Published online Oct 28, 2008. doi: 10.3748/wjg.14.6204
Revised: September 17, 2008
Accepted: September 24, 2008
Published online: October 28, 2008
AIM: To evaluate the prevalence of lactose intolerance (LI) following a load of 12.5 g in patients diagnosed as high-grade malabsorbers using the hydrogen breath test (HBT)-25.
METHODS: Ninety patients showing high-grade malabsorption at HBT-25 were submitted to a second HBT with a lactose load of 12.5 g. Peak hydrogen production, area under the curve of hydrogen excretion and occurrence of symptoms were recorded.
RESULTS: Only 16 patients (17.77%) with positive HBT-25 proved positive at HBT-12.5. Hydrogen production was lower as compared to HBT-25 (peak value 21.55 parts per million (ppm) ± 29.54 SD vs 99.43 ppm ± 40.01 SD; P < 0.001). Symptoms were present in only 13 patients. The absence of symptoms during the high-dose test has a high negative predictive value (0.84) for a negative low-dose test. The presence of symptoms during the first test was not useful for predicting a positive low-dose test (positive predictive value 0.06-0.31).
CONCLUSION: Most patients with a positive HBT-25 normally absorb a lower dose of lactose and a strict lactose restriction on the basis of a “standard” HBT is, in most instances, unnecessary. Thus, the 25 g lactose tolerance test should probably be substituted by the 12.5 g test in the diagnosis of LI, and in providing dietary guidelines to patients with suspected lactose malabsorption/intolerance.
- Citation: Argnani F, Camillo MD, Marinaro V, Foglietta T, Avallone V, Cannella C, Vernia P. Hydrogen breath test for the diagnosis of lactose intolerance, is the routine sugar load the best one? World J Gastroenterol 2008; 14(40): 6204-6207
- URL: https://www.wjgnet.com/1007-9327/full/v14/i40/6204.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6204
| LI (HBT 25) 65 patients | LM (HBT 25) 25 patients | |||
| Symptoms + | Symptoms - | Symptoms + | Symptoms - | |
| HBT 12.5 + | 5 | 7 | 0 | 4 |
| HBT 12.5 - | 8 | 45 | 0 | 21 |
The enzyme lactase phlorizin hydrolase, located at the intestinal brush border, is necessary for the hydrolysis of lactose, the main sugar in milk. Due to the genetically programmed decrease in intestinal lactase activity that occurs post-weaning (lactase non-persistence), a large proportion of the human population loses, in adult age, the possibility to digest and absorb lactose[1-3]. In Europe, its prevalence increases with a north-south and west-east gradient. Thus, about 50% of adult Italians cannot digest and absorb lactose normally[4,5].
Lactose malabsorption (LM) may be asymptomatic or induce symptoms similar to those of functional bowel disorders and irritable bowel syndrome, consisting of abdominal pain, gaseousness, flatulence and diarrhea. LM is not necessarily a predictor of the occurrence of symptoms, and the term “lactose intolerance (LI)” refers to a condition in which abdominal symptoms are experienced after the ingestion of lactose, in milk or dairy food.
The test for identifying the genotype responsible for lactase deficiency[6-8] is not widely available and its use for the diagnosis of LM is debatable. Thus, the diagnosis of LM is usually based on a positive hydrogen breath test (HBT) with an oral load of 25 g lactose (HBT-25) and is often followed by the institution of a lactose-free diet, also in those patients who do not experience abdominal symptoms. This approach is debatable, as a reduction of calcium intake below the recommended daily allowance (RDA) may ensue. Moreover, also in those cases in which symptoms are triggered by the 25 g of lactose ingested during the test, such a strict reduction of milk and dairy products is often unnecessary, as the amount of lactose administered during the test considerably exceeds the amount of lactose ingested daily, by most adults.
