Published online Jun 21, 2008. doi: 10.3748/wjg.14.3633
Revised: May 4, 2008
Accepted: May 11, 2008
Published online: June 21, 2008
AIM: To investigate the roles of the adipocytokines, ghrelin and leptin in gastric cancer cachexia.
METHODS: Resistin, ghrelin, leptin, adiponectin, insulin and insulin-like growth factor (IGF-I), were measured in 30 healthy subjects, and 60 gastric cancer patients of which 30 suffered from cancer-induced cachexia and 30 served as a control group. The relationships between hormones, body mass index (BMI) loss ratio, age, gender, and Glasgow Prognostic Score (GPS) were investigated.
RESULTS: Cachexia patients had higher tumor stage and GPS when compared with non-cachexia patients (P < 0.05). Ghrelin, resistin, leptin, adiponectin and IGF-I, showed a significant correlation with BMI loss ratio and GPS (P < 0.05). A strong correlation was seen between GPS and BMI loss (R = -0.570, P < 0.0001). Multivariate analysis indicated that BMI loss was significantly independent as a predictor of ghrelin, resistin, leptin and IGF-I (P < 0.05). Existence of an important significant relationship between resistin and insulin resistance was also noted.
CONCLUSION: These results showed that serum ghrelin, leptin, adiponectin, and IGF-I play important roles in cachexia-related gastric cancers. No relationship was found between resistin and cancer cachexia. Also, because of the correlation between these parameters and GPS, these parameters might be used as a predictor factor.
- Citation: Kerem M, Ferahkose Z, Yilmaz UT, Pasaoglu H, Ofluoglu E, Bedirli A, Salman B, Sahin TT, Akin M. Adipokines and ghrelin in gastric cancer cachexia. World J Gastroenterol 2008; 14(23): 3633-3641
- URL: https://www.wjgnet.com/1007-9327/full/v14/i23/3633.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3633
Cachexia, characterized by marked weight loss, anorexia, asthenia and anemia, is often associated with the presence and growth of a tumor and leads to malnutrition secondary to the induction of anorexia or decreased food intake[1]. As major mediators of metabolism; growth hormone (GH) and insulin-like growth factor-I (IGF-I) have attracted many researchers in the field of cachexia associated gastrointestinal cancer[23]. Recent evidence suggests that an intricate interplay between multiple hypothalamic effector pathways and afferent hormonal signals of diverse systemic origin (resistin, leptin and adiponectin from adipocytes, ghrelin and polypeptides from the gastrointestinal tract, and insulin from the pancreas) is important in the regulation of energy intake and expenditure[4].
Ghrelin was discovered as the peptide hormone that stimulates the release of GH from the anterior pituitary and has crucial roles in the regulation of food intake and energy homeostasis in both humans and rodents[5]. It is produced primarily by the mammalian gastric enteroendocrine cells of the oxyntic mucosa, likely the X/A-like cells[6]. It has been reported that there was an increase in the levels of total ghrelin in cachectic lung[7], breast and colon[8] cancer patients. Ghrelin infusion has recently been shown to increase appetite in subjects with cancer-induced cachexia[9]. Data on the association between ghrelin levels and gastric cancer cachexia are contradictory.
Leptin is a member of a group of adipocyte-secreted proteins, collectively known as the adipocytokines. Leptin acts in the central nervous system, particularly in the hypothalamus, to suppress food intake and stimulate energy expenditure[4]. Leptin levels were reported to be low in gastrointestinal[10] and pancreatic cancers[11], and high in breast and gynecologic cancer patients[12].
Resistin which is an 108-amino acid, 12.5-kDa peptide hormone member of the cysteine-rich secreted protein family, is also referred to as resistin-like molecules or “found in inflammatory zone” molecules. Resistin has mainly been studied in mice, in which there is compelling evidence linking the protein to insulin resistance, obesity, and type 2 diabetes mellitus[13]. There is only 55% amino acid homology between human and murine resistin, and findings have been inconclusive regarding a potential role of resistin in human insulin regulation, obesity and type 2 diabetes mellitus[14]. There is no information about the role of resistin in cancer cachexia.
Adiponectin is a member of the adipocytokine family. It is induced during adipocyte differentiation, and its secretion is stimulated by insulin and IGF-I[4]. A negative correlation between obesity and circulating adiponectin has been well established and adiponectin concentrations increase concomitantly with weight loss[1516]. Although low adiponectin levels were reported among patients with weight-loss in advanced lung cancer[16], there was no correlation between adiponectin levels and cachexia in breast and colon cancer patients[8]. A more recent study demonstrated that an impaired response of adiponectin, ghrelin, and leptin may play a role in the pathogenesis of cancer cachexia with breast and colon cancer[8]. However, the role of adiponectin in gastric cancer patients is not clearly understood.
There is increasing evidence that the presence of a systemic inflammatory response, as evidenced by elevated concentrations of C-reactive protein (CRP), is a prognostic factor independent of stage, performance status and weight loss in patients with advanced cancer. Recently, Forrest et al[1718] have shown that an elevated CRP and hypoalbuminemia may be combined to form a score, the Glasgow Prognostic score (GPS), which has prognostic value in patients with inoperable non-small-cell lung cancer.
