Published online Apr 28, 2008. doi: 10.3748/wjg.14.2561
Revised: January 21, 2008
Published online: April 28, 2008
AIM: To compare the effect of oral erythromycin vs no preparation with prokinetics on the transit time and the image quality of capsule endoscopy (CE) in evaluating small bowel (SB) pathology.
METHODS: We conducted a retrospective, blinded (to the type of preparation) review of 100 CE studies, 50 with no preparation with prokinetics from one medical center (Group A) and 50 from another center with administration of a single dose of 200 mg oral erythromycin 1 h prior to CE (Group B). Gastric, SB and total transit times were calculated, the presence of bile in the duodenum was scored, as was cleanliness within the proximal, middle and distal intestine.
RESULTS: The erythromycin group had a slightly shorter gastric transit time (21 min vs 28 min, with no statistical significance). SB transit time was similar for both groups (all P > 0.05). Total transit time was almost identical in both groups. The rate of incomplete examination was 16% for Group A and 10% for Group B (P = 0.37). Bile and cleanliness scores in different parts of the intestine were similar for the two groups (P > 0.05).
CONCLUSION: Preparation for capsule endoscopy with erythromycin does not affect SB or total transit time. It tends to reduce gastric transit time, but it does not increase the cecum-reaching rate. Erythromycin does not adversely affect image quality. We consider the routine use of oral erythromycin preparation as being unjustified, although it might be considered in patients with known prolonged gastric emptying time.
- Citation: Niv E, Bogner I, Barkay O, Halpern Z, Mahajna E, Depsames R, Kopelman Y, Fireman Z. Effect of erythromycin on image quality and transit time of capsule endoscopy: A two-center study. World J Gastroenterol 2008; 14(16): 2561-2565
- URL: https://www.wjgnet.com/1007-9327/full/v14/i16/2561.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2561
Capsule endoscopy (CE) is a well accepted methodology which provides a direct and noninvasive way to view the entire small bowel (SB) mucosa[1–6]. Capsule technology has enriched our knowledge about small bowel pathology and revolutionized the management of SB diseases[78]. The best way to ensure the most complete and high-quality visualization of the entire small bowel is, however, a subject of controversy in the literature. In addition to improving the technical characteristics of the capsule itself, two ways of achieving these aims are proper preparation and the use of prokinetics[9]. Decreasing gastric and SB transit times were expected to allow the capsule to successfully reach the cecum and to overcome the problem of the capsule’s too-short battery lifetime (7 ± 1 h).
Erythromycin is a well-known prokinetic agent. It induces high amplitude gastric propulsive contractions by the initiation of gastric interdigestive migrating motor complexes. As a result, it accelerates gastric emptying, including that of indigestible particles[10–12]. The effect of erythromycin on small bowel motility is less known. As such, its use to accelerate capsule transit time in the stomach and probably in the small intestine seemed to be an attractive idea, and several studies were conducted to explore this possibility[13–15]. The number of such studies is very small and each includes few patients. Another aspect that has not yet been investigated is whether or nor erythromycin stimulates the biliary-pancreatic secretions to the small intestine, thereby adversely affecting image quality.
Uncertainty about these preparation-related issues of CE led to the uses of different CE preparation protocols among medical centers. Two Israeli medical centers have been routinely following two different protocols: patients undergoing CE at the Tel-Aviv Sourasky Medical Center (TASMC) are instructed to undergo a standard 12-h fast while those is examined at the Hillel-Yaffe Medical Center (HYMC) fast for 12 h and also take oral erythromycin to accelerate gastric emptying. The objective of the current study was to compare the CE data from these two medical centers in order to determine whether the addition of erythromycin leads to a relative acceleration of gastric and small intestinal transit times of the capsule without adversely affecting its image quality.