The present study was aimed at evaluating whether a reduction in the daily intake of milk and lactose-containing food is really necessary in subjects with LM during a standard HBT-25. To this end, we performed the HBT with an oral load of 12.5 g lactose (HBT-12.5) in a group of patients with marked LM documented by means of HBT-25. Positivity of the test, occurrence and type of symptoms during the two tests were compared.
During the period January, 2001 to May, 2004, 913 outpatients underwent a lactose tolerance test in our laboratory. The HBT was performed after 24 h on a low-fiber diet and a 12-h fasting period with an oral load of lactose at a dose of 0.5 g/kg body weight, up to a maximum of 25 g. End-alveolar air samples were collected in syringes using a modified Haldane-Priestly tube[9], prior to the administration of lactose, and thereafter every 30 min for 4 h. Hydrogen (H2) and methane (CH4) concentrations were measured in parts per million (ppm) by means of a Quintron Model DP Microlyzer gas chromatograph (Quintron Instruments, Milwaukee, WI, USA). The test was defined as “positive” when a H2 peak exceeding 20 ppm over baseline values was observed in two or more samples. Tests not fulfilling the above-mentioned criteria were defined as negative. Those patients with a negative HBT, who did not excrete increased amounts of H2 after oral administration of 20 g lactulose in a subsequent HBT (24 patients), were defined as hydrogen non-producers. A positive test identified patients with LM, irrespective of the presence or absence of abdominal symptoms. Positivity of LM was arbitrarily defined as “high-grade” when H2 excretion exceeded 70 ppm in at least two samples, and “low-grade” in all other instances.
Of the 353 patients with positive HBT-25, 147 fulfilled the above-mentioned criteria for high-grade LM. Of these, 50 were excluded from the study due to the presence of small bowel diseases, such as Crohn’s disease and celiac disease, in which medical treatment or dietary modifications could result in variations in lactase activity. The remaining 97 patients were considered eligible for entry to the study and were required to undergo a further lactose tolerance test, with a lactose load of 12.5 g. Only seven refused to enter the study (compliance 92.78%) and the test was performed, 4-12 wk after the first test, in 90 patients (12 male, 78 female, mean age 41.81 ± 15 SD years). Of these, 65 had experienced symptoms during HBT-25 and 25 had not. The excretion of gas during HBT-12.5 was quantified as: (1) peak H2 concentration; (2) area under the curve (AUC) of H2 concentration from 60 to 240 min, calculated with the triangular rule and expressed in arbitrary units of ppm/h. During the test, occurrence and type of symptoms were recorded.
Data were analyzed using the χ2 test, the inference between proportions and the t-test for paired data, when appropriate.
Only 16 (17.7%) of the 90 patients enrolled still had a positive test during the HBT-12.5 while the remaining 74 (82.3%) were negative. The difference between HBT-12.5 and HBT-25, evaluated by means of the inference between proportions, was highly significant (P < 0.001). Of the 65 LI patients, only five experienced symptoms during the HBT-12.5, while another seven had a positive test, but reported no symptoms. Of the 25 patients with LM, only four had a positive test after an oral load of 12.5 g lactose. None reported symptoms.
Considering all 90 patients together, the mean value of peak H2 excretion during HBT-12.5 was 21.55 ppm ± 29.54 SD, whereas in HBT-25, the mean peak H2 excretion was 99.43 ppm ± 40.01 SD. As expected, the difference from HBT-25 was highly significant (P < 0.001). Considering only the data from the 16 patients who proved positive in both tests, the peak H2 excretion was 97.68 ppm ± 27.37 during HBT-25 and 69 ppm ± 36.53 SD during HBT-12.5. Thus, even in those patients who had LM during HBT-12.5, the amount of hydrogen excretion was significantly lower as compared to the first test (P < 0.01). No difference was found between patients who had symptoms during the test (LI) and those who were LM, but did not experience symptoms, as far as concerning the peak H2 excretion.