The aim of the present study was to investigate: (1) the nature of the relationship between serum levels of resistin, leptin, adiponectin, ghrelin, and cancer related cachexia; (2) the relationship of these three hormones, insulin, IGF-I and insulin-resistance; and (3) to evaluate the relation between the hormones and GPS.
The protocol was approved by the Gazi University Medical Faculty Ethics Committee and was conducted between October 2005 and December 2006. All clinical investigations described in this paper were conducted within the guidelines mentioned in The Declaration of Helsinki. Patients with a histopathological diagnosis of gastric adenocarcinoma were included in the study, while patients with gastric lymphoma and malignant stromal tumors were excluded from the study for homogenization. The contributors were informed of the nature of the study and informed consent was obtained.
Patients were excluded if there was evidence of drug or alcohol abuse defined as any use of reactional drugs or more than two drinks per day; presence of congestive heart failure (ejection fraction < 35% on a echocardiogram or signs such as edema, dyspnea, or jugular venous distension); severe liver disease; severe chronic obstructive pulmonary disease; diabetes with hemoglobin A1c levels greater than 7%; fasting plasma glucose greater than 160 mg/dL or random glucose levels greater than 200 mg/dL; presence of thyroid diseases or renal failure; active infection (temperature > 38°C or other signs or symptoms of infection); history of neuroendocrine tumor; use of glucocorticoids, progesterone, testosterone or other orexigenic agents; history of eating disorders or dysphagia; treatment by chemotherapy, radiotherapy, or a major operation within the last 6 mo prior to hospitalization; malignancy with an obstructing lesion in the cardia or antrum together or if patients refused consent.
Control group patients were chosen from healthy people over 40 years of age, because the majority of the patients were over 50 and most of the parameters studied in the study were affected by age.
Clinical parameters obtained in the study included age, gender, BMI, cancer localization and staging, cachexia, performance status, and GPS. All pathology reports were evaluated and data on tumor histology were recorded. The extent of tumor spread was recorded using the American Joint Cancer Committee TNM Classification and Stage System. Patient height and weight were measured and BMI was calculated as body weight divided by height squared (kg/m2). Patients were defined as cachectic, based on > 10% reduction in BMI within 6 mo prior to admission, as calculated from reported weight differences given by these subjects[1]. Performance status was evaluated by using the World Health Organization (WHO) performance status.
Blood samples were drawn from each subject between 8 and 9 AM, after an overnight bed rest for measurement of the hormones, CRP, and fasting sugar, as well as a complete blood count and chemistry. All samples were stored at -80°C until analytical measurements were performed, except for glucose, which was determined immediately after blood was drawn.
Routine laboratory measurements of hemoglobin, albumin and CRP were conducted. Serum glucose was measured using the glucose oxidase method. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated from fasting insulin and glucose levels [fasting glucose (millimoles per litre X fasting insulin microunits per millilitre)/22.5] as previously described[19].
Prealbumin concentrations in the serum were determined using a commercial solid phase sandwich enzyme linked immuno-sorbent assay (ELISA) kit from Immundiagnostic® kit (Bensheim, Germany).
Resistin, leptin, adiponectin, IGF-I, and insulin concentrations in serum were determined using a commercially available ELISA kit from RayBiotech® (Norcross, GA, USA). The intra- and interassay coeffi-cients of variation were less than 7%-10% for all parameters.
For human ghrelin assessment, we used a RIA kit (Linco Research®, Missouri, USA) which incorporates an antibody that is specific for active ghrelin. The sensitivity was 100 pg/mL (in a 100 &mgr;L sample size) with a range of 100 to 10 000 pg/mL. The intra- and inter assay coefficients of variation were 5.63% and 16%, respectively.
All calculations and statistical tests were performed using SPSS (version 13.00 software, SPSS, Inc., Chicago, IL, USA). Descriptive data were expressed as mean ± SD. Categorical parameters were expressed as percentage. The study variables were compared between the study groups using Student t-tests or ANOVA for continuous variables and Fisher exact test for categorical variables. For multiple comparisons, the Tukey test was used. Pearson or Kendall’s tau-b correlation coefficient was used to determine the relationship between continuous variables. Multiple linear regression analysis was performed to ascertain independent effects of BM, after adjustment for age, gender, and differentiation of tumor on adiponectin, ghrelin, leptin, and IGF-I levels. All significance tests were two-tailed.
Sixty patients (30 cachectic and 30 non-cachectic), who satisfied study criteria, were selected from 76 gastric cancer patients admitted to our unit between the study dates.