This retrospective study was conducted in two Israeli medical centers, TASMC and HYMC. The protocol of the study was approved by the local Helsinki committees of both centers. The films of 50 capsules were randomly chosen from each center’s Department of Gastroenterology’s archives between 2000 and 2007. All 50 of the TASMC patients underwent a standard 12-h overnight fast preparation protocol (Group A), while all 50 of the HYMC patients followed a protocol that involved a 12-h overnight fast and a single dose of 200 mg oral erythromycin taken 1 h prior to undergoing the test (Group B). The CE procedure was standard in both centers: all patients ingested a PilliCamTM SB wireless video capsule (Given® Diagnostic Imaging System, Yokneam, Israel) with a small amount of water. An array of 8 sensors was attached to the abdominal wall, and a belt holding a recorder with a battery was fastened around their waists. Patients were allowed to drink 2 h after ingesting the capsule and to eat a light meal 4 h later. Eight h after capsule ingestion, the recorder was disconnected and the sensors were removed. The recorded digital information was downloaded onto the computer and the images were analyzed using RAPID® software. The provenance of the films was concealed from an independent expert who reviewed all 100 films.
The following data were collected from the CE studies and the CE endoscopy reports of all participating patients: demographics, the indications for referral to the procedure, the findings in the small intestine, and the rate of cecal arrival (i.e., the completeness of evaluation). The gastric transit time was calculated from the first view of the gastric mucosa to the first image of the duodenum and it was expressed in minutes. The SB transit time was calculated from the first view of the duodenum up to the first cecal image and it, too, was expressed in minutes. If the capsule did not reach the cecum during the battery’s lifetime, the SB transit time was calculated as 480 min (8 h) minus gastric transit time.
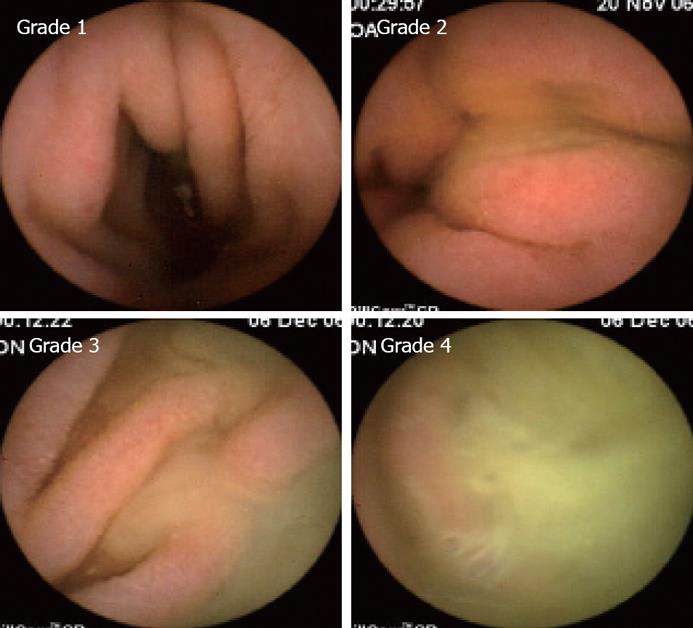
The presence of bile in the duodenum lumen was evaluated using a scale of 1 to 4, with 1 representing none and 4 indicating more than 90% of the lumen being full of bile (Figure 1). The quality of visualization of SB mucosa (i.e., cleanliness) was also evaluated by a 4-point scale, with 1 representing no residual material in the lumen and 4 indicating more than 90% of the lumen having residual material. The SB section of the CE study was divided into three parts, proximal, middle and distal intestine, and each was given a separate grading of cleanliness. A CE study was defined as having been completed if the capsule reached the cecum.
Comparisons between patients with and without erythromycin preparation with regard to demographic (age, gender) and clinical parameters (transit time, indications, diagnosis, etc) were performed using the Chi-square, Fisher’s Exact and unpaired t-tests. The non-parametric Mann-Whitney test was applied since the continuous parameters did not follow a normal distribution. The relationships between transit time and other continuous parameters were examined by the Spearman’s correlation coefficients. This was done for the entire sample and for each group separately (according to preparation type). The statistical analysis was performed by the SPSS for Windows software (Chicago, USA), version 14.0. The statistical tests were defined as having a confidence interval of 95%. A P value < 0.05 was considered significant for all tests performed.