The amount of H2 excreted by the entire population of 90 patients was 18 ppm/h ± 27.12 SD in the HBT-12.5 compared to 97.08 ppm/h ± 40.56 SD in the HBT-25 (P < 0.001). Again, taking into account only data from the 16 patients who proved positive in both tests, the amount of H2 excreted during HBT-12.5, the AUC was significantly lower compared to that of the HBT-25 (54.29 ppm/h ± 41.23 SD vs 99.21 ppm/h ± 35.58 SD, respectively; P < 0.01). Again no difference was observed between LI and LM.
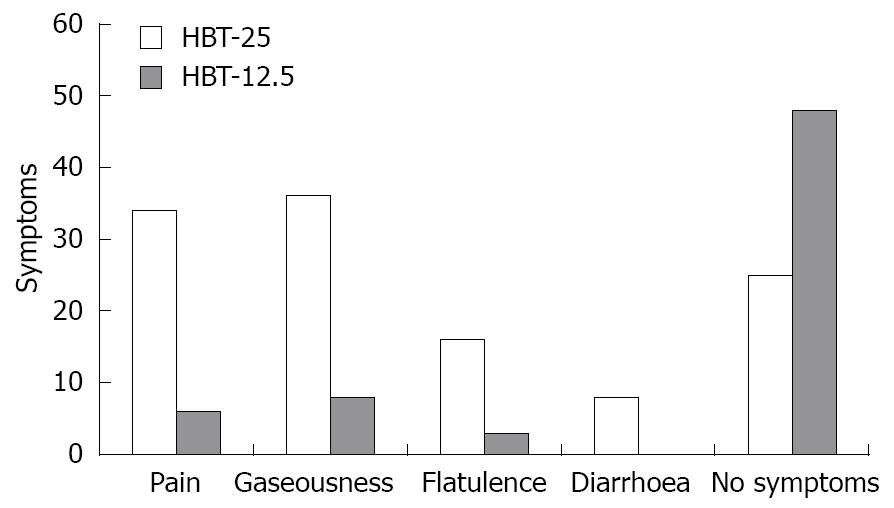
During the test with 25 g lactose in the 65 patients with LI, gaseousness was present in 36 (55.3%), abdominal pain in 34 (52.3%), flatulence in 16 (24.6%) and diarrhea in eight (13.8%), with some patients reporting more than one symptom (Figure 1). Only five patients with a positive HBT-12.5 experienced symptoms, namely gaseousness in three, flatulence in three and abdominal pain in one. None experienced diarrhea. Eight patients with a negative HBT-12.5 reported experiencing minor symptoms, consisting of gaseousness in five and abdominal pain in five. The relationship between positivity/negativity of the low-dose test (HBT-12.5) and occurrence of symptoms during HBT 12.5 is reported in Table 1.
Due to the small number of patients who proved positive during the low-dose test, positivity of HBT-25 does not help predict a positive HBT-12.5 (positive predictive value: 0.17). The occurrence of any symptom during HBT-25 showed only a slightly better positive predictive value for a positive HBT-12.5 (positive predictive value: 0.41). Taking into consideration the occurrence of individual symptoms during HBT-25, the positive predictive value was 0.06 for abdominal pain, 0.25 for gaseousness, 0.31 for flatulence and 0.12 for diarrhea. On the other hand, the absence of abdominal symptoms during HBT-25 had a negative predictive value of 0.84.