The patient demographic characteristics are presented in Table 1. There was no significant difference between the ages of cachectic (63.6 ± 13.8), non-cachectic (55.6 ± 13.3), and control patients (56.4 ± 3.0). There was no difference with respect to gender within the three groups. While 60% of non-cachectic patients had stage 1 and 2 cancers, this rate was only 10% in cachectic patients. The majority of cachectic patients were stage 3 (40%) or 4 (50%) and in non-cachectic patients, this ratio was 25% and 15%, respectively (P = 0.007). None of the non-cachectic patients were inoperable. On the other hand, there were 2 inoperable patients (5%) in the cachectic group (P < 0.05). There was no difference between the two groups according to tumor location and differentiation (P = 0.369). While the WHO score for gastric cancer patients was significantly worse than the control group (P < 0.001), there was no significant difference between the WHO scores of cachectic and non-cachectic groups (P = 0.108). Baseline biochemical findings and changes in BMI and weights are listed in Table 2. Changes in BMI and weight were significantly higher in cachectic patients than the non-cachectic patients (P < 0.001). Hb levels of cachectic patients were significantly lower than the levels of control and non cachectic patients (P < 0.001). There were no significant differences in the levels of TSH, AST, creatinine, WBC or lymphocytes in the three groups.
| Healthy controls | Patients with gastric cancer | P value | ||
| Non-cachexia | Cachexia | |||
| Age (mean ± SD) | 56.4 ± 3.0 | 55.8 ± 13.3 | 63.6 ± 13.8 | 0.0011 |
| Gender (%) | 0.34423 | |||
| Male | 20 (67) | 25 (84) | 23 (77) | |
| Female | 10 (33) | 5 (16) | 7 (23) | |
| Tumor stage (%) | 0.0072 | |||
| I | - | 6 (20) | 0 | |
| II | - | 12 (40) | 3 (10) | |
| III | - | 8 (25) | 12 (40) | |
| IV | - | 4 (15) | 15 (50) | |
| Tumor differentiation | 0.3472 | |||
| Differentiated | 26 (87) | 23 (77) | ||
| Undifferentiated | 4 (13) | 7 (23) | ||
| Operability | 0.3512 | |||
| Operable/No operable | - | 30/0 | 28/2 | |
| Localization (%) | 0.3692 | |||
| Cardia | - | 3 (10) | 2 (6) | |
| Corpus | - | 6 (20) | 10 (34) | |
| Fundus | - | 3 (10) | 0 | |
| Antrum | 18 (60) | 18 (60) | ||
| WHO performance status (%) | 0.1082, < 0.0013 | |||
| 0 | 30 (100) | 9 (30) | 3 (10) | |
| 1 | 0 | 14 (45) | 11 (36) | |
| 2 | 0 | 6 (20) | 6 (20) | |
| 3 | 0 | 0 | 7 (24) | |
| 4 | 0 | 1 (5) | 3 (10) | |
| GPS | < 0.00123 | |||
| 0 | 30 (100) | 18 (60) | 1 (5) | |
| 1 | 0 | 12 (40) | 17 (55) | |
| 2 | 0 | 0 | 12 (40) | |
| Healthy controls (n = 30) | Patients with gastric cancer | P value (between groups) (ANOVA) | ||
| Non-cachexia (n = 30) | Cachexia (n = 30) | |||
| Initial BMI (kg/m2) | 25.3 ± 2.3 | 27 ± 5.1 | 26.3 ± 2.5 | 0.985 |
| Final BMI (kg/m2) | 26.7 ± 2.8 | 24.8 ± 5.0 | 20.3 ± 2.5a | 0.002 |
| Change BMI (%) | 1.26 ± 2.6 | -4.0 ± 2.4c | -5.7 ± 5.2a | < 0.001 |
| Initial weight (kg) | 75.4 ± 11.0 | 73.8 ± 13.3 | 70.0 ± 12.3 | 0.436 |
| Final weight (kg) | 76.2 ± 10.2 | 70.8 ± 16.3c | 53.6 ± 13.0a | 0.001 |
| Change weight (kg) | 0.15 ± 0.76 | -2.9 ± 1.5c | -10.4 ± 3.3a | < 0.001 |
| Hb (gr/L) | 12.8 ± 2.8 | 12.6 ± 1.9 | 11.1 ± 1.3a | < 0.001 |
| TSH (mIU/mL) | 1.96 ± 0.28 | 1.98 ± 0.85 | 2.11 ± 0.11 | 0.492 |
| AST (IU/L) | 23.3 ± 8.3 | 28.7± 8.2 | 26.5 ± 5.1 | 0.088 |
| Creatinine (mg/dL) | 0.81 ± 0.36 | 1.03 ± 0.27 | 0.97 ± 0.28 | 0.085 |
| WBC (× 109) | 6.9 ± 1.2 | 7.1 ± 2.1 | 7.0 ± 2.2 | 0.230 |
| Lymphocytes (%) | 22.3 ± 4.2 | 24.3 ± 3.8 | 23.98 ± 4.8 | 0.156 |
Mean ghrelin levels were significantly elevated in cachectic patients compared with non-cachectic cancer patients and healthy control subjects (2 305 ± 818 ng/mL vs 1980 ± 913 ng/mL, and 1332 ± 620 ng/mL, respectively, P = 0.013; Table 3). No significant difference in ghrelin levels between non-cachexia and healthy control groups was observed.