A total of 100 CE studies were reviewed by an indepen-dent expert who was blinded to the center-belonging of the films. Fifty patients were from TASMC who used no preparation (Group A) and 50 patients were from HYMC who used an erythromycin preparation (Group B). In two studies (one from TASMC and one from HYMC), the gastric transit time was extremely prolonged (longer than 3 h) and, therefore, each of those studies was replaced by a randomly chosen one from the each center’s archives.
Table 1 summarizes the study cohort’s demographic and other relevant clinical data. Both groups were fairly similar, with the exception of the greater number of cases with a final diagnosis of Crohn’s disease belonging to Group B (i.e., 1 case in Group A vs 7 cases in Group B, P = 0.06).
| Parameter | No erythromycin | Erythromycin | P |
| (n = 50) | (n = 50) | ||
| Age, yr (mean ± SD) | 51.7 ± 21.6 | 52.1 ± 19.4 | 0.931 |
| Male/Female | 32/18 | 36/14 | 0.39 |
| Indications | 0.40 | ||
| Suspected Crohn’s | 19 | 18 | 0.84 |
| Obscure GI bleeding | 23 | 28 | 0.32 |
| Others2 | 8 | 4 | 0.22 |
| Final diagnosis in SB | 0.10 | ||
| Normal findings | 23 | 19 | 0.42 |
| Non-specific findings | 9 | 7 | 0.59 |
| Crohn’s disease | 1 | 7 | 0.06 |
| Angiodysplasia | 7 | 2 | 0.16 |
| Polyps | 1 | 3 | 0.62 |
| Others2 | 9 | 12 | 0.46 |
Table 2 compares the transit times, the rates at which the capsule reached the cecum, and the bile and cleanliness scores between the two groups. There was a trend towards a shorter gastric transit time for the patients in Group B (28 min in Group A vs 21 min in Group B, P = 0.07, unpaired t-test). The Mann-Whitney test (which is more accurate in groups with high variability) did not, however, confirm this finding (P = 0.16). Nevertheless, there was a higher variability of gastric transit times among the patients within Group A compared with the variability within Group B, which had more uniformity (P = 0.076 according to Levene’s test for equality of variances).
| No erythromycin | Erythromycin | P | |
| (n = 50) | (n = 50) | ||
| Gastric transit time (min) | 28.36 ± 23.56 | 21.08 ± 15.13 | 0.071 |
| 0.16 | |||
| SB transit time (min) | 270.42 ± 108.13 | 279.14 ± 103.89 | 0.83 |
| Total transit time (min) | 299.26 ± 108.18 | 300.22 ± 103.25 | 0.97 |
| Did not reach the cecum (%) | 8 (16%) | 5 (10%) | 0.37 |
| Bile presence score | 1.75 ± 0.61 | 1.76 ± 0.58 | 0.99 |
| Cleanliness score | |||
| Proximal SB | 1.78 ± 0.76 | 1.77 ± 0.74 | 1.00 |
| Middle SB | 2.22 ± 0.75 | 2.16 ± 0.77 | 0.73 |
| Distal SB | 2.79 ± 0.65 | 2.75 ± 0.74 | 0.73 |
There were no group differences in SB transit time (270 min in Group A vs 279 min in Group B) or in total transit time (299 min vs 300 min, respectively; Table 2). The capsule did not reach the cecum during the battery’s lifetime in 8 Group A cases (16%) compared with 5 Group B cases (10%, P > 0.05). There was no case of capsule retention. Even after excluding from the calculations those cases in which the capsule did not reached the cecum, there were still no significant differences between Group A and Group B in SB and total transit times: the respective SB transit times were 237.50 ± 82.69 min and 259.29 ± 89.28 min (P = 0.44), and the respective total transit times were 264.83 ± 79.99 min and 280.24 ± 88.27 min (P = 0.56).
A comparison of the presence of bile (a score of 1.75 in Group A vs 1.76 in Group B, P = 0.99) and of the cleanliness scores in different parts of the SB failed to show any differences as well (Table 2). As expected in each group, the presence of residual material was more prominent in the distal than in the proximal intestine, assuring a better visualization of the SB mucosa in the proximal intestine.