The diagnosis of LM is usually based upon the positivity of HBT after an oral load of lactose[10-15]. The most commonly used load of lactose is 20-25 g, corresponding to an intake of 400-500 mL of milk, which is rarely ingested in a single dose. Indeed, 400-500 mL of milk exceeds in most instances, the total daily intake of milk and diary products. As HBT has been found to correlate with lactase activity in duodenal biopsies, the HBT-25 is, indeed, useful for population studies[16,17]. HBT, however, is used in the clinical setting with the primary aim of diagnosing LM and LI, the rate of positive tests depending not only upon the degree of hypolactasia, but also the amount in the oral load used for the test. Moreover, the prevalence of symptoms, which is clinically relevant and of great importance for the patient, is dose-related. Thus, the traditional test with 25 g lactose likely overestimates the prevalence of LI. This may lead to unnecessary restrictions in the intake of foods that represent the main source of dietary calcium[18,19]. The present study was aimed at evaluating whether, and to what extent, the use of an oral load of 12.5 g lactose, instead of 25 g, could influence the prevalence of positive tests for diagnosing LI and LM. The present data confirm, in a large series of patients, previous observations showing that high loads of lactose (50 g, corresponding to 1 L of milk) induce abdominal pain and diarrhea in most lactose malabsorbers[20]. Conversely, small amounts of the sugar were usually well tolerated[21-24]. The present data indicates that the absence of abdominal symptoms during an HBT-25 is, in most instances, associated with a negative HBT-12.5. Unexpectedly, the presence of symptoms during HBT-25 was not useful for predicting a positive HBT-12.5. Less than 50% of the patients with abdominal symptoms (LI) display malabsorption of lactose in detectable amounts when the sugar load is reduced and the occurrence of symptoms are relatively rare. Thus, a moderate intake of lactose during a standard HBT-25 may prove harmless in the large majority of patients diagnosed as LI or LM[25].
Interestingly, during the HBT-12.5, eight patients reported symptoms despite a negative test with prevalence similar to that observed in a previous study performed in normal subjects and in patients with irritable bowel syndrome[26-29]. In the present series, symptoms consisted of gaseousness and mild abdominal pain, whereas none of the patients had diarrhea. As patients were asked to report even minor symptoms, a “nocebo”, or “inverse placebo”, effect may have been elicited by the investigators.
Finally, false-negative results cannot be completely ruled out in these patients, due to a better sensitivity of late (> 240 min) increases in hydrogen excretion, as suggested by Di Stefano et al[30]. These data, however, are debatable as these authors, using different hydrogen cutoff levels, considered definitely as lactose intolerant with a false-negative breath test those patients reporting symptoms during the HBT, irrespective of the test results. This is unlikely, as negative expectations often induce non-specific abdominal symptoms not only during lactose HBT, but also after a sham lactose load (personal unpublished data).
In conclusion, these data reaffirm that LI is dose-dependent. Considering the daily mean lactose intake in the general population, 50 or 25 g lactose tolerance breath tests may prove useful for epidemiological studies, looking for lactose deficiency. The widespread availability of genetic testing for lactase polymorphism may render obsolete this technique. Conversely, in the clinical setting, the use of the 12.5 g lactose tolerance test should be probably preferred to the 25 g test, at least in Caucasians and in the populations of the Mediterranean basin, as it may help to identify those patients who would profit from dietary restriction of lactose-containing food, minimizing the risk of inappropriately reducing calcium intake to those who do not need it.
Hydrogen breath test (HBT), after a lactose load of 25-50 g is widely used in the clinical setting for diagnosing lactose malabsorption (LM) and, when abdominal symptoms are present, of lactose intolerance (LI). The positivity of the test often induces dietary modifications, leading to the reduction of calcium intake.
The authors confirmed in a large series of patients previous findings suggesting that most patients with LM, documented by HBT, tolerate well small amounts of lactose.
The present data indicates that an oral load of 12.5 of lactose, corresponding to about 250 mL milk, is well tolerated by the majority of patients unable to completely digest and absorb 25 g lactose. Moreover, in the majority of them, an increased excretion of hydrogen was not documented after ingesting an isoosmolar solution of 12.5 g of lactose, indicating that they can normally digest lactose at least up to a dose corresponding to 250 mL milk.
The authors suggest that 12.5 g lactose HBT should be preferred to the usual oral load of 25-50 g, in order to identify those patients who could really profit from a reduction of lactose-containing food, and minimize the risk of unnecessary reductions of calcium intake.
HBTs are indicated by HBT. The positivity of the test defines a subject’s LM, irrespective of the occurrence of abdominal symptoms. The coincident occurrence of symptoms is required for defining LI patients.