| Healthy controls (n = 30) | Patients with gastric cancer | P-value (between groups) (ANOVA) | ||
| Non-cachexia (n = 30) | Cachexia (n = 30) | |||
| Albumin (gr/dL) | 4.2 ± 0.2 | 4.0 ± 0.5 | 2.9± 0.2a | 0.03 |
| CRP (mg/L) | 5.95 ± 0.8 | 9.72 ± 4.3e | 12.9 ± 2.2a | < 0.001 |
| Prealbumin (ng/mL) | 52.5 ± 8.3 | 49.2 ± 6.4 | 41.0 ± 12.3a | 0.001 |
| Fasting glucose (mg/dL) | 102.7 ± 8.3 | 105.7 ± 13.7 | 114.3 ± 13.8a | 0.005 |
| Insulin (&mgr;IU/mL) | 21.8 ± 9.0 | 24.4 ± 6.3 | 18.4 ± 6.0c | 0.04 |
| HOMA-IR | 5.58 ± 2.55 | 6.58 ± 1.89 | 5.05 ±1.68 | 0.07 |
| IGF-1 (pg/mL) | 95.0 ± 30.1 | 63.1 ± 13.1e | 43.8 ± 9.5a | < 0.001 |
| Resistin (ng/mL) | 18.1 | 43.4e | 66.7a | < 0.001 |
| Ghrelin (ng/ml) | 1332 ± 620 | 1980 ± 913 | 2305 ± 818a | < 0.001 |
| Adiponectin (&mgr;g/mL) | 22.6 ± 12.4 | 27.8 ± 11.9 | 36.5 ± 15.0a | 0.045 |
| Leptin (pg/mL) | 2810 ± 818 | 2623 ± 665 | 3405 ± 640 | 0.003 |
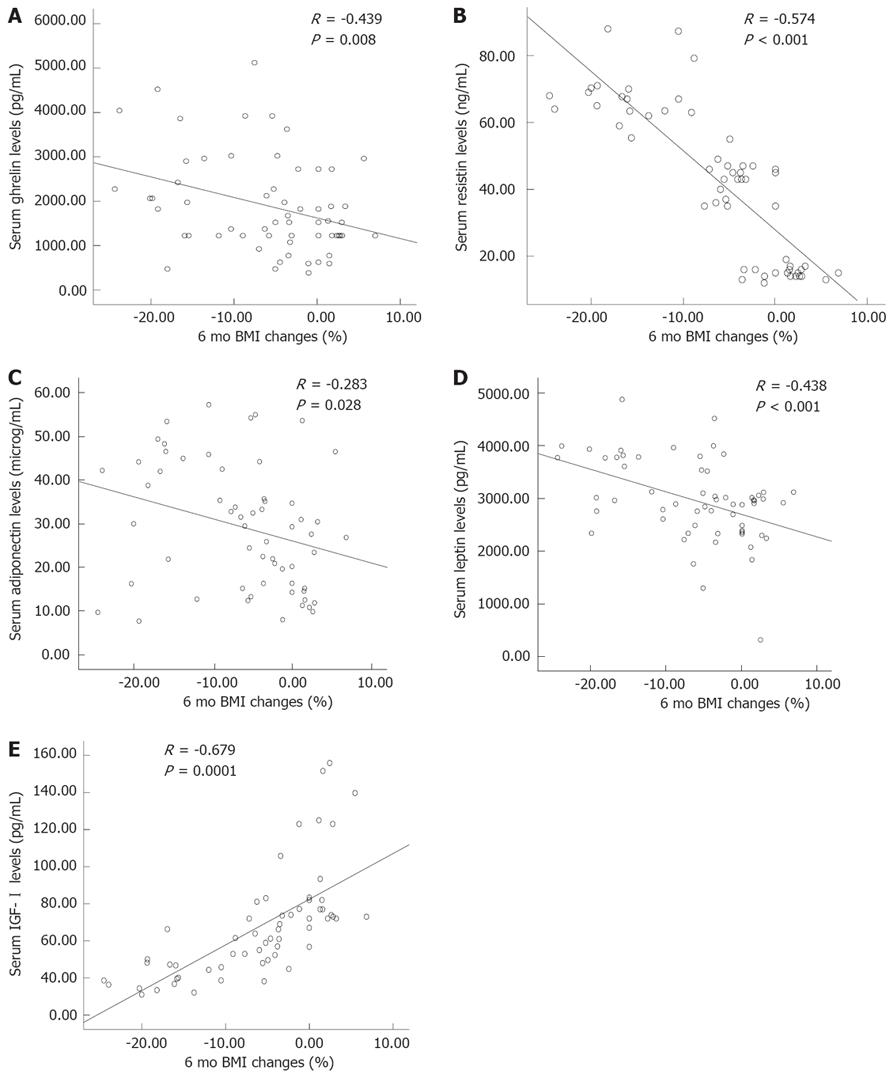
A significant negative correlation was found between the serum ghrelin levels and BMI loss in the previous 6 mo (R = -0.439, P = 0.008, Figure 1A). There was also a positive significant correlation between the serum ghrelin levels and age (R = 0.467, P = 0.039, Table 4), and GPS (R = 0.327, P = 0.002).