We sought to determine whether or not the gastric transit time in cases in which the capsule failed to reach the cecum was longer than in cases with completed tests, assuming that extended transit time may consume valuable battery time for gastric visualization than for the small intestine. It emerged that the gastric transit time was fairly similar for all subgroups, regardless of cecum reachability (Table 3).
| No erythromycin | Erythromycin | P | |
| (n = 50) | (n = 50) | value | |
| Gastric transit time in cases failing to reach cecum (min) | 36.75 ± 33.93 (n = 8) | 22.2 ± 14.45 (n = 5) | 0.52 |
| Gastric transit time in cases where cecum was reached (min) | 26.76 ± 21.22 (n = 42) | 20.96 ± 15.36 (n = 45) | 0.24 |
| P | 0.52 | 0.81 |
An additional analysis was performed to explore the relationships between the patient’s clinical picture and capsule transit times (Table 4). The two major indications for undergoing CE that were chosen for this assessment were a clinical picture suspicious for Crohn’s disease (abdominal pain, diarrhea, elevated CRP, anemia, etc) and for obscure gastrointestinal (GI) bleeding (OGIB). There were no differences in gastric transit times between these two indications for either Group A or Group B or between the two groups. SB transit time was, however, significantly longer in the cases suspected to have Crohn’s disease than OGIB in Group B and almost significantly longer in Group A (P = 0.04 and P = 0.063, respectively). Neither the preparation with erythromycin nor the indication for CE affected the cecum-reaching rates in both groups (P = 0.160 and P = 0.452, respectively).
| Transit time | Indication | No erythromycin | Erythromycin | P |
| (n = 50) | (n = 50) | |||
| Gastric transit time (min) | Crohn’s disease | 27.16 ± 26.11 | 16.72 ± 13.21 | 0.19 |
| OGIB | 29.82 ± 23.53 | 23.61 ± 16.49 | 0.43 | |
| P | 0.41 | 0.16 | ||
| Small bowel transit time (min) | Crohn's disease | 285.53 ± 96.31 | 324.50 ± 114.57 | 0.28 |
| OGIB | 230.39 ± 103.00 | 255.32 ± 90.81 | 0.44 | |
| P | 0.066 | 0.04 |
Age below or above 40 years old (decided arbitrarily) did not affect gastric or SB transit time, but there was some tendency for shorter total transit time in patients above age 40 only in Group A (P = 0.07). In addition, Group A demonstrated a borderline gender-dependent difference: females in Group A tended to have a shorter gastric transit time and a longer SB transit time (P = 0.052 and P = 0.069, respectively). No influence of age or gender on transit times was evidenced in Group B.
The results of our comparative two-center study demonstrate that preparation with oral erythromycin before a CE study does not affect SB transit time, total transit time or the rate of the capsule in reaching the cecum. The medication may have had a possible shortening effect on gastric transit time and it tended to reduce the variability of gastric transit times among the patients in the group that used it. The length of gastric time does not, however, predict the likelihood that the capsule will reach the cecum during the battery’s lifetime. We demonstrated that the indication for patients undergoing CE affects the SB transit time, but not any of the other examined parameters, and that the influences of age and gender are only marginal. Erythromycin does not affect adversely an image quality as measured by presence of bile and residual material in the intestine.
Our study is the largest thus far to address the issue of erythromycin preparation before a CE study. Leung et al[14] conducted a small prospective comparative study on 24 patients who received an oral erythromycin preparation vs 14 patients who were not given any prokinetics. While no differences were found in SB transit times, in cecum arrival rates or in the quality of images, there was a highly significant difference in gastric transit time between these two groups (16 min vs 70 min, respectively, P = 0.005). Caddy et al[13] performed a study of similar design (22 and 23 patients in each group) and demonstrated no significant difference in gastric and SB transit times, cecum arrival rate or image quality between the two groups. The third published study on this issue was conducted by Fireman et al[15]. Their investigation included one group prepared with polyethylene glycol (26 patients), another with erythromycin (29 patients) and a third with no preparation (40 patients). According to their results, erythromycin had no effect on SB transit time, but it did show some tendency for shortening gastric transit time. The erythromycin group in Fireman et al study[15] had significantly more residual material in the lumen and poorer image quality.
