretion are increased by fasting and decreased after refeeding[3,9-11]. Therefore, in a recent study, we determined the changes in gastric estrogen and leptin levels in relation to gastric ghrelin expression level under a fasting condition[27]. In that study, however, neither the expression level of gastric aromatase nor the concentration of estrogen in the portal vein was altered by fasting[27], although the expression level of gastric ghrelin was significantly elevated as predicted (Figure 4A). In contrast, both gastric leptin expression (Figure 4B) and concentrations were found to be significantly decreased by fasting[27]. In addition, refeeding of fasted animals induced an increase in gastric leptin expression level (Figure 4B), which was also opposite to the decreased gastric ghrelin expression level after food intake (Figure 4A).
In another study, relative changes in expressions of gastric ghrelin, aromatase and leptin in postnatal rats were investigated. We found that gastric ghrelin expression levels significantly increased from 2 wk of age to 4 wk of age. Similarly, gastric aromatase expression level was also significantly elevated in 4-wk-old rats compared with the level in 2-wk-old rats. In contrast, gastric leptin expression level decreased from 2 wk of age to 4 wk of age.
Although the mechanism underlying the fluctuations in gastric estrogen and leptin levels in a certain physiological state remains to be elucidated, these fluctuations in gastric estrogen and leptin levels together with the fact that estrogen and leptin produced in the stomach directly regulate gastric ghrelin expression led us to propose a regulatory model of stomach ghrelin expression in some physiological states. Under a basal (fed) condition, the expression of gastric ghrelin is maintained at a certain level due to a balance between positive regulation from gastric estrogen and negative regulation from gastric leptin. In postnatal development, gastric estrogen level increases and gastric leptin level decreases with increasing age, resulting in gradual elevation of ghrelin expression level during postnatal development. Similarly, under a fasting condition, when fasting reduces gastric leptin level with no change in gastric estrogen level, this balance is also broken by attenuated negative regulation due to decreased gastric leptin level and finally results in increased ghrelin expression. These two models do not, however, exclude the possibility of involvement of other factors such as neural control through the vagal nerve system or other hormones inside and outside the stomach such as gastric somatostatin and insulin. But, we believe that the balance control through gastric estrogen and leptin at least partially contributes to the altered ghrelin expression level in some physiological states.
CONCLUSION
In the gastrointestinal tract, ghrelin cells were identified as opened- and closed-type cells. The greatest number of ghrelin cells was found in the stomach, and it was found that the number of opened-type cells gradually increases in the direction from the stomach to the lower intestine. Stomach ghrelin cells in female rats differentiate at an earlier stage of development than they do in male rats. Gastric estrogen and leptin directly regulate ghrelin expression and production in the stomach, and the balance control through positive regulation from gastric estrogen and negative regulation from gastric leptin contributes to the altered ghrelin expression level in some physiological states. These findings provide new insights into the physiological regulation of stomach ghrelin, and may be important for the development of methods for controlling high ghrelin expression levels in some negative energy balance states, which directly contribute to increased food intake and adiposity. However, the regulatory mechanism of ghrelin is thought to be more complicated, and may involve other factors such as vagal activity and hormones outside the stomach. Further studies are necessary to elucidate the roles of these factors.
S- Editor Xiao LL E- Editor Lin YP