| Resistin | Ghrelin | Leptin | Adiponectin | IGF-I | ||
| Age | R | 0.115 | 0.267a | 0.253 | -0.092 | -0.331a |
| P | 0.687 | 0.039a | 0.051 | 0.486 | 0.010a | |
| GPS | R | 0.387a | 0.327a | 0.303a | 0.241a | 0.363a |
| P | 0.0017a | 0.002a | 0.003a | 0.019a | 0.004a | |
| Insulin | R | 0.348a | -0.126 | -0.225 | 0.197 | 0.06 |
| P | 0.016a | 0.339 | 0.084 | 0.132 | 0.905 | |
| Glucose | R | 0.418a | 0.194 | -0.324a | 0.172 | -0.358a |
| P | 0.0018a | 0.138 | 0.012a | 0.190 | 0.005a | |
| HOMA-IR | R | 0.518a | -0.100 | -0.181 | -0.094 | 0.073 |
| P | 0.0001a | 0.449 | 0.166 | 0.475 | 0.581 | |
| Prealbumin | R | -0.218 | -0.223 | -0.097 | -0.341a | 0.287a |
| P | 0.082 | 0.087 | 0.461 | 0.008a | 0.026a |
Mean resistin levels were significantly elevated in cachectic patients compared with non-cachectic cancer patients and healthy control subjects (P = 0.013). It was found that resistin levels in non-cachectic gastric cancer patients were significantly higher than the control group (P = 0.042, Table 3). A significant negative correlation was found between the serum ghrelin levels and BMI loss in the previous 6 mo (R = -0.574, P < 0.001, Figure 1B), and GPS (R = 0.387, P < 0.01, Table 4). There was also a significant correlation between the serum resistin levels and insulin (R = 0.348, P = 0.016), glucose (R = 0.418, P = 0.0018), and HOMA-IR (R = 0.518, P = 0.0001, Table 4).
The average serum adiponectin levels were 36.5 ± 15.0 &mgr;g/mL in patients with cachectic gastric cancer, 22.6 ± 12.4 &mgr;g/mL in healthy controls, and 27.8 ± 11.9 &mgr;g/mL in the non-cachexia group. Thus, there was a significant difference between the cachexia group and healthy and non-cachexia groups (P = 0.006, Table 3). However, there was no significant difference between the serum adiponectin levels of healthy and non-cachectic controls.
The association between adiponectin levels and BMI loss in the last 6 mo is plotted in Figure 1C. A significant negative correlation was found between the serum adiponectin levels and BMI changes in the previous 6 mo (R = -0.283, P = 0.028). Adiponectin showed a strong positive correlation with GPS (R = 0.241, P = 0.008).
Cachectic gastric cancer patients had significantly higher serum leptin levels than healthy controls and non-cachectic gastric cancer patients (3 405 ± 640 pg/mL vs 2623 ± 665 pg/mL and 2 810 ± 818 pg/mL, respectively, P = 0.003, Table 3). No significant differences in serum leptin levels between non-cachexia and healthy control groups were observed. A significant negative correlation was found between leptin levels and BMI loss in the previous 6 mo (R = -0.438, P < 0.001, Figure 1D). Leptin also showed a strong positive correlation with GPS (R = 0.303, P = 0.003).
Albumin and prealbumin, markers of nutritional status, were lower in gastric cancer patients when compared with healthy and non-cachectic subjects (P = 0.03, P = 0.001, respectively). The mean CRP levels were significantly higher in cachexia patients than the levels of healthy and non-cachexia controls (P < 0.001). CRP levels also increased significantly in non-cachectic and healthy subjects (P < 0.05, Table 3). The average GPS was significantly higher in cachexia patients than non-cachectic cancer patients and healthy controls (P < 0.001). BMI loss in last 6 mo showed a strong negative correlation with GPS (R = -0.758, P < 0.0001) and CRP (R = -0.570, P < 0.0001), however, a positive correlation with prealbumin (R = 0.302, P = 0.019).
Fasting glucose levels increased borderline significantly in cachectic patients compared with the healthy and non-cachectic patients (P = 0.05). Insulin levels were increased in non-cachectic gastric cancer patients when compared with cachectics (P = 0.04); however, there was no significant difference between non-cachectic cancer patients and healthy subjects (P > 0.05). After adjusting for the presence of diabetes mellitus, HOMA-IR values were not significantly different between groups. Only glucose showed an inverse significant correlation with BMI loss in 6 mo (R = -0.324, P = 0.012).
IGF-I levels significantly decreased in cachectic subjects compared with healthy and non-cachectic subjects (43.8 ± 9.5 pg/mL vs 95.0 ± 30.1 pg/mL and 63.1 ± 13.1 pg/mL; P < 0.05). Moreover, there was a significant difference in IGF-I levels between healthy and non-cachectic cancer patients (63.1 ± 13.1 pg/mL vs 43.8 ± 9.5 pg/mL; P < 0.05, Figure 1E). IGF-I also showed a positive correlation with BMI loss in the previous 6 mo (R = -0.679, P < 0.0001, Figure 1E) and GPS (R = 0.363, P = 0.004); negative correlations with age (R = -0.331, P =0.01) and fasting glucose (R = -0.358, P = 0.005, Table 4) were observed.