Altogether, there is general agreement among the available studies that erythromycin does not affect SB transit time but that it may or may not shorten gastric time. There are some minor differences in design between those earlier studies and our current one. We excluded the patients with extremely prolonged gastric emptying time (> 3 h) in order to prevent skewing of the data during the statistical analysis (1 patient from Group A and 1 patient from Group B). In addition, the dosage of erythromycin used in the other studies was slightly higher than in ours (250 mg vs 200 mg, respectively) although the route of administration (oral) and timing (1 h prior to CE study) were identical. Our study was retrospective, but all the CE studies were re-examined by an independent expert who was blind to the hospital in which any given CE study had been performed.
The question about whether or not erythromycin stimulates the secretion of bile and by doing so impairs the image quality in the duodenum had not been previously explored in CE studies. Using a bile score, we could now clearly show that there is no such stimulation of bile secretion. As for the controversial issue of erythromycin’s affecting the cleanliness score, we used a separate score for the proximal, middle and distal parts of the intestine and found no negative impact of the medication on the cleanliness of the intestine.
In conclusion, we suggest that the routine use of oral erythromycin 200-250 mg for SB or total transit time in CE is unfounded. It should, however, be considered as part of the preparation for patients with known prolonged gastric emptying time (i.e. either from a previous CE study or other imaging tests) or in patients with symptoms characteristic of gastroparesis (e.g. feeling of upper abdominal fullness, vomiting, etc). The optimal dosage (probably higher dosage) and the preferred route of administration of erythromycin (intravenous vs oral) in the setting of CE await further study.
Capsule endoscopy (CE) is a well accepted methodology which provides a direct and noninvasive way to view the entire small bowel (SB) mucosa. The best way to ensure the most complete and high-quality visualization of the entire small bowel is, however, a subject of controversy in the literature. The aim of this study is to compare the effect of oral erythromycin vs no preparation with prokinetics on the transit time and the image quality of CE in evaluating SB pathology.
We conducted a retrospective, blinded (to the type of preparation) review of 100 CE studies, 50 with no preparation with prokinetics from one medical center (Group A) and 50 from another center with administration of a single dose of 200 mg oral erythromycin 1 h prior to CE (Group B). The results of the study demonstrated that preparation for capsule endoscopy with erythromycin does not affect SB or total transit time (P > 0.05). It tends to reduce gastric transit time (P = 0.07), but it does not increase the cecum-reaching rate (P = 0.37). Erythromycin does not adversely affect image quality (both bile score and the score of cleanliness). We consider the routine use of oral erythromycin preparation as being unjustified, although it might be considered in patients with known prolonged gastric emptying time.
Our study is the largest thus far to address the issue of erythromycin preparation before a CE study. In addition, we used a new bile score and a separate score of cleanliness for different parts of the small intestine. These innovations made the conclusions more precise. Our study was retrospective, but all the CE studies were re-examined by an independent expert who was blind to the hospital in which any given CE study had been performed. We concluded that the routine use of oral erythromycin 200 mg-250 mg for SB or total transit time in CE is unfounded. It should, however, be considered as part of the preparation for patients with known prolonged gastric emptying time (i.e. either from a previous CE study or other imaging tests) or in patients with symptoms characteristic of gastroparesis (e.g. feeling of upper abdominal fullness, vomiting, etc).
The conclusions of our study may spare to the patients an unnecessary use of antibiotics before the capsule procedure.