This is an interesting study in which the authors argue that the use of 25 g of lactose to test for LM may be inappropriate, as this is higher than the average dietary intake, and the removal of lactose from the diet may have other deleterious consequences such as reduced calcium intake.
Peer reviewer: Phillip S Oates, PhD, Department of Physiology, School of Biomedical and Chemical Sciences, the University of Western Australia, Western Australia 6009, Australia
S- Editor Li DL L- Editor Rippe RA E- Editor Lin YP
| 1. | Gilat T, Russo S, Gelman-Malachi E, Aldor TA. Lactase in man: a nonadaptable enzyme. Gastroenterology. 1972;62:1125-1127. [Cited in This Article: ] |
| 2. | Simoons FJ. The geographic hypothesis and lactose malabsorption. A weighing of the evidence. Am J Dig Dis. 1978;23:963-980. [Cited in This Article: ] |
| 3. | Friedl J. Lactase deficiency: distribution, associated problems and implications for nutritional policy. Ecol Food Nutr. 1981;11:37-48. [Cited in This Article: ] |
| 4. | Burgio GR, Flatz G, Barbera C, Patane R, Boner A, Cajozzo C, Flatz SD. Prevalence of primary adult lactose malabsorption and awareness of milk intolerance in Italy. Am J Clin Nutr. 1984;39:100-104. [Cited in This Article: ] |
| 5. | Bozzani A, Penagini R, Velio P, Camboni G, Corbellini A, Quatrini M, Conte D, Bianchi PA. Lactose malabsorption and intolerance in Italians. Clinical implications. Dig Dis Sci. 1986;31:1313-1316. [Cited in This Article: ] |
| 6. | Enattah NS, Sahi T, Savilahti E, Terwilliger JD, Peltonen L, Jarvela I. Identification of a variant associated with adult-type hypolactasia. Nat Genet. 2002;30:233-237. [Cited in This Article: ] |
| 7. | Bodlaj G, Stocher M, Hufnagl P, Hubmann R, Biesenbach G, Stekel H, Berg J. Genotyping of the lactase-phlorizin hydrolase -13910 polymorphism by LightCycler PCR and implications for the diagnosis of lactose intolerance. Clin Chem. 2006;52:148-151. [Cited in This Article: ] |
| 8. | Szilagyi A, Malolepszy P, Hamard E, Xue X, Hilzenrat N, Ponniah M, MacNamara E, Chong G. Comparison of a real-time polymerase chain reaction assay for lactase genetic polymorphism with standard indirect tests for lactose maldigestion. Clin Gastroenterol Hepatol. 2007;5:192-196. [Cited in This Article: ] |
| 9. | Metz G, Gassull MA, Leeds AR, Blendis LM, Jenkins DJ. A simple method of measuring breath hydrogen in carbohydrate malabsorption by end-expiratory sampling. Clin Sci Mol Med. 1976;50:237-240. [Cited in This Article: ] |
| 10. | Levitt MD, Donaldson RM. Use of respiratory hydrogen (H2) excretion to detect carbohydrate malabsorption. J Lab Clin Med. 1970;75:937-945. [Cited in This Article: ] |
| 11. | Solomons NW, Viteri FE, Hamilton LH. Application of a simple gas chromatographic technique for measuring breath hydrogen. J Lab Clin Med. 1977;90:856-862. [Cited in This Article: ] |
| 12. | Corazza GR, Sorge M, Strocchi A, Lattanzi MC, Benati G, Gasbarrini G. Methodology of the H2 breath test. II. Importance of the test duration in the diagnosis of carbohydrate malabsorption. Ital J Gastroenterol. 1990;22:303-305. [Cited in This Article: ] |
| 13. | Levitt MD, Bond JH Jr. Volume, composition, and source of intestinal gas. Gastroenterology. 1970;59:921-929. [Cited in This Article: ] |