Multiple regression analysis was used to evaluate the role of BMI loss as a continuous variable, along with age, gender, and BMI change to predict ghrelin, resistin, leptin, adiponectin, and IGF-I levels. The results of the regression model indicated that age was not a significant predictor of hormone levels. Gender was a negative independent significant predictor for leptin, and BMI was found to be a negative independent significant predictor for all parameters, except adiponectin (Table 5).
In this prospective study, BMI loss in gastric cancer patients negatively correlated with serum active ghrelin, resistin, adiponectin and leptin levels, but positively correlated with the level of serum IGF-I. It was also noted that there was a correlation between resistin which was found to be high in cachectic patients, and insulin resistance, insulin and blood glucose.
Ghrelin an anabolic hormone, has several roles in metabolism, appetite, nutrition, weight gain, gastric motility, and gastric emptying. In addition, it has an important role in the regulation of synthesis of GH, IGF-I, insulin, and leptin[4–6]. Total ghrelin levels in cachectic patients with colon, breast and lung cancer were significantly higher than the levels in non-cachectic patients[78]. Garcia et al[20] showed that the ratio of active to total ghrelin levels increased in cancer-induced cachexia. In the same study, it was stated that the increase in active ghrelin levels could have been explained by ghrelin resistance. As the ratio of active/total ghrelin levels were in favor of active ghrelin in cancer cachexia, we evaluated active ghrelin levels. Our study showed that active ghrelin levels were higher in healthy subjects and non-cachectic cancer patients, especially females. Although we were not able to confirm the ghrelin resistance mentioned by Garcia et al[20], we saw the indirect effects. Under normal conditions, endogenous ghrelin increases GH secretion and indirectly increases IGF-I by stimulation of its own receptors. However in our study, the existence of a negative correlation between decreased IGF-I and ghrelin may show that efficiency decreases while the levels of active ghrelin increase. These findings support the presence of ghrelin resistance. It is also possible that ghrelin levels could increase to compensate for the increased metabolic rate and energy need, which was hypothesized by Nagaya et al[21] Several experimental studies have shown that ghrelin has an important role in the regulation of insulin via controlling pancreatic endocrine functions[2223]. No significant relationship between ghrelin and insulin, fasting glucose level and HOMA-IR were found in the present study.
Resistin is a member of the newly discovered family of cysteine-rich secretory proteins, called resistin-like proteins. The role of resistin in pathogenesis of insulin resistance remains questionable, with conflicting data in animal models and negative findings in clinical observation[1314]. The role of insulin resistance in cancer cachexia occurrence is not fully understood[12]. From this point of view, it came to mind whether resistin can have effect in gastric cancer cachexia occurrence. There are no clinical studies of serum resistin levels in cancer cachexia. It has been shown that serum resistin levels are high in lymphoma patients[24], but the resistin-tumor cachexia relationship was not investigated in this study. From this point of view, our study is the first study in the literature. We found that serum resistin levels in cachectic gastric cancer patients were significantly higher than the noncachectic patients and healthy controls. Resistin showed negative correlation with BMI loss. The effect of resistin on cachexia is probably due to insulin resistance and ineffective usage of glucose. The existence of a correlation between serum resistin levels and insulin, insulin resistance and blood glucose levels supports this idea[25]. The role of leptin in modulating the immune response and inflammation has become increasingly evident and has been reviewed recently[2627]. Complex interactions among the nervous, endocrine and immune systems affect the leptin loop and the potential role of these mediators in cancer-related cachexia-anorexia syndrome[81112]. Wallace et al[10] showed that serum levels of leptin did not differ between normal subjects and patients with gastrointestinal cancer. Other studies have shown that there is a relationship between cachexia and leptin levels in pancreatic, lung, breast, and colon cancer patients[81112]. Wolf et al[8] showed that changes in leptin levels in cancer cachexia were significantly higher. Our results were similar to this last study. It is known that leptin receptors are found in β islet cells of the pancreas and inhibit insulin secretion[2829]. However, our study revealed that there was a reverse correlation between leptin and fasting glucose levels, whereas no relation was found between leptin, insulin resistance, and insulin levels.
It also has been showed that insulin resistance and low serum IGF-I levels are important factors for cancer cachexia. These hormones are strongly anabolic and increase muscle protein synthesis[12]. IGF-I concentrations increase with growth hormone and testosterone administration, thereby accounting for some of the effects of these hormones on muscle bulk and strength. Low IGF-I concentrations in malnourished humans suggest a role for IGF-I in the pathogenesis of cachexia[30]. These findings showed that IGF-I was one of the most important factors in the gastric cancer cachexia.