This is a retrospective comparative study comparing two preparation regimens for capsule endoscopy. One group received oral erythromycin and the second did not receive a prokinetic preparation. The study aimed to look for differences in capsule transit time, but was not able to demonstrate an advantage for the erythromycin group. The paper is well written and the study design although retrospective adequate.
| 1. | Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature. 2000;405:417. [Cited in This Article: ] |
| 2. | Scapa E, Jacob H, Lewkowicz S, Migdal M, Gat D, Gluckhovski A, Gutmann N, Fireman Z. Initial experience of wireless-capsule endoscopy for evaluating occult gastrointestinal bleeding and suspected small bowel pathology. Am J Gastroenterol. 2002;97:2776-2779. [Cited in This Article: ] |
| 3. | Costamagna G, Shah SK, Riccioni ME, Foschia F, Mutignani M, Perri V, Vecchioli A, Brizi MG, Picciocchi A, Marano P. A prospective trial comparing small bowel radiographs and video capsule endoscopy for suspected small bowel disease. Gastroenterology. 2002;123:999-1005. [Cited in This Article: ] |
| 4. | Ell C, Remke S, May A, Helou L, Henrich R, Mayer G. The first prospective controlled trial comparing wireless capsule endoscopy with push enteroscopy in chronic gastrointestinal bleeding. Endoscopy. 2002;34:685-689. [Cited in This Article: ] |
| 5. | Fireman Z, Mahajna E, Broide E, Shapiro M, Fich L, Sternberg A, Kopelman Y, Scapa E. Diagnosing small bowel Crohn's disease with wireless capsule endoscopy. Gut. 2003;52:390-392. [Cited in This Article: ] |
| 6. | Mylonaki M, Fritscher-Ravens A, Swain P. Wireless capsule endoscopy: a comparison with push enteroscopy in patients with gastroscopy and colonoscopy negative gastrointestinal bleeding. Gut. 2003;52:1122-1126. [Cited in This Article: ] |
| 7. | Rey JF, Ladas S, Alhassani A, Kuznetsov K. European Society of Gastrointestinal Endoscopy (ESGE). Video capsule endoscopy: update to guidelines (May 2006). Endoscopy. 2006;38:1047-1053. [Cited in This Article: ] |
| 8. | Mishkin DS, Chuttani R, Croffie J, Disario J, Liu J, Shah R, Somogyi L, Tierney W, Song LM, Petersen BT. ASGE Technology Status Evaluation Report: wireless capsule endoscopy. Gastrointest Endosc. 2006;63:539-545. [Cited in This Article: ] |
| 9. | Villa F, Signorelli C, Rondonotti E, de Franchis R. Preparations and prokinetics. Gastrointest Endosc Clin N Am. 2006;16:211-220. [Cited in This Article: ] |
| 10. | Prather CM, Camilleri M, Thomforde GM, Forstrom LA, Zinsmeister AR. Gastric axial forces in experimentally delayed and accelerated gastric emptying. Am J Physiol. 1993;264:G928-G934. [Cited in This Article: ] |
| 11. | Keshavarzian A, Isaac RM. Erythromycin accelerates gastric emptying of indigestible solids and transpyloric migration of the tip of an enteral feeding tube in fasting and fed states. Am J Gastroenterol. 1993;88:193-197. [Cited in This Article: ] |
| 12. | Bruley des Varannes S, Parys V, Ropert A, Chayvialle JA, Roze C, Galmiche JP. Erythromycin enhances fasting and postprandial proximal gastric tone in humans. Gastroenterology. 1995;109:32-39. [Cited in This Article: ] |
| 13. | Caddy GR, Moran L, Chong AK, Miller AM, Taylor AC, Desmond PV. The effect of erythromycin on video capsule endoscopy intestinal-transit time. Gastrointest Endosc. 2006;63:262-266. [Cited in This Article: ] |
| 14. | Leung WK, Chan FK, Fung SS, Wong MY, Sung JJ. Effect of oral erythromycin on gastric and small bowel transit time of capsule endoscopy. World J Gastroenterol. 2005;11:4865-4868. [Cited in This Article: ] |
| 15. | Fireman Z, Paz D, Kopelman Y. Capsule endoscopy: improving transit time and image view. World J Gastroenterol. 2005;11:5863-5866. [Cited in This Article: ] |