| 14. | Corazza GR, Sorge M, Maurino E, Strocchi A, Lattanzi MC, Gasbarrini G. Methodology of the H2 breath test. I. Collection and storage for gas measurement. Ital J Gastroenterol. 1990;22:200-204. [Cited in This Article: ] |
| 15. | Romagnuolo J, Schiller D, Bailey RJ. Using breath tests wisely in a gastroenterology practice: an evidence-based review of indications and pitfalls in interpretation. Am J Gastroenterol. 2002;97:1113-1126. [Cited in This Article: ] |
| 16. | Newcomer AD, McGill DB, Thomas PJ, Hofmann AF. Prospective comparison of indirect methods for detecting lactase deficiency. N Engl J Med. 1975;293:1232-1236. [Cited in This Article: ] |
| 17. | Davidson GP, Robb TA. Value of breath hydrogen analysis in management of diarrheal illness in childhood: comparison with duodenal biopsy. J Pediatr Gastroenterol Nutr. 1985;4:381-387. [Cited in This Article: ] |
| 18. | Savaiano D. Lactose intolerance: a self-fulfilling prophecy leading to osteoporosis? Nutr Rev. 2003;61:221-223. [Cited in This Article: ] |
| 19. | Prentice A. Diet, nutrition and the prevention of osteoporosis. Public Health Nutr. 2004;7:227-243. [Cited in This Article: ] |
| 20. | Shaw AD, Davies GJ. Lactose intolerance: problems in diagnosis and treatment. J Clin Gastroenterol. 1999;28:208-216. [Cited in This Article: ] |
| 21. | Vesa TH, Korpela RA, Sahi T. Tolerance to small amounts of lactose in lactose maldigesters. Am J Clin Nutr. 1996;64:197-201. [Cited in This Article: ] |
| 22. | Suarez FL, Savaiano D, Arbisi P, Levitt MD. Tolerance to the daily ingestion of two cups of milk by individuals claiming lactose intolerance. Am J Clin Nutr. 1997;65:1502-1506. [Cited in This Article: ] |
| 23. | Suarez FL, Adshead J, Furne JK, Levitt MD. Lactose maldigestion is not an impediment to the intake of 1500 mg calcium daily as dairy products. Am J Clin Nutr. 1998;68:1118-1122. [Cited in This Article: ] |
| 24. | Suarez FL, Savaiano DA, Levitt MD. A comparison of symptoms after the consumption of milk or lactose-hydrolyzed milk by people with self-reported severe lactose intolerance. N Engl J Med. 1995;333:1-4. [Cited in This Article: ] |
| 25. | Hertzler SR, Huynh BC, Savaiano DA. How much lactose is low lactose? J Am Diet Assoc. 1996;96:243-246. [Cited in This Article: ] |
| 26. | Savaiano DA, Boushey CJ, McCabe GP. Lactose intolerance symptoms assessed by meta-analysis: a grain of truth that leads to exaggeration. J Nutr. 2006;136:1107-1113. [Cited in This Article: ] |
| 27. | Vernia P, Di Camillo M, Marinaro V. Lactose malabsorption, irritable bowel syndrome and self-reported milk intolerance. Dig Liver Dis. 2001;33:234-239. [Cited in This Article: ] |
| 28. | Carroccio A, Montalto G, Cavera G, Notarbatolo A. Lactose intolerance and self-reported milk intolerance: relationship with lactose maldigestion and nutrient intake. Lactase Deficiency Study Group. J Am Coll Nutr. 1998;17:631-636. [Cited in This Article: ] |
| 29. | Vernia P, Di Camillo M, Marinaro V. Lactose malabsorption, irritable bowel syndrome and self-reported milk intolerance. Dig Liver Dis. 2001;33:234-239. [Cited in This Article: ] |
| 30. | Di Stefano M, Missanelli A, Miceli E, Strocchi A, Corazza GR. Hydrogen breath test in the diagnosis of lactose malabsorption: accuracy of new versus conventional criteria. J Lab Clin Med. 2004;144:313-318. [Cited in This Article: ] |