GPS was found to be an important parameter for determining the prognosis of advanced cancers[1718]. In our study, besides the strong correlation between GPS and BMI loss, significant correlations were found between the GPS and the levels of hormones and cytokines which could be very important for clinical evaluation. GPS which is calculated using routine measurements of albumin and CRP can help us in evaluation and management of the cancer cachexia in clinical practice.
Adiponectin, which has an obvious anti-inflamma-tory effect, is inversely related to weight gain[4]. Serum adiponectin levels were low in cases with increased insulin resistance like obesity, type 2 diabetics, and non-alcoholic fatty liver diseases[30]. However, serum adiponectin levels decreased in patients who lost weight voluntarily. High adiponectin levels are risk factors for endometrial and breast cancer, whereas low levels are risk factors for gastric cancers[3132]. No relationship was found between adiponectin and cachexia in breast and colon cancer. In our study, a significant positive correlation was found between adiponectin and BMI loss, but multivariate analysis did not show BMI loss as predictive for adiponectin. Adiponectin, which is predominantly secreted from adipose tissues, might have increased due to lipolysis which occurred with muscle loss in the catabolic state. No correlation was found between adiponectin, whose close relationship with insulin resistance was known, and insulin, blood glucose levels, HOMA-IR, or IGF-I[4].
As a result, cachexia in gastric cancer is a complex process in which ghrelin, resistin, leptin, adiponectin, and IGF-I function. It is of note that these hormones have important roles in occurrence of cachexia in gastric cancer patients. This study will be one of the corner stones of the further studies about prevention and treatment of cachexia.
Adipocytokines are the peptide hormones secreted from adipocytes and have special roles in regulation of metabolism, glucose metabolism, and inflammation. Ghrelin which is secreted from especially from the gastric fundus has roles in regulation of blood glucose level, appetite and secretion of growth hormone. In this study, the role of adiponectin, ghrelin, and insulin like growth factor in gastric cancer patients with cachexia is evaluated.
Significant correlation between cachexia and ghrelin, leptin adiponectin and IGF-I can inform more sophisticated studies about the mechanism of cachexia. Despite sufficient nutritional support, cachexia is always an expected morbidity in cancer patients. This study will help future studies about the pathophysiology of the cachexia.
Although there have been several studies concerning nutritional support and measurement of cachexia in cancer patients, there have not been many studies about the pathophysiology. It is important to understand the behaviour of cancer with cachexia and also to support the patient. Several factors contributing to cancer biology demand attention. However, this study is original for being the first evaluating the correlation between cancer cachexia and resistin. The relationship between adipocytokines and colon, lung and breast cachexia have been studied before. This study has investigated the relationship between gastric cancer cachexia and adipocytokines.
To date, the treatment of cancer cachexia has gone no further than nutritional and fluid-electrolyte support. Alternative treatment modalities may be identified if the role of hormones and peptides in cachexia is well-understood. For example, administration of recombinant ghrelin induces growth hormone secretion and appetite.
Adipocytokines are secreted from adipocytes. They are peptide hormones that have systemic effect. For example: resistin, adiponectin, leptin, etc. Cachexia means loss of lipid, carbohydrate and protein in a short time.
This paper investigated resistin, ghrelin, leptin, adiponectin, insulin, IGF-I in gastric cancer subjects with and without cachexia and healthy controls. Ghrelin, resistin, adiponectin significantly differed between subjects with and without cachexia. The study was well described and appropriately presented. It’s an important study.
| 1. | Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2:862-871. [Cited in This Article: ] |
| 2. | Yoshikawa T, Noguchi Y, Doi C, Makino T, Nomura K. Insulin resistance in patients with cancer: relationships with tumor site, tumor stage, body-weight loss, acute-phase response, and energy expenditure. Nutrition. 2001;17:590-593. [Cited in This Article: ] |
| 3. | Huang Q, Nai YJ, Jiang ZW, Li JS. Change of the growth hormone-insulin-like growth factor-I axis in patients with gastrointestinal cancer: related to tumour type and nutritional status. Br J Nutr. 2005;93:853-858. [Cited in This Article: ] |
| 4. | Meier U, Gressner AM. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem. 2004;50:1511-1525. [Cited in This Article: ] |
| 5. | Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656-660. [Cited in This Article: ] |
| 6. | Gualillo O, Lago F, Gomez-Reino J, Casanueva FF, Dieguez C. Ghrelin, a widespread hormone: insights into molecular and cellular regulation of its expression and mechanism of action. FEBS Lett. 2003;552:105-109. [Cited in This Article: ] |
| 7. | Shimizu Y, Nagaya N, Isobe T, Imazu M, Okumura H, Hosoda H, Kojima M, Kangawa K, Kohno N. Increased plasma ghrelin level in lung cancer cachexia. Clin Cancer Res. 2003;9:774-778. [Cited in This Article: ] |
| 8. | Wolf I, Sadetzki S, Kanety H, Kundel Y, Pariente C, Epstein N, Oberman B, Catane R, Kaufman B, Shimon I. Adiponectin, ghrelin, and leptin in cancer cachexia in breast and colon cancer patients. Cancer. 2006;106:966-973. [Cited in This Article: ] |
| 9. | Neary NM, Small CJ, Wren AM, Lee JL, Druce MR, Palmieri C, Frost GS, Ghatei MA, Coombes RC, Bloom SR. Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89:2832-2836. [Cited in This Article: ] |
| 10. | Wallace AM, Kelly A, Sattar N, McArdle CS, McMillan DC. Circulating concentrations of "free" leptin in relation to fat mass and appetite in gastrointestinal cancer patients. Nutr Cancer. 2002;44:157-160. [Cited in This Article: ] |
| 11. | Brown DR, Berkowitz DE, Breslow MJ. Weight loss is not associated with hyperleptinemia in humans with pancreatic cancer. J Clin Endocrinol Metab. 2001;86:162-166. [Cited in This Article: ] |
| 12. | Bolukbas FF, Kilic H, Bolukbas C, Gumus M, Horoz M, Turhal NS, Kavakli B. Serum leptin concentration and advanced gastrointestinal cancers: a case controlled study. BMC Cancer. 2004;4:29. [Cited in This Article: ] |
| 13. | Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307-312. [Cited in This Article: ] |
| 14. | Kusminski CM, McTernan PG, Kumar S. Role of resistin in obesity, insulin resistance and Type II diabetes. Clin Sci (Lond). 2005;109:243-256. [Cited in This Article: ] |
| 15. | Faraj M, Havel PJ, Phelis S, Blank D, Sniderman AD, Cianflone K. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2003;88:1594-1602. [Cited in This Article: ] |
| 16. | Jamieson NB, Brown DJ, Michael Wallace A, McMillan DC. Adiponectin and the systemic inflammatory response in weight-losing patients with non-small cell lung cancer. Cytokine. 2004;27:90-92. [Cited in This Article: ] |
| 17. | Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. Br J Cancer. 2004;90:1704-1706. [Cited in This Article: ] |
| 18. | Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2003;89:1028-1030. [Cited in This Article: ] |
| 19. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [Cited in This Article: ] |
| 20. | Garcia JM, Garcia-Touza M, Hijazi RA, Taffet G, Epner D, Mann D, Smith RG, Cunningham GR, Marcelli M. Active ghrelin levels and active to total ghrelin ratio in cancer-induced cachexia. J Clin Endocrinol Metab. 2005;90:2920-2926. [Cited in This Article: ] |
| 21. | Nagaya N, Uematsu M, Kojima M, Date Y, Nakazato M, Okumura H, Hosoda H, Shimizu W, Yamagishi M, Oya H. Elevated circulating level of ghrelin in cachexia associated with chronic heart failure: relationships between ghrelin and anabolic/catabolic factors. Circulation. 2001;104:2034-2038. [Cited in This Article: ] |
| 22. | Prado CL, Pugh-Bernard AE, Elghazi L, Sosa-Pineda B, Sussel L. Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proc Natl Acad Sci USA. 2004;101:2924-2929. [Cited in This Article: ] |
| 23. | Wierup N, Yang S, McEvilly RJ, Mulder H, Sundler F. Ghrelin is expressed in a novel endocrine cell type in developing rat islets and inhibits insulin secretion from INS-1 (832/13) cells. J Histochem Cytochem. 2004;52:301-310. [Cited in This Article: ] |
| 24. | Pamuk GE, Demir M, Harmandar F, Yesil Y, Turgut B, Vural O. Leptin and resistin levels in serum of patients with hematologic malignancies: correlation with clinical characteristics. Exp Oncol. 2006;28:241-244. [Cited in This Article: ] |
| 25. | Burcelin R. Leptin and resistin: master enemy adipokines unified in brain to control glucose homeostasis. Endocrinology. 2008;149:443-444. [Cited in This Article: ] |
| 26. | La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4:371-379. [Cited in This Article: ] |
| 27. | Kieffer TJ, Heller RS, Habener JF. Leptin receptors expressed on pancreatic beta-cells. Biochem Biophys Res Commun. 1996;224:522-527. [Cited in This Article: ] |
| 28. | Ahren B, Havel PJ. Leptin inhibits insulin secretion induced by cellular cAMP in a pancreatic B cell line (INS-1 cells). Am J Physiol. 1999;277:R959-R966. [Cited in This Article: ] |
| 29. | Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79-83. [Cited in This Article: ] |
| 30. | Dal Maso L, Augustin LS, Karalis A, Talamini R, Franceschi S, Trichopoulos D, Mantzoros CS, La Vecchia C. Circulating adiponectin and endometrial cancer risk. J Clin Endocrinol Metab. 2004;89:1160-1163. [Cited in This Article: ] |
| 31. | Miyoshi Y, Funahashi T, Kihara S, Taguchi T, Tamaki Y, Matsuzawa Y, Noguchi S. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res. 2003;9:5699-5704. [Cited in This Article: ] |
| 32. | Ishikawa M, Kitayama J, Kazama S, Hiramatsu T, Hatano K, Nagawa H. Plasma adiponectin and gastric cancer. Clin Cancer Res. 2005;11:466-472. [Cited in This Article: ] |